Abstract
Adipose-derived stem cells (ASCs) offer a potential alternative for tissue repair and regeneration. We have recently shown that hypoxia stimulates ASCs and enhances the regenerative potential of ASCs, which is beneficial for ASC therapy. In the present study, we further investigated a key mediator and a signal pathway involved in the stimulation of ASC during hypoxia. Culturing ASC in a hypoxic incubator (2% oxygen tension) increased the proliferation and migration, and this was mediated by Akt and ERK pathways. To determine the generation of reactive oxygen species (ROS), 2′,7′-dichlorofluorescin diacetate intensity was detected by fluorescence-activated cell sorting. Hypoxia significantly increased the dichlorofluorescin diacetate intensity, which was greatly reduced by N-acetyl-cysteine and diphenyleneiodonium treatment. Likewise, the hypoxia-induced proliferation and migration of ASCs were reversed by N-acetyl-cysteine and diphenyleneiodonium treatment, suggesting the involvement of ROS generation in ASC stimulation. Further, we examined the activation of receptor tyrosine kinases and observed that hypoxia stimulated the phosphorylation of platelet-derived growth factor receptor-β. In summary, the ROS produced by ASCs in response to hypoxia was mostly likely due to NADPH oxidase activity. The increased cellular ROS was accompanied by the phosphorylation of platelet-derived growth factor receptor-β as well as by the activation of ERK and Akt signal pathways. Our results suggest a pivotal role for ROS generation in the stimulation of ASCs by hypoxia.
Introduction
Adipose-derived stem cells (ASCs) have recently been considered as a substitute for other stem cell sources to offer a potential alternative for tissue repair and regeneration [1–6]. For example, we have demonstrated that ASCs promote wound healing and hair growth [5,7]. In those studies, the treatment of a conditioned medium of ASCs (ASC-CM) stimulated dermal fibroblasts and papilla cells, and ASC transplantation accelerated wound healing and hair regeneration in vivo. Of note, the hypoxia-cultured ASCs and CM induced a significant increase in wound-healing and hair-growth potential compared with normal culture conditions [8,9]. Likewise, the beneficial effects of culturing ASC under hypoxic conditions has been reported in various experimental systems [10–14]. Therefore, hypoxia appears to play a key stimulating role during ASC expansion, although the expansion and regenerative potential of ASCs are influenced by multiple factors such as serum contents, basal medium type, glucose concentration, stable glutamine, cell-plating density, and plastic surface quality.
ASCs reside in anatomical sites that are relatively oxygen deficient (although ASCs reside in a perivascular location, the vessels might be associated with venous structures and a partial pressure of oxygen at 40–60 mmHg), and hypoxia may provide signals conducive to the maintenance of definitive ASC properties [15,16]. Despite the low oxygen preference, ASCs are usually cultured under normoxia (20%–21% O2 condition). Therefore, an appropriate hypoxic condition may be beneficial and invaluable for developing novel cell therapy with ASCs. For example, Rehman et al. reported that hypoxia increased antiapoptotic and angiogenic growth factor secretion of ASC, which increased the recovery from hind-limb ischemia [11]. Our group also demonstrated that hypoxia-expanded ASCs enhanced antioxidant and angiogenic growth factor secretion to accelerate skin regeneration [8,9]. However, all those studies focused on the chronic response to hypoxia whereby stabilization of HIF-1α enhanced the secretion of target proteins and increased the regenerative potential of ASCs. On the contrary, the acute intra-cellular responses of ASCs (ie, involved membrane receptors and signal pathways) during hypoxia have not yet been clearly identified.
Evidence suggests that membrane receptors and signal pathways are stimulated by acute hypoxia in various cell systems. For example, hypoxia increased proliferation of cancer and endothelial cells by activating the Akt and ERK1/2 pathways [17–19]. Further, hypoxia-induced epithelial growth factor receptor and platelet-derived growth factor receptor (PDGFR) tyrosine kinase activation have been demonstrated in some cell types [19–21]. Wang et al. reported the signal pathway involved in the growth factor secretion of mesenchymal stem cells, and that hypoxia-induced secretion was associated with increased activation of p38 mitogen-activated protein kinase [22]. In addition, involvement of phosphatidylinositol 3 kinase/Akt, a mammalian target for rapamycin, focal adhesion kinase, and Src phosphorylation has been demonstrated in the hypoxia-induced proliferation and migration of embryonic stem cells [23]. It is unknown, however, if they are involved in an acute response to hypoxia and in the stimulation of ASCs. In the present work, we investigated if there is a key stimulating factor that mediates and initiates the cellular responses of ASCs during hypoxia and the signal pathways involved in the stimulation of ASCs.
