Abstract
Objective:
To determine whether preterm birth is associated with epilepsy in a national cohort of adults aged 25–37 years.
Methods:
We conducted a national cohort study of 630,090 infants born in Sweden from 1973 through 1979, including 27,953 born preterm (<37 weeks), followed from 2005 to 2009 for 1) hospitalization for epilepsy and 2) outpatient and inpatient prescription of antiepileptic drugs. Epilepsy diagnoses and medication data were obtained from all hospitals and pharmacies throughout Sweden.
Results:
We found a strong association between preterm birth and epilepsy that increased by earlier gestational age. After adjusting for fetal growth and potential confounders, odds ratios for hospitalization for epilepsy were 4.98 (95%confidence interval [CI] 2.87–8.62) for those born at 23–31 weeks, 1.98 (95% CI 1.26–3.13) for those born at 32–34 weeks, and 1.76 (95% CI 1.30–2.38) for those born at 35–36 weeks, relative to those born full-term (37–42 weeks). A similar but slightly weaker trend was observed for the association between preterm birth and antiepileptic drug prescription. These associations persisted after excluding individuals with cerebral palsy, inflammatory diseases of the CNS, cerebrovascular disease, and brain tumors.
Conclusions:
These findings suggest that preterm birth, including late preterm birth, is strongly associated with epilepsy in Swedish adults aged 25–37 years. This association was independent of fetal growth and was not mediated by cerebral palsy or other comorbidities.
Epilepsy affects 50 million people worldwide1 and is the most common neurologic condition affecting individuals of all ages.2 A growing body of evidence has shown that individuals who are born preterm have an increased risk of epilepsy in early life, but the longer-term risk of epilepsy in these individuals is unclear. One recent study reported an association between preterm birth or intrauterine growth restriction and an increased risk of epilepsy in individuals in Denmark who were followed up to 24 years of age.3 The association between very preterm birth (gestational age 22–32 weeks) and epilepsy in that study weakened over time but persisted between 15 and 24 years of age. There are no sufficiently powered studies to date, however, that have examined the association between preterm birth and epilepsy in adults beyond this age range.
We conducted a national cohort study to examine the association between preterm birth and epilepsy in Swedish adults aged 25 to 37 years. Epilepsy was ascertained using nationwide hospital discharge diagnoses as well as nationwide outpatient and inpatient antiepileptic drug prescription data. We hypothesized that preterm birth is independently associated with an increased risk of epilepsy in adults in this age range.
METHODS
Study population.
We identified 648,276 individuals in the Swedish Birth Registry who were born from 1973 through 1979. We excluded 6,553 (1.0%) individuals who were no longer living in Sweden at the time of follow-up (2005–2009); 7,926 (1.2%) who had significant congenital anomalies (i.e., other than undescended testicle, preauricular appendage, congenital nevus, or hip dislocation); 1,637 (0.3%) who had missing information on gestational age at birth; and 245 (<0.1%) who had missing information on birthweight. In order to remove possible coding errors, we also excluded 6 (<0.01%) individuals who had a reported gestational age <23 weeks, and 1,819 (0.3%) who had a reported birthweight more than 4 standard deviations above or below the mean birthweight for gestational age and gender from a Swedish reference growth curve.4 A total of 630,090 individuals (97.2% of the original cohort) remained for inclusion in the study.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Ethics Committee of Lund University in Malmö, Sweden.
Study period.
To assess the longer-term risk of epilepsy among individuals born preterm, we focused on the age range of 25 to 37 years (the maximum ages of this cohort) because no previous study has had adequate statistical power to examine this association in adults >25 years of age. Study participants were followed for hospital discharge diagnoses from January 1, 2005, through December 31, 2009; and for medication prescriptions from July 1, 2005, through December 31, 2009 (the first 4.5 years that the national pharmacy register was kept).
Outcome measurement.
Epilepsy was ascertained by 1) a primary hospital discharge diagnosis of epilepsy, and 2) any outpatient or inpatient prescription of phenytoin, levetiracetam, or phenobarbital. These particular antiepileptic drugs were examined because they are used mainly for epilepsy and therefore are expected to have a higher positive predictive value for epilepsy than many other antiepileptic drugs that are also commonly used for other conditions.
Hospital discharge diagnoses were obtained using the Swedish hospital discharge registry, which was started in 1969 and has operated on a nationwide basis since 1987. The primary outcome for this study was a primary discharge diagnosis of epilepsy (codes G40–G41 in the International Classification of Diseases, tenth revision [ICD-10]) from any hospital nationwide during 2005–2009.
