Traffic at the trans-Golgi network (TGN) and endosomes is regulated by glucose via an unknown mechanism that depends on protein kinase A (PKA). TGN–endosomal clathrin adaptors exhibit specific responses to glucose starvation that likely are coordinated with other cell behaviors regulated by PKA.
Abstract
Glucose is a rich source of energy and the raw material for biomass increase. Many eukaryotic cells remodel their physiology in the presence and absence of glucose. The yeast Saccharomyces cerevisiae undergoes changes in transcription, translation, metabolism, and cell polarity in response to glucose availability. Upon glucose starvation, translation initiation and cell polarity are immediately inhibited, and then gradually recover. In this paper, we provide evidence that, as in cell polarity and translation, traffic at the trans-Golgi network (TGN) and endosomes is regulated by glucose via an unknown mechanism that depends on protein kinase A (PKA). Upon glucose withdrawal, clathrin adaptors exhibit a biphasic change in localization: they initially delocalize from the membrane within minutes and later partially recover onto membranes. Additionally, the removal of glucose induces changes in posttranslational modifications of adaptors. Ras and Gpr1 signaling pathways, which converge on PKA, are required for changes in adaptor localization and changes in posttranslational modifications. Acute inhibition of PKA demonstrates that inhibition of PKA prior to glucose withdrawal prevents several adaptor responses to starvation. This study demonstrates that PKA activity prior to glucose starvation primes membrane traffic at the TGN and endosomes in response to glucose starvation.
INTRODUCTION
Unicellular organisms detect and respond to numerous signals, including nutrients, hydration, salinity, oxygen, and even the presence of other cells. Cellular responses to changes in the environment or to signaling ligands can entail comprehensive remodeling of numerous cell behaviors. In the yeast Saccharomyces cerevisiae, glucose acts as a signaling molecule to direct a developmental program that utilizes glucose for rapid growth. Transcriptional responses to glucose have been extensively investigated and play a prominent role in the differences in cell physiology between cells grown on glucose and on other carbon sources (Zaman et al., 2009). There are also extremely rapid responses to glucose starvation that are unlikely to be mediated by transcriptional responses. These responses include inhibition of translation, loss of cell polarity, and changes in protein localization (Ashe et al., 2000; Gorner et al., 2002; Uesono et al., 2004; De Wever et al., 2005; Faulhammer et al., 2007; Demmel et al., 2008a). Some of these rapid responses are transient; translation reinitiates and cells repolarize within 2 h of starvation. These rapid and transient changes likely allow cells to pause nutrient consumption while remodeling to a transcriptional program better suited for survival during glucose starvation.
Traffic at the trans-Golgi network (TGN) and endosomes may play an important role in mediating cellular responses to extracellular nutrient levels. Many receptors, transporters, and enzymes that are regulated by nutrient availability are sorted at the TGN and endosomes for transport to either the cell surface or to the lytic vacuole for degradation. For example, in S. cerevisiae, the trafficking of the general amino acid permease Gap1 is regulated by nitrogen availability (Scott et al., 2004). On poor nitrogen sources, cells express Gap1 and route it to the plasma membrane. When shifted to media containing a preferred nitrogen source, such as ammonium, newly synthesized and cell surface-associated Gap1 are rerouted for degradation in the vacuole. Failure to reroute Gap1 can result in toxic accumulation of amino acids in the cytoplasm (Risinger et al., 2006). Thus regulation of traffic can be necessary for cell survival and is essential for cellular adaptation to changing conditions. A key question is how the signaling pathways that sense changing nutrients regulate traffic.
Glucose-dependent regulation of traffic has been recently demonstrated. During glucose starvation, the H+-ATPase Pma1 redistributes from the plasma membrane to the vacuole (Huang and Chang, 2011). This rerouting depends on clathrin adaptors acting at the TGN and endosomes. Clathrin adaptor proteins recognize and sort specific cargo at the TGN and endosomes, and recruit clathrin and other accessory proteins required for vesicle formation and transport (Owen et al., 2004). There are three classes of clathrin adaptors at the TGN and endosomes: AP-1, Gga proteins (Gga1 and Gga2), and epsin-like Ent3 and Ent5 (Panek et al., 1997; Boman et al., 2000; Dell'Angelica et al., 2000; Duncan et al., 2003; Bonifacino, 2004). Adaptors are recruited to the membrane via interactions with phosphatidylinositol 4-phosphate (PI4P), Arf1, cargo, and one another. Because of their central role in selecting cargo and recruiting clathrin, adaptors are likely candidates for regulation of TGN–endosome traffic. Indeed, Gga1 and Gga2 are specifically required for rerouting Pma1 after glucose starvation (Huang and Chang, 2011). However, it is unclear which signaling pathways sense glucose and how these pathways communicate glucose starvation to Gga and other membrane traffic adaptors.