Materials and Methods
Cell culture and inhibition conditions
Sampling of human subcutaneous adipose tissue and isolation of ASCs were previously reported [7,24]. ASCs were characterized by flow cytometry using cell surface markers (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). ASCs were grown in a minimum essential medium alpha medium (Gibco, Invitrogen) with 10% fetal bovine serum (Gibco), and 1% penicillin and streptomycin (Gibco) at 37°C in a humidified atmosphere containing either 5% CO2 plus 20% O2 (Normoxia) or 5% CO2 plus 2% O2 with the balanced N2 (Hypoxia). ASCs were used between passage 5 and passage 9. ASCs were characterized using cell surface markers such as CD34, CD73, CD90, and CD105 (data not shown). Various inhibition conditions such as 5 μM LY294002 (Calbiochem), 5 μM U0126 (Calbiochem), 100 μM–1 mM N-acetyl-cysteine (NAC; Sigma-Aldrich, 100–500 nM diphenyleneiodonium (DPI; Sigma), and 5–20 μM AG1296 (Calbiochem) were used in the present study.
Proliferation assay
ASCs were plated overnight in triplicate 48-well plates at a density of 5,000–7,000 cells per well in the complete medium. After 24 h, the medium was replaced with serum-free medium containing 1% penicillin and streptomycin. The following day after seeding, cells were incubated in either normoxia (20% O2, 5% CO2) or hypoxia (2% O2, 5% CO2 and balanced N2) and either with or without chemical inhibitors for 48–72 h. After incubation, the medium was removed and the cell number was measured using a CCK-8 assay kit (Dojindo). CCK-8 solution (150 μL) was added to each well followed by incubation for 2 h. After incubation, the absorbance was measured at 450 nm using a microplate reader (TECAN).
Migration assay
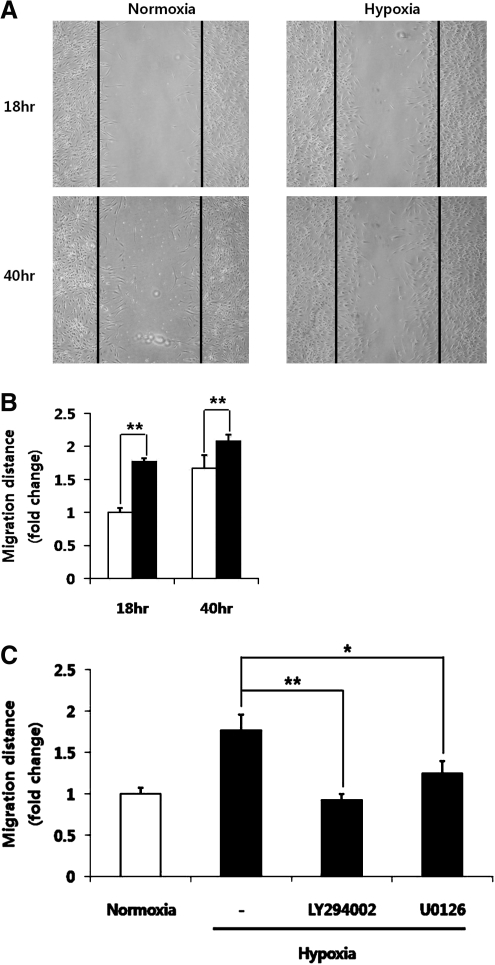
For the migration assay, ASCs (5×105 cells/well) were seeded in 6-well plates with a complete medium. The following day, confluent ASCs were kept in the serum-free medium for 12–24 h. Serum-starved ASCs were wounded using a migration micropipette tip and were incubated with or without inhibitors in hypoxia and normoxia. Cell migration was quantified by microscopic examination at 18 and 40 h after wounding. For the evaluation of ASC migration, 5 randomly selected points along each wound were marked, and the horizontal distances of migrating cells from the initial wound (black line in Fig. 2A) were measured. Relative migration distance was compared with that of control (normoxia) and fold change was evaluated.
FIG. 2.