To evaluate whether any association between preterm birth and epilepsy is mediated by comorbidities, we obtained all primary and secondary hospital discharge diagnoses for the following conditions from birth through 2009: cerebral palsy (code 343 in the International Classification of Diseases, eighth revision [ICD-8] or the International Classification of Diseases, ninth revision [ICD-9], and G80 in ICD-10); inflammatory diseases of the CNS (codes 320–326 in ICD-8 or ICD-9, and G00–G09 in ICD-10); cerebrovascular disease (codes 430–438 in ICD-8 or ICD-9, and I60–I69 in ICD-10); and brain tumors, whether malignant, benign, or uncertain behavior (codes 191–192, 225, and 237, respectively, in ICD-8 or ICD-9; and C70–C72, D33, and D42-D43, respectively, in ICD-10).
Medication prescription data were obtained using the national pharmacy register maintained by the Swedish National Board of Health and Welfare.5–10 This register contains a record of each medication prescription dispensed to a patient by any outpatient or inpatient pharmacy in Sweden. All medication data are categorized according to the Anatomic Therapeutic Chemical (ATC) Classification System developed by the WHO Collaborating Centre for Drug Statistics Methodology.11 We obtained records of all outpatient and inpatient prescriptions for phenytoin (ATC code N03AB02), levetiracetam (N03AX14), and phenobarbital (N03AA02). These data were linked to the Birth Registry using an anonymous, serial number version of each individual's unique identification number, allowing complete and accurate linkage between the different registers.
Exposure measurement.
Gestational age at birth was obtained from prenatal and birth records in the Birth Registry, and was based on maternal report of last menstrual period. In the current study this was categorized into 5 groups (23–31 weeks, 32–34 weeks, 35–36 weeks, 37–42 weeks, ≥43 weeks) to allow for a nonlinear response. Cutpoints were chosen in order to have adequate numbers in each category for statistical analysis.
Adjustment variables.
Other characteristics that may be associated with preterm birth and with epilepsy2 were obtained using the Birth Registry and the Swedish Population and Housing Census of 1990. The following were included as adjustment variables and were 100% complete for this cohort:
Fetal growth.
Measured as the number of standard deviations from the mean birthweight for gestational age and gender from a Swedish reference growth curve,4 categorized into 6 groups (<−2 SD; −2 SD to <−1 SD; −1 SD to <0 SD; 0 SD to <1 SD; 1 SD to <2 SD; ≥2 SD).
Age.
Modeled as a continuous variable by date of birth.
Gender.
Female or male.
Multiple gestation status.
Singleton or twin. Included because multiple gestation is associated with low fetal growth and preterm delivery, and some12 but not all13 evidence has suggested that it is associated with an increased risk of epilepsy in the offspring.
Birth order.
1, 2, or ≥3. Included because low birth order has been associated with preterm birth14 and high birth order may be associated with an increased risk of epilepsy.15
Maternal age at birth.
<20 years, 20–24 years, 25–29 years, 30–34 years, or ≥35 years. Included because very early or advanced maternal age is associated with preterm delivery, and increasing maternal age may be associated with an increased risk of epilepsy in the offspring.15,16
Maternal marital status in 1990.
Married/cohabiting, never married, divorced, or widowed.
Maternal education in 1990.
Compulsory high school or less (≤9 years), practical high school or some theoretical high school (10–11 years), or theoretical high school or college (≥12 years).
Family income in 1990.
Calculated as the annual family income divided by the number of people in the family, or family income per capita, using a weighted system whereby small children were given lower weights than adolescents and adults. The final variable was categorized in quartiles.
Maternal or paternal history of epilepsy.
Primary or secondary hospital discharge diagnosis of epilepsy (code 345 in ICD-8 or ICD-9, and G40-G41 in ICD-10) during 1969–2009, dichotomized as ever vs never and entered into the model separately for mothers and fathers.
Statistical analysis.
Generalized estimating equations were used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between gestational age at birth and hospitalization for epilepsy or prescription of antiepileptic drugs (as defined above). Analyses were conducted unadjusted and then were adjusted for the model covariates. Robust standard errors were used in all models to account for correlation among siblings.