In yeast, the presence of glucose activates protein kinase A (PKA; Jiang et al., 1998; Thevelein and de Winde, 1999). PKA activity is the primary determinant of whether cells adopt the transcriptional profile of glucose-starved or -activated cells (Zaman et al., 2009). PKA activity also regulates nontranscriptional features of cell physiology. It inhibits activation of autophagy and promotes association of proteins to the vacuole, consistent with its role in inhibiting starvation responses (Hedbacker et al., 2004; Stephan et al., 2009). Despite the fact that PKA functions in high glucose, it is required for diverse, immediate responses to glucose starvation. In cells with reduced PKA, translation is not inhibited nor is actin polarity lost upon glucose starvation. A role for PKA activity in glucose-dependent regulation of TGN–endosome traffic has not been investigated.
In this study, we demonstrate that the localization and phosphorylation of clathrin adaptors at the TGN and endosomes are rapidly and reversibly regulated by glucose availability. We find that the modulation of TGN–endosome traffic in response to glucose starvation is biphasic. In the first phase, TGN–endosomal clathrin adaptors are rapidly redistributed to cytosol. In the second phase, long-term depletion of glucose leads to partial relocalization of clathrin adaptors to membranes. We find that posttranslational modifications of adaptors change in response to glucose. These effects are reversible. Reintroduction of glucose restores adaptor localization and reverses the changes in posttranslational modifications of adaptors. We also show that glucose-sensing pathways that converge on PKA are required for the redistribution of adaptors and changes in adaptor modification during glucose starvation. We demonstrate that PKA activity in the presence of glucose primes cells to allow adaptors to respond to glucose starvation. Taken together, our data indicate that PKA signaling is required to allow regulation of TGN–endosomal trafficking, and that this regulation may play a central role in coordinating membrane traffic with other global responses. These results suggest a mechanism for how glucose-sensing pathways influence changes in protein localization in response to glucose starvation.
RESULTS
TGN–endosomal adaptors rapidly delocalize in response to glucose starvation
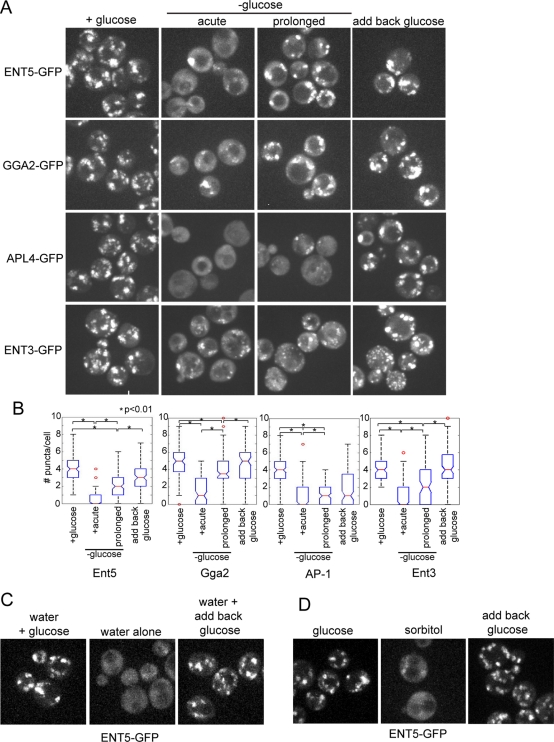
We investigated the effect of glucose starvation on adaptors by monitoring changes in the localization of green fluorescent protein (GFP)-tagged adaptors after acute glucose starvation. As previously reported, in cells grown to mid-log phase in the presence of glucose, the clathrin adaptors Ent5, Gga2, AP-1, and Ent3 localized to numerous, bright puncta throughout the cell volume (Figure 1A; Costaguta et al., 2006; Fernandez and Payne, 2006). Within a few minutes of glucose removal, all adaptors became delocalized with increased diffuse, cytosolic localization and drastically decreased number of puncta per cell (Figure 1B). By first resuspending mid-log-phase cells in 2% glucose in water with no other supplements, we determined this effect was strictly due to the removal of glucose. Adaptors were retained in numerous bright puncta in 2% glucose (Figure 1, C and D, and Supplemental Figure S1). However, when washed into water alone or 2% sorbitol, adaptors became delocalized. Furthermore, adaptor delocalization was readily reversible. After a brief incubation in water or 2% sorbitol, adaptors returned to puncta upon the addition of 2% glucose. These data demonstrate the localization of adaptors responds to withdrawal of glucose but not other nutrients.
FIGURE 1:
Adaptors relocalize in response to glucose withdrawal. (A) Fluorescence microscopy of adaptors in cells expressing Ent5-GFP (DLY3), Gga2-GFP (DLY4), the AP-1 subunit Apl4-GFP (DLY36), or Ent3-GFP (DLY6). Adaptors localize to puncta in the presence of glucose. Immediately after glucose washout, adaptors are localized to cytoplasm. After prolonged starvation, adaptors are both diffusely localized and found in puncta. Five minutes after glucose is reintroduced following prolonged starvation, Ent5, Gga2, and Ent3 are found predominately in puncta, while AP-1 is found both diffusely localized and in puncta. (B) The number of puncta per cell was quantitated during growth in glucose, acute glucose starvation, or prolonged glucose starvation, or 5 min after the addition of 2% glucose to starved cells. (C) Glucose alone can regulate adaptor localization. Cells expressing Ent5-GFP were grown in the presence of glucose for 2 h then transferred to water with 2% glucose (left) or into water (Center), or first washed into water for 15 min and then transferred into 2% glucose (right). (D) Cells expressing Ent5-GFP were grown in the presence of glucose for 2 h, then transferred to water with 2% glucose (left) or into 2% sorbitol (center), or first washed into 2% sorbitol for 15 min and then transferred into 2% glucose (right).