Effect of hypoxia on the migration of ASCs. Note that hypoxia significantly increased the migration of ASCs. Photographs were taken to measure the migration distance of ASCs at 18 and 40 h after scraping (A, black line represents an initial wound), and the hypoxia-induced migration distance was evaluated. (B, normoxia: white bars, hypoxia: black bars). LY294002 (5 μM) and U0126 (5 μM) significantly decreased the hypoxia-induced migration of ASCs (C). *P<0.05, **P<0.01.
Antibodies
Antibodies recognizing Akt (1:3,000), phospho-Akt (1:2,000), ERK (1:3,000), phospho-ERK (1:3,000), PDGFR-β (1:2,000), and phosphor-PDGFR-β (Y1009 and Y1021; 1:1,000) were purchased from Cell signaling Technology. Horseradish-peroxidase (HRP)-conjugated secondary mouse antibody (1:10,000) and HRP-conjugated secondary rabbit antibody (1:10,000) were purchased from Santa Cruz Biotechnology.
Western blotting
Proteins were solubilized using sodium dodecyl sulfate sampling buffer. Lysates were examined either by 10% or 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and were transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was blocked with 5% skim milk for 1 h at room temperature and was then incubated with primary antibody overnight at 4°C. The following day, the membrane was washed with TBS-T buffer (0.1% Tween 20 in Tris-buffered saline), followed by incubation with HRP-conjugated secondary antibody for 1 h at room temperature. The membrane was reacted to ECL solution (Millipore) and was exposed.
Assay of reactive oxygen species generation
Reactive oxygen species (ROS) production in ASCs was measured using 2′,7′-dichlorofluorescin diacetate (DCF-DA; Molecular probes). ASCs (7×105 cells) were seeded in 100 mm culture dish with a serum-free medium for overnight. Cells were pretreated with NAC and DPI for 30–60 min and were incubated with DCF-DA (20 μM) for 10 min at 37°C (in the dark). Then, cells were incubated in normoxia and hypoxia for 20 min and incubated cells were harvested by trypsin–EDTA. Fluorescence was measured using a flow cytometer (Becon Dickinson).
In vitro wound-healing assay
Human dermal fibroblast (HDF) migration assay was adopted for the measurement of in vitro wound healing. HDF was isolated and cultured as previously described [7,24]. Confluent HDFs kept in the serum-free medium for 24 h were wounded with a plastic micropipette tip with a large orifice. Then, the medium was added with concentrated proteins from CM of ASCs in normoxia (Nor-CM), in hypoxia (hypo-CM), in hypoxia with 100 mM NAC treatment to scavenge ROS (NAC-CM), and in hypoxia with 100 nM DPI to inhibit ROS generation (DPI-CM). Photographs of the wounded areas were taken every 24 h by phase-contrast microscopy. In vitro wound-healing potential was evaluated by migrated distance of HDF.
Statistical analysis
All data were representative of triplicate independent experiments and subjected to statistical analysis with P<0.05 or P<0.01 being considered significant.
Results
Hypoxia increased the proliferation of ASCs
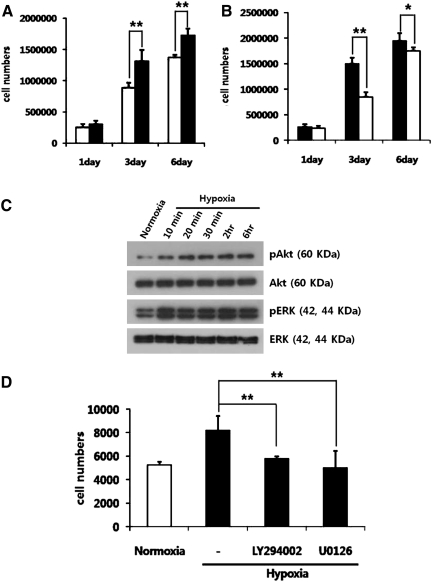
The effect of oxygen concentration on the proliferation of ASCs had been examined in the preliminary study, which showed that 1%–5% hypoxia significantly increased the proliferation of ASCs. However, survival of ASCs under serum-free conditions was not increased by hypoxia. Therefore, a condition of 2% hypoxia with 10% fetal bovine serum was used throughout the present study. ASCs cultured under normoxic conditions were transferred to a hypoxic incubator wherein 2% hypoxia significantly increased proliferation at days 3 and 6 (Fig. 1A). To the contrary, when hypoxia-cultured ASCs were transferred to a normoxic incubator, it led to a significant decrease in the proliferation of ASCs at days 3 and 6 (Fig. 1B). To test the roles of cell survival/proliferation pathways in hypoxia-increased proliferation, the Akt and ERK1/2 pathways were examined. Hypoxia increased the phosphorylation of Akt and ERK1/2 for 6 h (Fig. 1C). Involvement of these signal molecules was confirmed by the fact that their specific inhibitors, LY294002 and U0126, significantly decreased the proliferation of ASCs at day 3 (Fig. 1D).