We also performed the same adjusted analyses as above after excluding all 1,306 (0.2%) individuals with cerebral palsy, 2,667 (0.4%) with inflammatory diseases of the CNS, 1,177 (0.2%) with cerebrovascular disease, and 1,014 (0.2%) with brain tumors. These conditions were not prevalent enough to allow adjustment for them as covariates. Therefore, in a secondary analysis we excluded individuals with these diagnoses to assess whether associations observed in the main analyses, if any, were mediated by these conditions. Pearson χ2 goodness-of-fit tests were used to confirm that the final models appropriately fit the data.
We explored first-order interactions between gestational age at birth and each of the model covariates with respect to hospitalization for epilepsy or antiepileptic drug prescription, using a likelihood ratio test to evaluate for statistical significance. All analyses were conducted using Stata statistical software, version 11.0.17
RESULTS
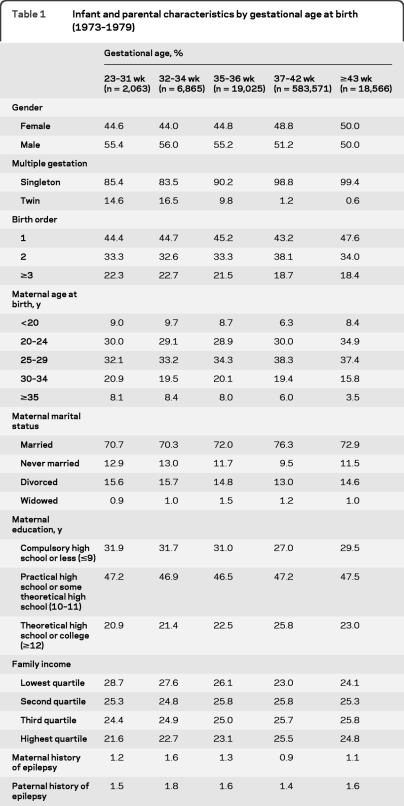
Of the 630,090 individuals in this cohort, 27,953 (4.4%) were born prematurely (<37 weeks), including 2,063 (0.3%) born at 23–31 weeks; 6,865 (1.0%) born at 32–34 weeks; and 19,025 (3.0%) born at 35–36 weeks (table 1). Preterm infants were more likely than full-term infants to be male, be a twin, or have a birth order ≥3; their mothers were more likely to be <20 or ≥35 years old at the time of delivery, be unmarried, and have the lowest educational attainment or the lowest family incomes; and their mothers or fathers were more likely to have an inpatient diagnosis of epilepsy (p < 0.01 for each of these comparisons by Kruskal-Wallis test).
Table 1.
Infant and parental characteristics by gestational age at birth (1973–1979)
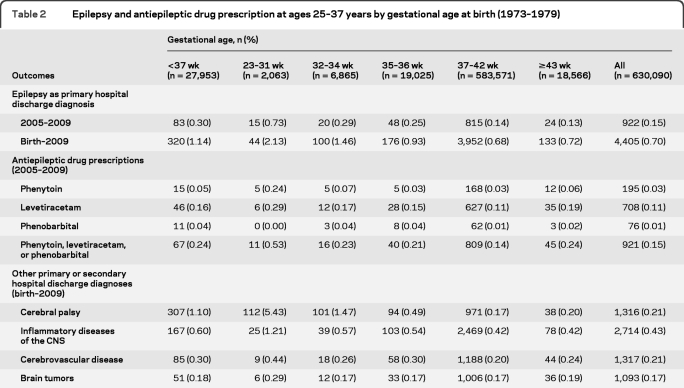
A total of 4,405 (0.70%) individuals from the entire cohort had been hospitalized for epilepsy during their lifetime, and 922 (0.15%) were hospitalized for epilepsy during 2005–2009 (table 2). Hospitalization for epilepsy was more common across the full range of preterm gestational ages than among those born full- or post-term. The proportion of individuals hospitalized for epilepsy during 2005–2009 was 0.73% among those born at 23–31 weeks, 0.29% among those born at 32–34 weeks, and 0.25% among those born at 35–36 weeks, compared to 0.14% among those born full-term (37–42 weeks). A higher proportion of individuals born preterm or post-term were prescribed phenytoin, levetiracetam, or phenobarbital than those born full-term. Comorbidities were also more prevalent in individuals born preterm, especially cerebral palsy, as well as inflammatory diseases of the CNS and cerebrovascular disease.
Table 2.