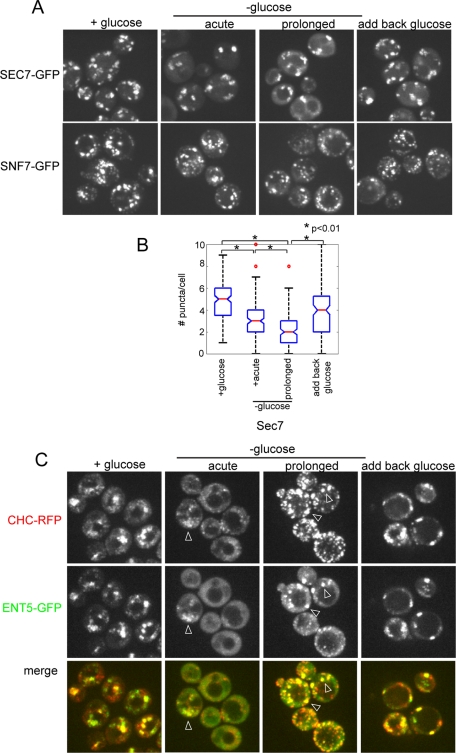
Delocalization of the adaptors could reflect redistribution of adaptors into the cytosol or fragmentation of the TGN and endosomes into structures too small to be resolved by light microscopy. To determine whether the TGN and endosomes fragment in response to glucose starvation, we visualized markers of the TGN and endosomes. Sec7 is an Arf-GEF that colocalizes with clathrin-rich puncta that correspond to the TGN and endosomes (Huh et al., 2003). Unlike the adaptors, Sec7 retained strong punctate localization after glucose withdrawal (Figure 2A). Although there was no obvious relocalization of Sec7 to cytosol, the number of Sec7-containing puncta per cell decreased, suggesting a reduction of TGN/endosome structures (Figure 2, A and B). Similarly, Snf7, a marker of late endosomes, retained a punctate localization after glucose withdrawal (Figure 2A). Thus fragmentation of the TGN and endosomes does not cause the apparent delocalization of the clathrin adaptors.
FIGURE 2:
Glucose starvation does not delocalize other markers of the TGN and endosomes, but affects clathrin localization. (A) Fluorescence microscopy of cells expressing Sec7-GFP (DLY35) or Snf7-GFP (DLY22). Both Sec7 and Snf7 are predominantly localized to puncta during growth in glucose, immediately following or after prolonged glucose washout, and following reintroduction of glucose to starved cells. (B) The number of Sec7 puncta per cell was quantitated during growth in glucose, acute glucose starvation, or prolonged glucose starvation, or 5 min after the addition of 2% glucose to starved cells. (C) Clathrin colocalizes with Ent5 during glucose starvation. Fluorescence microscopy of cells coexpressing clathrin heavy chain (Chc1-red fluorescent protein [RFP]) and Ent5-GFP (DLY7). Cells were imaged during growth in glucose, immediately following or after prolonged glucose washout. Glucose was then added to the cells, which were imaged 5 min later. Arrowheads indicate examples of colocalization.
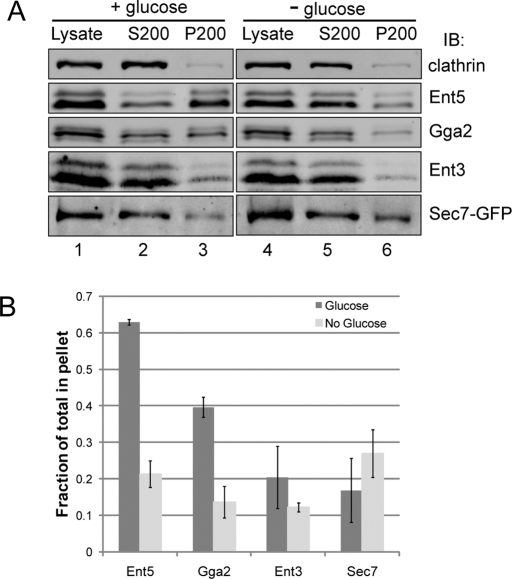
We next directly examined using sedimentation analysis the effect of glucose starvation on the association of adaptors with membranes. We modified existing cell lysis procedures to maintain glucose throughout the steps required to remove the yeast cell wall. Under these conditions, Gga2 and Ent5 were found to be enriched in the membrane pellet (Figure 3A, lane 3). In contrast, when glucose was removed 15 min before lysis, Gga2 and Ent5 substantially redistributed to the supernatant fraction (Figure 3A, lane 5). Ent3 was not substantially enriched in the membrane pellet, even in the presence of glucose, and its enrichment did not dramatically change in response to glucose starvation. In contrast to the adaptors, the TGN marker Sec7 was slightly more enriched in the membrane pellet following glucose starvation. These results indicate clathrin adaptors at the TGN and endosomes rapidly redistribute from membranes to cytosol in response to glucose starvation.