FIG. 1.
Effect of hypoxia on the proliferation of ASCs. Note that hypoxia significantly increased the proliferation of ASCs. Normoxia-cultured ASCs (white bars) were transferred to an hypoxic condition (black bars), which led to a significant increase in cell proliferation (A). On the contrary, when hypoxia-cultured ASCs were transferred to normoxia, the cell proliferation was decreased (B, white bars: normoxia, black bars: hypoxia). Expression levels of phosphorylated Akt and ERK1/2 were detected by Western blot analysis (C). Hypoxia-increased proliferation was attenuated by specific Akt (LY294002, 5 μM) and ERK1/2 (U0126, 5 μM) inhibitors at day 3 (D). *P<0.05, **P<0.01. ASC, adipose-derived stem cell.
Hypoxia increased the migration of ASCs
After removing the confluent ASCs with a scraper, the migration distances of ASCs were measured. Compared with normoxia, hypoxia significantly increased the migration of ASCs at 18 and 40 h after scraping (Fig. 2A, B). The involvement of Akt and ERK1/2 signal molecules was investigated under the same conditions by which inhibitions of these signal pathways by LY294002 and U0126 had significantly decreased the hypoxia-induced migration of ASCs (Fig. 2C).
Hypoxia induced ROS generation
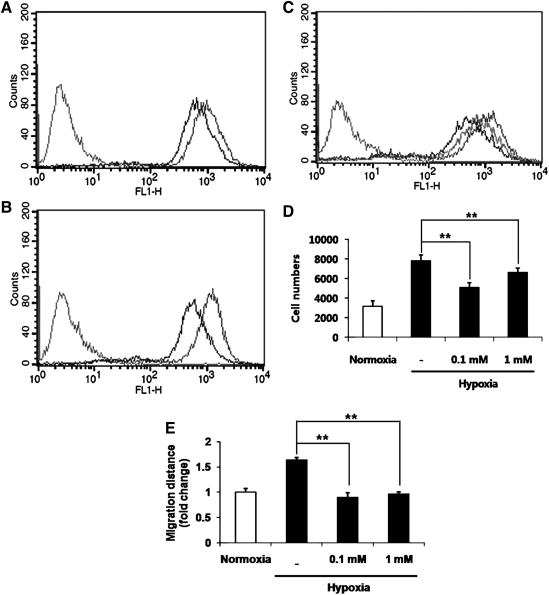
We further examined which factor(s) increased proliferation and migration of ASCs during hypoxia. Since low-level ROS generation was reportedly involved in signal transduction, we hypothesized that ROS generated by acute hypoxia may be involved in the stimulation of ASCs [25,26]. Therefore, generation of ROS was examined using DCF-DA florescence dye. Acute hypoxia significantly increased the fluorescence intensity of DCF-DA in a time-dependent manner (10 min: Fig. 3A; 20 min: Fig. 3B). The role of hypoxia-generated ROS in the stimulation of ASCs was examined using NAC, an ROS scavenging agent. Hypoxia-induced ROS generation and functional enhancement was reversed by NAC treatment. As shown in Fig. 3C and Table 1, NAC treatment reduced the signal intensity of DCF-DA in ASCs. In addition, NAC treatment significantly decreased the hypoxia-induced proliferation (Fig. 4D) and migration (Fig. 4E) of ASCs. However, NAC treatment did not significantly reduce the proliferation and migration of ASCs in normoxia, which implies a critical role of ROS generation in hypoxia-induced functional enhancement (data not shown).
FIG. 3.
Hypoxia-induced ROS generation and its involvement in ASC stimulation. Note that hypoxia generates ROS and that hypoxia-induced stimulation was reversed by NAC treatment. The fluorescence intensity of DCF-DA in ASCs at 10 min (A, the first peak, negative control; the second peak, normoxia; the third peak, hypoxia) and 20 min after hypoxia (B, the first peak, negative control; the second peak, normoxia; the third peak, hypoxia). NAC (0.1 mM) treatment decreased the fluorescence intensity of DCF-DA in ASCs (C, the first peak, negative control; the second peak, normoxia; the third peak, hypoxia; the forth peak, NAC treatment). Hypoxia-induced proliferation (D) and migration (E) of ASCs was reversed by NAC treatment. **p<0.01.