Epilepsy and antiepileptic drug prescription at ages 25–37 years by gestational age at birth (1973–1979)
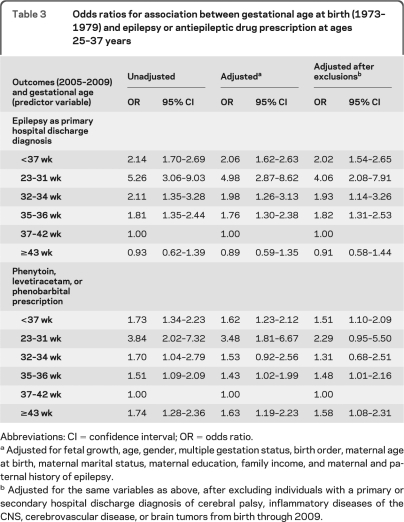
The relative odds of hospitalization for epilepsy were markedly increased among adults who were born preterm. After adjusting for fetal growth and potential confounders, ORs were 4.98 (95% CI 2.87–8.62) for those born at 23–31 weeks, 1.98 (95% CI 1.26–3.13) for those born at 32–34 weeks, and 1.76 (95% CI 1.30–2.38) for those born at 35–36 weeks, relative to those born full-term (table 3). Adjustment for any combination of covariates had little effect on the ORs. Furthermore, excluding individuals with cerebral palsy, inflammatory diseases of the CNS, cerebrovascular disease, and brain tumors had only modest effects on the ORs.
Table 3.
Odds ratios for association between gestational age at birth (1973–1979) and epilepsy or antiepileptic drug prescription at ages 25–37 years
Abbreviations: CI = confidence interval; OR = odds ratio.
Adjusted for fetal growth, age, gender, multiple gestation status, birth order, maternal age at birth, maternal marital status, maternal education, family income, and maternal and paternal history of epilepsy.
Adjusted for the same variables as above, after excluding individuals with a primary or secondary hospital discharge diagnosis of cerebral palsy, inflammatory diseases of the CNS, cerebrovascular disease, or brain tumors from birth through 2009.
The results for antiepileptic drug prescription were generally similar to those above, except that the relative odds were increased also among individuals born post-term (table 3). When these medications were examined separately, the highest relative odds was for phenytoin prescription among individuals born at 23–31 weeks relative to those born full-term (adjusted OR 8.19; 95% CI 2.79–24.0; table e-1 on the Neurology® Web site at www.neurology.org). Comparing all individuals born preterm to those born full-term, the highest relative odds was observed for phenobarbital prescription (adjusted OR 3.57; 95% CI 1.78–7.14; table e-1). We also examined the outcome defined as both hospitalization for epilepsy and prescription of phenytoin, levetiracetam, or phenobarbital, but the risk estimates were very similar to those reported above.
Among the covariates in the adjusted model, the only strong predictors of hospitalization for epilepsy were maternal and paternal history of epilepsy (adjusted ORs 3.63 [95% CI 2.55–5.16] and 2.01 [95% CI 1.33–3.02], respectively). Low fetal growth was not a significant predictor after adjusting for gestational age at birth and the other variables included in the model (table e-2). No first-order interactions were significant at the p < 0.05 level.
DISCUSSION
These findings suggest that preterm birth is strongly associated with epilepsy in Swedish adults at 25 to 37 years of age. The relative odds of hospitalization for epilepsy was markedly increased even for late preterm births (35–36 weeks), and increased monotonically by earlier gestational ages, including a fivefold increased risk among those born very preterm (23–31 weeks). The results for outpatient and inpatient antiepileptic drug prescription were consistent with these findings except that the relative odds were increased also among individuals born post-term. All of these associations were independent of fetal growth and broadly measured potential confounders, and did not appear to be mediated by cerebral palsy, inflammatory diseases of the CNS, cerebrovascular disease, or brain tumors. In contrast, no association was observed between low fetal growth and epilepsy after adjusting for gestational age at birth.
The associations found in the current study are notably stronger than those previously reported for a younger population in Denmark. Among individuals aged 15 to 24 years in that study, the epilepsy incidence rate was increased twofold among those born at 22–32 weeks compared to those born at 39–41 weeks, and no association was found between late preterm birth and epilepsy.3 The relatively few other studies that have examined the association between preterm birth and epilepsy in adulthood have had limited statistical power and were unable to examine the full range of gestational ages.15,18–20 Studies involving children have generally been small and most,16,21,22 but not all,23 have suggested an association between preterm birth and epilepsy.