FIGURE 3:
Adaptors are released from membranes during glucose starvation. Wild-type or Sec7-GFP-expressing cells (DLY35) were grown to mid-log phase and then incubated in the presence (left) or absence (right) of glucose for 15 min. The cells were then lysed by mechanical disruption, and the lysate was centrifuged at 200,000 × g to give the S200 supernatant and P200 membrane pellet. (A) Lysates were subject to immunoblot analysis and probed with antibodies to clathrin, Ent5, Gga2, Ent3, or GFP to detect Sec7. (B) Quantitative analysis of the fraction of adaptors found in P200 pellet to total from samples treated +/− glucose. Error bars represent SD (n = 3 for Ent5, Gga2, and Ent3; n = 2 for Sec7-GFP).
TGN–endosomal adaptors relocalize after prolonged starvation
Because yeast cells exhibit both immediate and extended responses to glucose withdrawal, we also examined the localization of adaptors after prolonged starvation (Martinez-Pastor and Estruch, 1996; Ashe et al., 2000; Uesono et al., 2004). Following prolonged glucose starvation, adaptors returned to clathrin-rich punctate structures (Figures 1A and 2B). However, adaptor recovery was not uniform. For example, the numbers of Ent5 and Gga2 structures were increased, whereas the number of AP-1 structures remained low. Although the number of puncta clearly increased during starvation, there were still significantly fewer structures than in the presence of glucose, even after 2 h (Figure 1B). The number of Sec7 puncta was also reduced at this time point. Therefore reduction in adaptor puncta likely reflects a reduction in the number of TGN/endosomal compartments upon prolonged glucose starvation (Figure 2A). We also noted that there were more clathrin structures after prolonged starvation (Figure 2B). The increase in clathrin structures and decrease in Sec7 structures likely indicates a shift in clathrin distribution in starved cultures. Together, these results demonstrate that clathrin adaptors transiently delocalize in response to glucose starvation, but they return to fewer clathrin-rich endosomal structures after prolonged starvation.
Even after prolonged starvation, when puncta numbers had increased, cytosolic levels of adaptors remained higher than in cells grown in glucose. We tested whether adding back glucose reversed this diffuse localization after prolonged starvation. Within a few minutes of glucose reintroduction, Ent5, Gga2, and Ent3 in the majority of cells were found mainly on puncta, with little diffuse localization (Figure 1A). In addition, the numbers of adaptor and Sec7 puncta increased following addition of glucose (Figure 1B and 2A). AP-1, however, did not recover as rapidly, and was both diffuse and found in puncta. Furthermore, quantitation of the number of puncta before and after glucose addition indicated slow recovery of AP-1. Taken with the results above, our results demonstrate that glucose rapidly and reversibly regulates the localization of adaptors and the numbers of TGN and endosomal structures.
Glucose starvation alters posttranslational modifications of TGN–endosomal clathrin adaptors
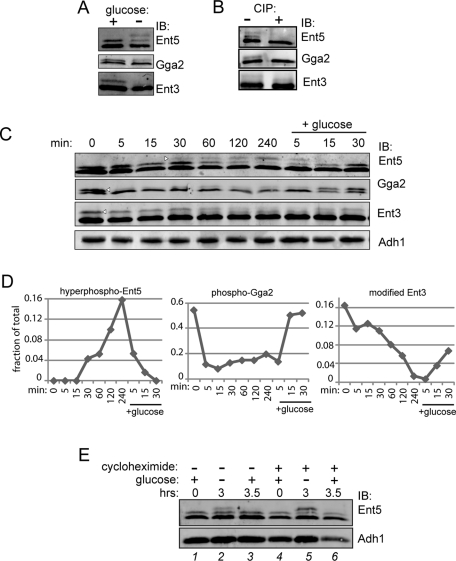
To determine whether adaptor levels change in response to glucose starvation, we performed Western blot analysis on glucose-starved cells. Adaptor levels were unchanged by glucose starvation (Figure 4C). However, the apparent molecular weight of adaptors was regulated by glucose status. In exponentially growing cells, Ent5, Gga2, and Ent3 ran as doublets (Figure 4, A and C). After prolonged starvation, Ent5 ran as a triplet, while Gga2 and Ent3 lost a substantial fraction of their higher-molecular-weight form. We tested to determine whether the higher-molecular-weight forms reflected phosphorylated forms. Phosphatase treatment of immunoprecipitated adaptors eliminated the high-molecular-weight forms of Ent5 and Gga2, demonstrating that these forms are phosphorylated (Figure 4B). The size of the high-molecular-weight form of Ent3 was reduced by phosphatase treatment, suggesting this form contains both a phosphorylation and a nonphosphorylation modification. Thus phosphorylation of Ent5 and Gga2, and possibly Ent3, is regulated by glucose.