Table 1.
The Fluorescence Intensity of Dichlorofluorescin Diacetate Adipose-Derived Stem Cells After N-Acetyl-Cysteine Treatment
| Condition | Geometric mean | |
|---|---|---|
| Negative | 3.0±0.3 | |
| Normoxia | 387.6±83.3 | |
| Hypoxia NAC | 0 mM | 667.2±109.2 |
| 0.1 mM | 477.3±69.0* | |
| 0.5 mM | 533.4±139.5 | |
| 1 mM | 494.1±74.0* |
n=2, *P<0.05.
NAC, N-acetyl-cysteine.
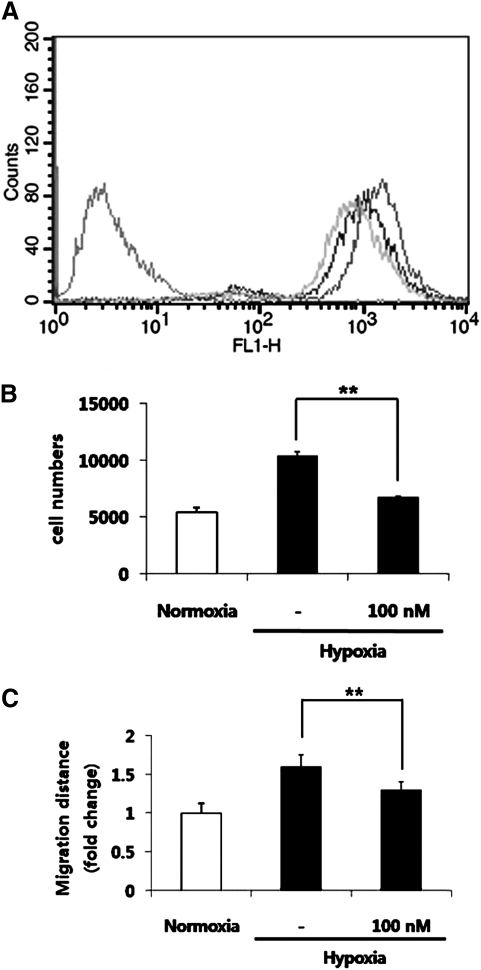
FIG. 4.
Involvement of NADPH oxidase in ROS generation. Note that hypoxia-induced stimulation was reversed by a NADPH oxidase inhibitor. The fluorescence intensity of DCF-DA in ASCs was decreased by 100 nM DPI treatment in Facs analysis (A, the first peak, negative control; the second peak, normoxia; the third peak, hypoxia; the forth peak, DPI treatment). Hypoxia-induced proliferation (B) and migration (C) of ASCs were reversed by DPI treatment. *p<0.05, **p<0.01.
Possible involvement of NADPH oxidase in the hypoxia-induced ROS generation
Several enzymes are now recognized as being potentially able to produce ROS; perhaps the most important of these is NADPH oxide. Therefore, we determined the possible involvement of NADPH oxidase in ROS generation using DPI (an inhibitor of NADPH oxidase by inhibiting electron transport). The fluorescence intensity of DCF-DA in ASCs was significantly reduced by 100 nM DPI treatment (Fig. 4A). In addition, 100 nM DPI treatment significantly decreased the hypoxia-induced proliferation (Fig. 4B) and migration (Fig. 4C) of ASCs. Collectively, these results suggest the possible involvement of NADPH oxidase in ROS generation during hypoxia.