The current study's results for antiepileptic drug prescription, but not those for epilepsy ascertained from hospital discharge diagnoses, suggest an association with post-term birth as well as with preterm birth. This finding was unexpected and needs confirmation in other populations. It is consistent with an earlier Swedish case-control study that reported an association between either low or high gestational age at birth and afebrile seizures in children <15 years of age.22 Most other studies, however, have either not examined post-term birth or have done so with insufficient statistical power.
The mechanisms underlying the association between preterm birth and epilepsy may involve intrauterine hypoxic brain injury resulting from pregnancy complications that predispose to preterm birth or impaired brain development resulting directly from preterm birth itself. Preeclampsia and intrauterine infection are associated with hypoxic-ischemic fetal brain injury and with preterm delivery.24 Preterm birth itself may also impair normal development of brain structures at their most critical period. MRI data have shown a normal fourfold increase in cortical gray matter between 29 and 41 weeks postconception, and a fivefold increase in myelinated white matter between 35 and 41 weeks postconception.25 Diffuse white and gray matter abnormalities have been documented in preterm infants26 which persist when they reach the equivalent age of term,27 and such abnormalities have also been associated with epilepsy.28
A limitation of the current study is the ascertainment of epilepsy by hospital discharge diagnoses and antiepileptic drug prescriptions, without clinical data with which to validate the diagnoses. A previous study found that 25% of children with epilepsy were treated as outpatients only; however, the age-specific associations between preterm birth and epilepsy were similar whether based only on inpatient diagnoses or on the combined use of outpatient and inpatient diagnoses.3 In the current study, we assessed for potential bias resulting from the use of hospital data by also examining outpatient and inpatient antiepileptic drug prescription data. The results from these complementary data sources were similar, providing further evidence that the observed associations are real and that any bias resulting from the use of hospital discharge data are likely to be small.
Phenytoin, levetiracetam, and phenobarbital were examined because they are expected to have a higher positive predictive value for epilepsy than many other antiepileptic drugs. Ethosuximide is mainly used for absence seizures but was not prescribed in large enough numbers for analysis. Clinical data were unavailable to determine the proportion of adults with epilepsy who are managed with these or other medications. We did not include other antiepileptic drugs that are commonly used for other conditions because this would have increased misclassification of the outcome, biasing the results toward the null hypothesis. Clinical data for specific types of seizures were also unavailable for this cohort.
Other limitations include the estimation of gestational age at birth by maternal report of last menstrual period rather than by ultrasound, which was not yet widely used at the time these individuals were born (1973–1979). A few of the adjustment variables were measured during childhood or adolescence rather than at birth. Residual confounding by these factors is possible but seems unlikely to account for the main findings.
The most important strength of this study is its ability to examine the association between gestational age at birth and epilepsy in a national cohort of adults using nationwide hospital data as well as outpatient and inpatient pharmacy data. Information on broadly measured perinatal and demographic factors enabled adjustment for potential confounders. In addition, information on cerebral palsy and other comorbidities enabled the evaluation and exclusion of these conditions as potential mediators of the observed associations.
This study shows that preterm birth, including late preterm birth, is strongly associated with an increased risk of epilepsy in Swedish adults at 25 to 37 years of age. This association was independent of fetal growth and did not appear to be mediated by cerebral palsy or other comorbidities. Clinicians will increasingly encounter adult survivors of preterm birth and will need to be aware of the magnitude and duration of the neurologic sequelae. These findings suggest that the association between preterm birth and epilepsy may be stronger and extend later in adulthood than had been previously reported. More effective prevention of preterm birth is urgently needed to reduce the burden of epilepsy in later life.
Supplementary Material
GLOSSARY
- ATC
Anatomic Therapeutic Chemical
- CI
confidence interval
- ICD
International Classification of Diseases
- OR
odds ratio
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
C.C. conceived the study, analyzed and interpreted the data, and drafted the article. M.W., K.S., and J.S. made substantial contributions to the study design and data interpretation and critically revised the manuscript for important intellectual content. All authors approved the final version.
DISCLOSURE
Dr. Crump and Dr. K. Sundquist report no disclosures. Dr. Winkleby serves on scientific advisory boards for the NIH CTSA, University of California, San Diego, and the Robert Wood Johnson Latino Network, University of California, Los Angeles; serves as a consultant for Health Point Institute, Mountain View, CA; and receives research support from the NIH (NHLBI, NICHD, NIDA), the Howard Hughes Medical Institute and Health Trust. Dr. J. Sundquist reports no disclosures.