FIGURE 4:
Posttranslational modifications of adaptors are regulated by glucose. (A) Glucose depletion causes hyperphosphorylation of Ent5, dephosphorylation of Gga2, and a change in Ent3 posttranslational modification. Wild-type cells (BY4742) were grown to mid-log phase, then washed with and resuspended in media without glucose for 2 h. Cell lysates were then probed with antibodies to adaptors. (B) Phosphorylation of adaptors. Adaptors were immunoprecipitated from wild-type cells grown to stationary phase (Ent5) or mid-log phase (Gga2 and Ent3). Immunoprecipitated samples were then treated +/− calf intestinal alkaline phosphatase (CIP) and subjected to immunoblot analysis. (C) Time course showing changes in adaptor modification in response to glucose. Wild-type cells were grown to mid-log phase and then washed with and incubated in media without glucose for 4 h. Following starvation, glucose was added to the culture media for 30 min. Samples were taken at indicated time points, lysed, and subjected to immunoblot analysis using antibodies to Ent5, Gga2, Ent3, or Adh1 as a loading control. The fraction of the highest-molecular-weight band (arrowheads) to total was quantitated and is presented graphically in (D). (E) Ent5 phosphorylation does not induce protein turnover during glucose starvation. Cells were grown in the absence (lanes 1–3) or presence (lanes 4–6) of 35 μg/ml cycloheximide to mid-log phase in glucose for 2 h (lanes 1 and 4) and then washed and starved for glucose for an additional 3 h (lanes 2 and 5). Glucose (2%) was then added back to cells for 30 min (lanes 3 and 6).
We examined the kinetics of timing for changes in adaptor modification occurring after glucose withdrawal. We monitored adaptor phosphorylation during several hours of glucose starvation. Within 5 min of glucose removal, Gga2 was immediately and severely dephosphorylated. On the other hand, hyperphosphorylation of Ent5 and modification of Ent3 changed slowly during glucose starvation (Figure 4, C and D). Therefore changes in adaptor modification vary depending on the specific adaptor and the duration of glucose starvation.
We tested to determine whether these changes in phosphorylation were reversible upon addition of glucose. After we added back glucose, Ent5 and Gga2 phosphorylation returned to prestarvation levels within 15 min. Modification of Ent3 was also reversed, although not as rapidly (Figure 4, C and D).
Phosphorylation can target proteins for degradation. Because Ent5 became hyperphosphorylated in glucose starvation, we tested whether glucose starvation was associated with increased degradation of Ent5. We treated cells with cycloheximide to inhibit translation. Cycloheximide did not alter the levels of either the hyperphosphorylated or other forms of Ent5 during glucose starvation or Ent5 levels after reintroduction of glucose (Figure 4E). Thus Ent5 hyperphosphorylation does not reflect a form that is targeted for constitutive degradation during glucose starvation. Together, these results show that Gga2, Ent3, and Ent5 undergo changes in posttranslational modification in response to glucose starvation and readdition, but that the kinetics of onset and recovery of these changes differ for the three adaptors.
PKA activity during growth in glucose-rich media is required for adaptor relocalization upon acute glucose withdrawal
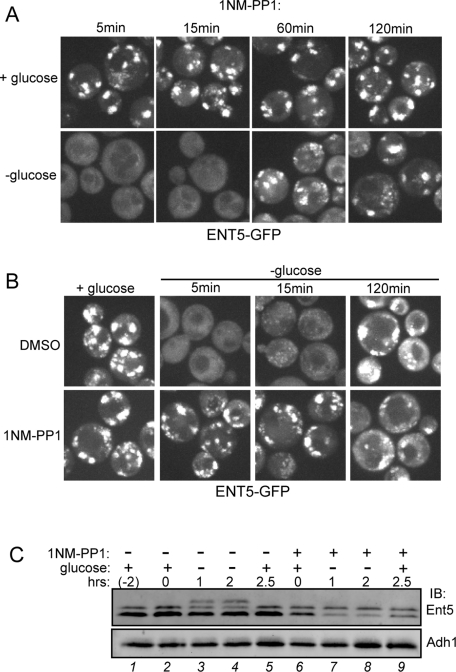
In yeast, the activity of protein kinase A (PKA) is regulated by glucose status (Zaman et al., 2009). In cells with reduced levels of PKA, the rapid responses of actin polarity and translation to acute glucose starvation do not occur (Ashe et al., 2000; Uesono et al., 2004). Because the biphasic response of adaptors to glucose starvation was reminiscent of the biphasic responses of cell polarity and translation to glucose starvation, we tested whether PKA was required for the changes in adaptor localization in response to glucose. PKA is a heterotetramer consisting of two regulatory subunits encoded by Bcy1 and two catalytic subunits encoded by one of three alternate genes, TPK1, -2, or -3 (Toda et al., 1987a, 1987b). Deletion of all Tpk genes is lethal; therefore we used an analogue-sensitive version of PKA (Tpk1-as; Toda et al., 1987b; Bishop et al., 2000; Zaman et al., 2009). We used a strain in which both the TPK2 and TPK3 loci are deleted, and the TPK1 locus is mutated, such that it can be inhibited by the cell-permeable molecule 1NM-PP1. To test the effects of inhibiting PKA, we incubated these cells with 2 μM 1NM-PP1 to completely inhibit PKA phosphorylation activity, and monitored adaptor localization prior to and after glucose starvation. PKA function is blocked in the presence of 2 μM 1NM-PP1, as shown previously, and in our study inhibits cell growth (Stephan et al., 2009).