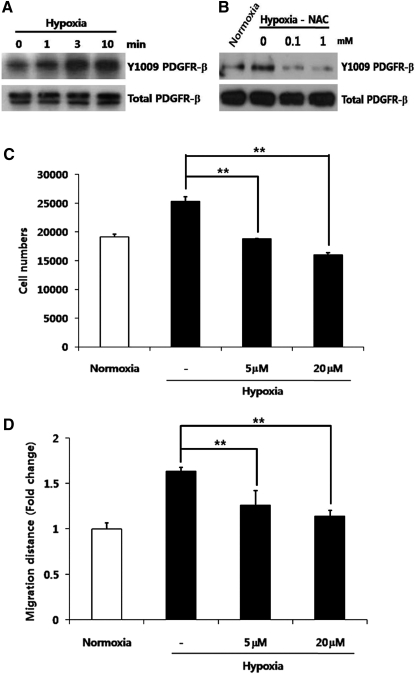
Hypoxia phosphorylates PDGFR-β
To investigate the signal pathways involved in the stimulation of ASC by hypoxia, a phospho-receptor tyrosine kinase (RTK) array was used to screen the involved RTKs. In a phospho-RTK chip array, the phosphorylation of PDGFR-β was primarily increased by hypoxia (data not shown). In addition, expression of Y1009- phosphorylated PDGFR-β was significantly increased by hypoxia compared with total PDGFR-β expression in Western blot analysis (Fig. 5A). In addition, scavenging ROS by NAC treatment significantly reduced the phosphorylation of PDGFR-β (Fig. 5B), which suggests that hypoxia-generated ROS mediates the phosphorylation of PDGFR-β. To confirm the involvement PDGFR-β in hypoxia-induced stimulation of ASC, a chemical inhibitor of PDGFR-β, AG1296, was used. As expected, inhibition of PDGFR-β by AG1296 significantly reduced the hypoxia-enhanced proliferation (Fig. 5C) and migration (Fig. 5D) of ASCs.
FIG. 5.
Phosphorylation of PDGFR-β by hypoxia. Note that PDGFR-β was primarily activated by hypoxia. Expression of Y1009-phosphorylated PDGFR-β was increased compared with total PDGFR-β in Western blot analysis (A). Scavenging ROS by NAC treatment (0.1 and 1 mM) attenuated the phosphorylation of PDGFR-β (B). In addition, AG1296 (a PDGFR-β inhibitor) significantly decreased the hypoxia-enhanced proliferation (C) and migration (D) of ASCs. **P<0.01. PDGFR, platelet-derived growth factor receptor.
Hypoxia enhanced wound-healing potential of ASCs in vitro
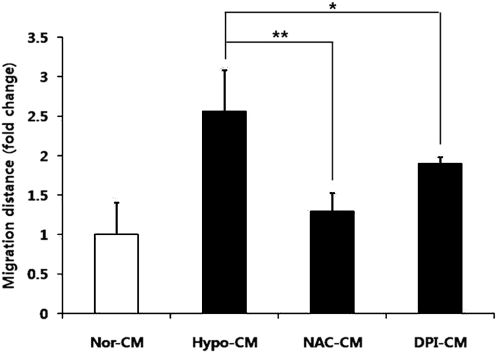
To confirm the increased regenerative potential of ASCs by ROS generation (ie, paracrine effect), we obtained concentrated proteins from the CM of ASCs in various conditions and compared the wound-healing potential in vitro. Compared with Nor-CM, concentrated proteins from hypo-CM significantly enhanced the migration of HDFs. However, the enhanced function was attenuated by the addition of concentrated proteins from NAC-CM (Fig. 6), which suggests that scavenging and reducing ROS generation by NAC decreased the wound-healing potential of ASCs. In addition, the addition of concentrated proteins from DPI-CM also resulted in the reduced migration of HDFs (Fig. 6). Therefore, ROS generation is potentially a key event for the hypoxia-induced wound-healing potential of ASCs.
FIG. 6.
Effect of hypoxia on the wound-healing potential of ASCs in vitro. Note that hypoxia enhanced the wound-healing potential of ASCs in vitro via paracrine mechanism. Compared with Nor-CM, the addition of concentrated proteins from hypo-CM increased the migration of dermal fibroblasts. However, enhanced function was attenuated by the addition of concentrated proteins from NAC-CM and DPI-CM. *P<0.05, **P<0.01. CM, conditioned medium.
Discussion
ROS are species of oxygen that are in a more reactive state than molecular oxygen. ROS such as superoxide anion (O2−) and hydrogen peroxide (H2O2) play an important role in normal cell growth, migration, differentiation, apoptosis, and senescence [27,28]. Excess amounts of ROS are toxic and involved in stem cell senescence and apoptosis [29], thereby contributing to oxidative stress-dependent diseases [30,31]. By contrast, ROS at low levels function as signaling molecules to enhance the proliferation and migration of stem cells by serving as second messengers for signal transduction and by triggering the expression of early response genes, such as Akt and ERK1/2, as well as the activation of tyrosine kinases and the deactivation of tyrosine phosphatases [25,26]. Further, ROS are reportedly involved in the secretion of diverse growth factors (ie, vascular endothelial growth factor) from stem cells by stabilizing the HIF-1α levels [23,32,33]. This evidence suggests that the controlled production of ROS by hypoxia has a beneficial effect on the cellular homeostasis of ASCs.