REFERENCES
- 1. Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia 2002;43(suppl 6):21–25 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy: a review. Epilepsy Res 2009;85:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun Y, Vestergaard M, Pedersen CB, Christensen J, Basso O, Olsen J. Gestational age, birth weight, intrauterine growth, and the risk of epilepsy. Am J Epidemiol 2008;167:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848 [DOI] [PubMed] [Google Scholar]
- 5. Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and risk of allergic rhinitis in young adulthood. J Allergy Clin Immunol 2011;127:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics 2011;127:e913–e920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crump C, Winkleby MA, Sundquist J, Sundquist K. Preterm birth and risk of medically treated hypothyroidism in young adulthood. Clin Endocrinol Epub 2011 Mar 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crump C, Winkleby MA, Sundquist K, Sundquist J. Preterm birth and psychiatric medication prescription in young adulthood: a Swedish national cohort study. Int J Epidemiol 2010;39:1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am J Epidemiol 2011;173:797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of diabetes among young adults born preterm in Sweden. Diabetes Care 2011;34:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO Guidelines for ATC classification and DDD assignment, 2010. In: Methodology CCfDS, ed. Oslo: WHO; 2009 [Google Scholar]
- 12. Sharma K. Higher risk of epilepsy in twins. Indian J Pediatr 1986;53:515–519 [DOI] [PubMed] [Google Scholar]
- 13. Berkovic SF, Howell RA, Hay DA, Hopper JL. Twin birth is not a risk factor for seizures. Neurology 1993;43:2515–2519 [DOI] [PubMed] [Google Scholar]
- 14. Astolfi P, Zonta LA. Risks of preterm delivery and association with maternal age, birth order, and fetal gender. Hum Reprod 1999;14:2891–2894 [DOI] [PubMed] [Google Scholar]
- 15. Monetti VC, Granieri E, Casetta I, et al. Risk factors for idiopathic generalized seizures: a population-based case control study in Copparo, Italy. Epilepsia 1995;36:224–229 [DOI] [PubMed] [Google Scholar]
- 16. Degen R. Epilepsy in children: an etiological study based on their obstetrical records. J Neurol 1978;217:145–158 [DOI] [PubMed] [Google Scholar]
- 17. StataCorp Stata Statistical Software: release 11.0. College Station, TX: StataCorp; 2010 [Google Scholar]
- 18. Rocca WA, Sharbrough FW, Hauser WA, Annegers JF, Schoenberg BS. Risk factors for complex partial seizures: a population-based case-control study. Ann Neurol 1987;21:22–31 [DOI] [PubMed] [Google Scholar]
- 19. Rocca WA, Sharbrough FW, Hauser WA, Annegers JF, Schoenberg BS. Risk factors for generalized tonic-clonic seizures: a population-based case-control study in Rochester, Minnesota. Neurology 1987;37:1315–1322 [DOI] [PubMed] [Google Scholar]
- 20. Leone M, Bottacchi E, Beghi E, et al. Risk factors for a first generalized tonic-clonic seizure in adult life. Neurol Sci 2002;23:99–106 [DOI] [PubMed] [Google Scholar]
- 21. Lilienfeld AM, Pasamanick B. Association of maternal and fetal factors with the development of epilepsy. I. Abnormalities in the prenatal and paranatal periods. JAMA 1954;155:719–724 [DOI] [PubMed] [Google Scholar]
- 22. Sidenvall R, Heijbel J, Blomquist HK, Nystrom L, Forsgren L. An incident case-control study of first unprovoked afebrile seizures in children: a population-based study of pre- and perinatal risk factors. Epilepsia 2001;42:1261–1265 [DOI] [PubMed] [Google Scholar]
- 23. Rocca WA, Sharbrough FW, Hauser WA, Annegers JF, Schoenberg BS. Risk factors for absence seizures: a population-based case-control study in Rochester, Minnesota. Neurology 1987;37:1309–1314 [DOI] [PubMed] [Google Scholar]
- 24. Berger R, Garnier Y, Jensen A. Perinatal brain damage: underlying mechanisms and neuroprotective strategies. J Soc Gynecol Investig 2002;9:319–328 [PubMed] [Google Scholar]
- 25. Huppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol 1998;43:224–235 [DOI] [PubMed] [Google Scholar]
- 26. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 2003;143:171–179 [DOI] [PubMed] [Google Scholar]
- 27. Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005;115:286–294 [DOI] [PubMed] [Google Scholar]
- 28. Tosun D, Dabbs K, Caplan R, et al. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain 2011;134:1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.