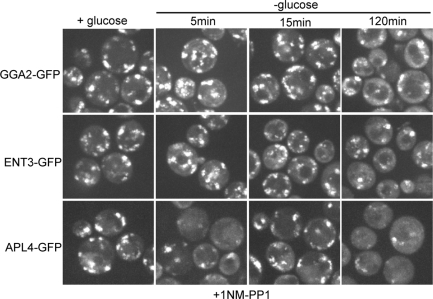
We first examined the localization of Ent5 in Tpk1-as cells in glucose-rich media. Inhibition of PKA did not alter the localization of Ent5 in glucose-rich media. Ent5 remained punctate after 5, 15, 60, or 120 min of 1NM-PP1 treatment, indicating that PKA activity is not required for the localization of Ent5 in glucose-rich media (Figure 5, A and B). In contrast, PKA was required for Ent5 delocalization in glucose-starved cells. Ent5 did not redistribute upon glucose withdrawal when cells were incubated with 1NM-PP1 for 60 or 120 min prior to glucose withdrawal. In control Tpk1-as cells treated with dimethyl sulfoxide (DMSO) for 120 min, Ent5 became delocalized in response to glucose starvation, indicating the defect in Ent5 redistribution is due to inhibition of Tpk1 by 1NM-PP1. PKA activity was also required for Ent3 and Gga2 delocalization in response to glucose starvation (Figure 6). In contrast, a portion of AP-1 appeared to initially be removed from membranes, only to be re-recruited within 15 min of glucose starvation. Thus PKA is required for the immediate response of Ent5, Gga2, and Ent3 to glucose starvation, and plays a complex role in the immediate response of AP-1.
FIGURE 5:
PKA is required for Ent5 relocalization and hyperphosphorylation. (A) Prolonged inhibition of PKA is required to prevent Ent5 relocalization during glucose starvation. ENT5-GFP TPK1-as tpk2Δ tpk3Δ cells (DLY13) were incubated with 2 μM 1NM-PP1 for indicated times, and then imaged immediately after glucose removal. (B) PKA is required for starvation-induced changes in Ent5 localization. Cells were grown in glucose in the presence of DMSO (top) or 2 μM 1NM-PP1 (bottom) for 2 h. Cells were imaged just before starvation or at indicated times following glucose washout. (C) When PKA is inhibited, Ent5 is not hyperphosphorylated during glucose starvation. Cells were treated with DMSO or 2 μM 1NM-PP1 for 2 h during growth in glucose and then starved for glucose. Samples were taken from cells just before DMSO or 1NM-PP1 treatment (lane 1) or just prior to glucose starvation (lanes 2 and 6) or at indicated time-points after starvation. Cell lysates were subject to SDS–PAGE and Western blot analysis with antibodies to Ent5 or Adh1.
FIGURE 6:
PKA is required for starvation-induced relocalization of all TGN–endosome adaptors. TPK1-as tpk2Δ tpk3Δ cells expressing GGA2-GFP (DLY14), ENT3-GFP (DLY17), and the AP-1 subunit APL4-GFP (DLY16) were imaged in the presence of 2 μM 1NM-PP1 during growth in glucose or at indicated times following glucose washout.
We next examined the localization of adaptors following prolonged starvation in the absence of PKA activity. After prolonged starvation in the presence of 1NM-PP1, adaptors were found in puncta, but also had notable diffuse cytosolic localization (Figures 5, A and B, and 6) The localization of Gga2, AP-1, and Ent3 after prolonged starvation in the presence of 1NM-PP1 was similar to the localization of these adaptors in untreated cells after prolonged starvation. However, Ent5 localization was different from that in untreated cells after prolonged starvation (Figure 5B). Ent5 puncta were notably smaller in the presence of 1NM-PP1 after prolonged starvation than were those seen in untreated cells. These data suggest that the initial response of all TGN–endosomal adaptors to glucose withdrawal requires PKA activity. However, adaptors retain some ability to respond to prolonged glucose starvation by partially relocalizing, even in the absence of PKA activity.
Because Ent5 becomes hyperphosphorylated after prolonged starvation and behaves differently in 1NM-PP1 treated cells, we tested to determine whether inhibiting PKA altered Ent5 phosphorylation. In the presence of glucose, inhibition of PKA had no effect on Ent5 phosphorylation (Figure 5C, lane 6). Interestingly, inhibiting PKA prevented Ent5 hyperphosphorylation upon glucose starvation (Figure 5C, lanes 7 and 8). Thus PKA is required for the hyperphosphorylation of Ent5 that occurs upon prolonged starvation.