ROS are generated from a number of sources, including NADPH oxidase, the mitochondrial electron-transport system, xanthine oxidase, cytochrome p450, uncoupled nitric oxide synthase, and myeloperoxidase [28,34]. Complex interactions may occur among different sources of ROS, as well as feed-back and feed-forward regulation of ROS accumulation [34]. However, NADPH oxidase has emerged as a major source of ROS in receptor-activated signal transduction, and is activated by growth factors and hypoxia [35,36]. Several homologs of gp91phox (also termed Nox2), including Nox1, Nox3, Nox4, and Nox5, as well as the dual oxidases (Duox)1 and 2, have been identified [37–39]. Nox1, Nox2, Nox4, and Nox5 are expressed in endothelial cells, whereas Nox2 and Nox4 are found expressed in mesenchymal stem cells [40–42]. In addition, increasing evidence shows that ROS are generated by cytosolic NADPH oxidase in stem/progenitor cells, which is involved in the differentiation and proliferation of stem cells [33]. Using DPI, a chemical inhibitor of NADPH oxidases, our results suggested the possible involvement of NADPH oxidases in ROS generation. However, the most critical NADPH oxidase involved in hypoxia-induced ASC activation has not been characterized. Therefore, identification of NADPH oxidase expressed in ASCs, and study of the kinetic profile of ROS generation by these NADPH oxidases will be the next topic of our research.
Culturing ASCs in hypoxia has diverse advantages over normoxia. Hypoxia increased the proliferation and regenerative potential of ASCs by enhancing the secretion of growth factors. In the present study, we investigated the key factor that initiates and mediates the cellular responses of ASCs during hypoxia and demonstrated the pivotal role of ROS and its downstream signaling pathways. To summarize, as an acute response to hypoxia, ASCs generated ROS. Then, an increased cellular ROS level activated PDGFR-β, followed by phosphorylation of Akt and ERK1/2 signal pathways. Collectively, these events increased proliferation, migration, and the regenerative potential of ASCs.
Supplementary Material
Acknowledgments
This study was supported by a grant from CHA University (CHAIACF-2009-A008). Ying Xia was supported by the grants NIH-HD34852 and NIH-AT004422.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Altman AM. Yan Y. Matthias N. Bai X. Rios C. Mathur AB. Song YH. Alt EU. IFATS Series: human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250–258. doi: 10.1634/stemcells.2008-0178. [DOI] [PubMed] [Google Scholar]
- 2.Park HJ. Kim IT. Won JH. Jeong SH. Park EY. Nam JH. Choi J. Lee KT. Anti-inflammatory activities of ent-16alphaH,17-hydroxy-kauran-19-oic acid isolated from the roots of Siegesbeckia pubescens are due to the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB inactivation. Eur J Pharmacol. 2007;558:185–193. doi: 10.1016/j.ejphar.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Hong SJ. Traktuev DO. March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Olmo D. Garcia-Arranz M. Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn's disease. Expert Opin Biol Ther. 2008;8:1417–1423. doi: 10.1517/14712598.8.9.1417. [DOI] [PubMed] [Google Scholar]
- 5.Won CH. Yoo HG. Kwon OS. Sung MY. Kang YJ. Chung JH. Park BS. Sung JH. Kim WS. Kim KH. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci. 2010;57:134–137. doi: 10.1016/j.jdermsci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Kim WS. Park BS. Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9:879–887. doi: 10.1517/14712590903039684. [DOI] [PubMed] [Google Scholar]
- 7.Kim WS. Park BS. Sung JH. Yang JM. Park SB. Kwak SJ. Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY. Xia Y. Kim WS. Kim MH. Kim TH. Kim KJ. Park BS. Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 9.Park BS. Kim WS. Choi JS. Kim HK. Won JH. Ohkubo F. Fukuoka H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31:27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 10.Song SY. Chung HM. Sung JH. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther. 2010;10:1529–1537. doi: 10.1517/14712598.2010.522987. [DOI] [PubMed] [Google Scholar]
- 11.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove CJ. Bovenkerk JE. Pell CL. Johnstone BH. Considine RV. March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 12.Oh JS. Ha Y. An SS. Khan M. Pennant WA. Kim HJ. Yoon do H. Lee M. Kim KN. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett. 2010;472:215–219. doi: 10.1016/j.neulet.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen JG. Frobert O. Pilgaard L. Kastrup J. Simonsen U. Zachar V. Fink T. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2010;26:1–11. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 14.He J. Genetos DC. Yellowley CE. Leach JK. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem. 2010;110:87–96. doi: 10.1002/jcb.22514. [DOI] [PubMed] [Google Scholar]