PKA activity prior to glucose withdrawal is required for starvation responses of adaptors
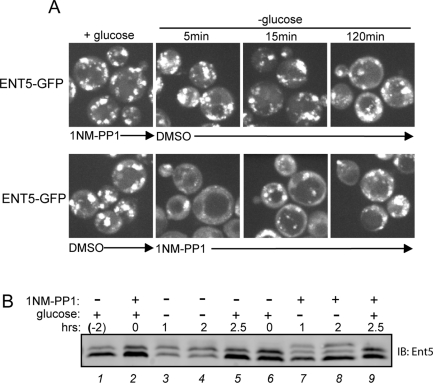
Although PKA is thought to be inactive in the absence of glucose, its role in membrane traffic at the TGN and endosomes had not previously been examined (Zaman et al., 2009). Therefore we examined whether PKA is active and regulates membrane traffic during glucose starvation. Because 1NM-PP1 acts to rapidly inhibit PKA activity, we could perform order-of-addition experiments to determine whether PKA activity was required before or after the onset of glucose starvation (Yorimitsu et al., 2007). We treated cells with 1NM-PP1 for 2 h in the presence of glucose and then washed cells into media lacking both glucose and 1NM-PP1. Pretreating cells with 1NM-PP1 prior to starvation was sufficient to prevent redistribution of Ent5 upon glucose removal (Figure 7A, top). Next we grew cells in glucose without pretreatment with 1NM-PP1, and then washed cells into media lacking glucose but containing 1NM-PP1. Without prior inhibition of PKA, Ent5 redistributed normally in response to glucose starvation (Figure 7A, bottom). Furthermore, when cells were pretreated with 1NM-PP1 for 15 min, Ent5 still redistributed (Figure 5A). These results suggest PKA activity in the presence of glucose is required to establish a cell status that can sense and respond to glucose starvation to direct delocalization of adaptors.
FIGURE 7:
Glucose-stimulated PKA activity is a prerequisite for Ent5 relocalization and hyperphosphorylation during starvation. (A) Glucose-stimulated PKA activity permits relocalization of Ent5 during starvation. ENT5-GFP TPK1-as tpk2Δ tpk3Δ cells (DLY13) were treated with 2 μM 1NM-PP1 or DMSO for 2 h prior to glucose starvation (top) or coincident with glucose starvation (bottom). Cells were imaged in the presence of glucose or at indicated times following glucose washout. (B) PKA must be active prior to glucose removal to allow hyperphosphorylation of Ent5. Cell lysates from TPK1-as tpk2Δ tpk3Δ cells treated with 2 μM 1NM-PP1 prior to (lanes 2–5) or coincident with (lanes 6–9) glucose starvation. Samples were taken from cells grown prior to treatment with DMSO or 1NM-PP1 (lane 1), 2 h after growth in glucose in the presence of DMSO or 1NM-PP1 (lanes 2 and 6), or at indicated times after glucose removal, or 30 min after glucose readdition (lanes 5 and 9). Cell lysates were subject to SDS–PAGE and Western blot analysis with antibodies to Ent5 or Adh1.
Because Ent5 was previously identified as a target of PKA catalytic subunits in a high-throughput approach, we tested to determine if PKA phosphorylation of Ent5 contributes to Ent5 phosphorylation in response to glucose (Ptacek et al., 2005). As described in the preceding paragraph, preincubation of Tpk1-as cells with 1NM-PP1 blocked hyperphosphorylation of Ent5 during glucose starvation (Figure 5C). Similarly, in cells pretreated with 1NM-PP1 and then washed into media lacking glucose and lacking 1NM-PP1, Ent5 did not become hyperphosphorylated (Figure 7B, lanes 2–4). However, when cells were treated with 1NM-PP1 after glucose removal, Ent5 still became hyperphosphorylated (Figure 7B, lanes 6–8). Thus PKA does not mediate hyperphosphorylation of Ent5 after glucose starvation. To determine if PKA phosphorylates Ent5 prior to glucose starvation, and if this phosphorylation of Ent5 is required to allow Ent5 hyperphosphorylation, we identified the sites at which PKA phosphorylates Ent5 (Figure S2). Mutation of the two sites required for PKA-mediated phosphorylation of Ent5 in vitro did not prevent Ent5 hyperphosphorylation in response to glucose starvation in vivo. Although total phosphorylation of Ent5 is reduced in the PKA-site mutant, the hyperphosphorylated form is still clearly apparent after starvation (Figure S2). Thus, although PKA activity is required prior to glucose starvation for Ent5 hyperphosphorylation, it does not directly phosphorylate Ent5 during glucose starvation nor is PKA-dependent phosphorylation of Ent5 a prerequisite for hyperphosphorylation of Ent5.