- 15.Suga H. Eto H. Inoue K. Aoi N. Kato H. Araki J. Higashino T. Yoshimura K. Cellular and molecular features of lipoma tissue: comparison with normal adipose tissue. Br J Dermatol. 2009;161:819–825. doi: 10.1111/j.1365-2133.2009.09272.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto A. Matsumoto S. Sowers AL. Koscielniak JW. Trigg NJ. Kuppusamy P. Mitchell JB. Subramanian S. Krishna MC. Matsumoto K. Absolute oxygen tension (pO(2)) in murine fatty and muscle tissue as determined by EPR. Magn Reson Med. 2005;54:1530–1535. doi: 10.1002/mrm.20714. [DOI] [PubMed] [Google Scholar]
- 17.Luo HY. Wei W. Shi YX. Chen XQ. Li YH. Wang F. Qiu MZ. Li FH. Yan SL. Zeng MS. Huang P. Xu RH. Cetuximab enhances the effect of oxaliplatin on hypoxic gastric cancer cell lines. Oncol Rep. 2010;23:1735–1745. doi: 10.3892/or_00000819. [DOI] [PubMed] [Google Scholar]
- 18.Murphy DA. Makonnen S. Lassoued W. Feldman MD. Carter C. Lee WM. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006) Am J Pathol. 2006;169:1875–1885. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen EY. Mazure NM. Cooper JA. Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433. [PubMed] [Google Scholar]
- 20.Toby IT. Chicoine LG. Cui H. Chen B. Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L600–L606. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eng E. Holgren C. Hubchak S. Naaz P. Schnaper HW. Hypoxia regulates PDGF-B interactions between glomerular capillary endothelial and mesangial cells. Kidney Int. 2005;68:695–703. doi: 10.1111/j.1523-1755.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang M. Crisostomo PR. Herring C. Meldrum KK. Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH. Lee YJ. Song CH. Ahn YK. Han HJ. Role of FAK phosphorylation in hypoxia-induced hMSCS migration: involvement of VEGF as well as MAPKS and eNOS pathways. Am J Physiol Cell Physiol. 2010;298:C847–C856. doi: 10.1152/ajpcell.00418.2009. [DOI] [PubMed] [Google Scholar]
- 24.Kim WS. Park BS. Park SH. Kim HK. Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Knebel A. Rahmsdorf HJ. Ullrich A. Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 26.Rao GN. Protein tyrosine kinase activity is required for oxidant-induced extracellular signal-regulated protein kinase activation and c-fos and c-jun expression. Cell Signal. 1997;9:181–187. doi: 10.1016/s0898-6568(96)00139-8. [DOI] [PubMed] [Google Scholar]
- 27.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 28.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 29.Case J. Ingram DA. Haneline LS. Oxidative stress impairs endothelial progenitor cell function. Antioxid Redox Signal. 2008;10:1895–1907. doi: 10.1089/ars.2008.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao EH. Yu Y. Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006;7:101–108. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 31.Du W. Adam Z. Rani R. Zhang X. Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal. 2008;10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmelter M. Ateghang B. Helmig S. Wartenberg M. Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006;20:1182–1184. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 33.Ushio-Fukai M. Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JM. Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 35.Gao Q. Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2008;295:H978–H989. doi: 10.1152/ajpheart.00316.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupte SA. Wolin MS. Hypoxia promotes relaxation of bovine coronary arteries through lowering cytosolic NADPH. Am J Physiol Heart Circ Physiol. 2006;290:H2228–H2238. doi: 10.1152/ajpheart.00615.2005. [DOI] [PubMed] [Google Scholar]
- 37.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 39.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 40.Schroder K. Kohnen A. Aicher A. Liehn EA. Buchse T. Stein S. Weber C. Dimmeler S. Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res. 2009;105:537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- 41.Urao N. Inomata H. Razvi M. Kim HW. Wary K. McKinney R. Fukai T. Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J. Stouffs M. Serrander L. Banfi B. Bettiol E. Charnay Y. Steger K. Krause KH. Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.