Clathrin adaptors are regulated by glucose-sensing pathways
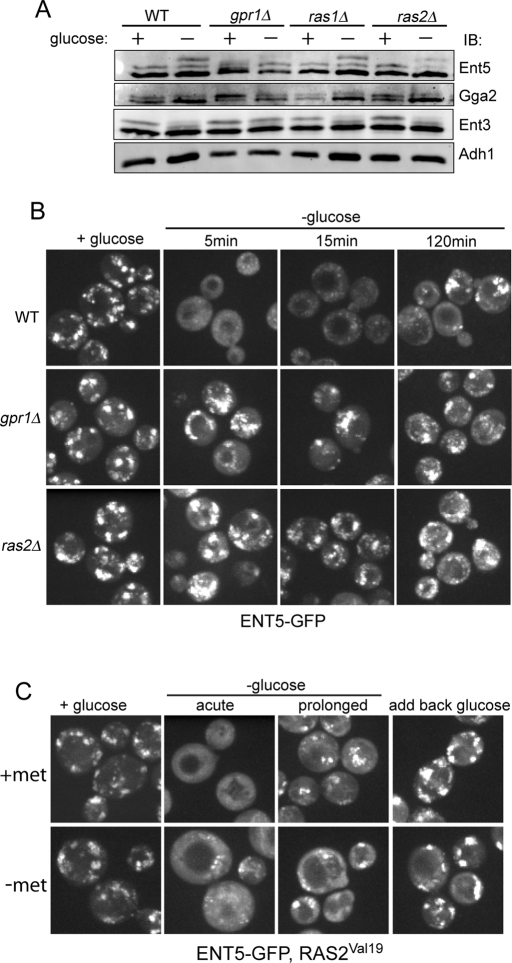
To better understand how glucose status regulates adaptors, we determined whether pathways known to activate PKA were required for changes in adaptor localization during glucose starvation. Two glucose-sensing pathways activate PKA. The first pathway utilizes the G protein–coupled receptor, Gpr1, to sense extracellular glucose. The second pathway utilizes Ras proteins to sense intracellular glucose through an unknown mechanism. Both pathways activate adenylate cyclase to make cAMP that in turn activates PKA (Toda et al., 1985; Kraakman et al., 1999). We first tested whether adaptors were regulated by either pathway by utilizing the readily detectable changes in adaptor phosphorylation as a facile readout. Deletion of Gpr1 reduced glucose responses of all three adaptors. Compared to wild-type cells, hyperphosphorylation of Ent5 was severely reduced after prolonged glucose starvation in gpr1Δ (Figure 8A). Similarly, both Ent3 and Gga2 retained a significant proportion of their high-molecular-weight forms in gpr1Δ after prolonged glucose starvation. Thus Gpr1 is required for maximal changes in posttranslational modifications of Ent5, Ent3, and Gga2 in response to glucose starvation.
FIGURE 8:
Gpr1 and Ras2 regulate adaptor modification and localization. (A) Gpr1 and Ras2, but not Ras1, affect changes in adaptors' phosphorylation. Wt, gpr1Δ, ras1Δ, and ras2Δ cells were grown to mid-log phase in the presence of glucose or subsequently washed and incubated with glucose-free media for 2 h. Cells were lysed, and cell lysates were analyzed by immunoblotting with antibodies to Ent5, Gga2, Ent3, or Adh1 as a loading control. (B) Gpr1 and Ras2 are required for starvation-induced relocalization of Ent5. Fluorescence microscopy of ENT5-GFP in wild-type (DLY3), gpr1Δ (DLY11), or ras2Δ cells (DLY12). Cells were imaged during growth in glucose, or at indicated times following glucose washout. (C) Hyperactivation of PKA does not inhibit relocalization of Ent5 during glucose starvation. Cells expressing ENT5-GFP (DLY3) were transformed with a plasmid containing the dominant active Ras2Val19 allele under the control MET3 promoter. Ras2Val19 expression was induced by growth in media without methionine (−met; bottom). In cells with hyperactive PKA activity, Ent5 localization is not significantly affected during growth in glucose. Following acute glucose starvation, Ent5 is predominantly diffusely localized and found in some punctate structures in cells with hyperactive PKA activity. During prolonged starvation, and following reintroduction of glucose, localization of Ent5 in hyperactive cells is similar to that in uninduced cells.
In contrast, the RAS pathway was only important for maximal changes in Ent5. In ras2Δ but not ras1Δ cells, the total amount of phosphorylated Ent5 was decreased (Figure 8A). Dephosphorylation of Gga2 and Ent3 was unaffected in ras1Δ or ras2Δ cells. Therefore phosphorylation of Ent5 is sensitive to perturbation in both Gpr1 and Ras2 signaling, while phosphorylation of Gga2 and Ent3 is sensitive only to Gpr1 signaling.
We next examined the role of Gpr1 and Ras2 on the localization of Ent5. In the presence of glucose, Ent5 localized to many puncta in gpr1Δ and ras2Δ cells. The distribution of Ent5 in gpr1Δ and ras2Δ cells in glucose was largely similar to that in wild-type cells grown in the presence of glucose, although the number of puncta was reduced (Figure 8B). In contrast to wild-type cells, Ent5 did not redistribute immediately in response to glucose starvation in the mutant cells, but rather continued to be retained on puncta even after 15 min of glucose starvation. Following prolonged starvation, cytoplasmic staining of Ent5 was increased, although it is still found in many puncta. Notably, after prolonged starvation, many Ent5 puncta in ras2Δ or gpr1Δ cells were smaller than those found in wild-type cells under the same conditions These results were strikingly similar to what occurred when PKA itself was inhibited (Figure 5). Therefore activation of PKA through both the Gpr1 and Ras pathways is required for the immediate redistribution of Ent5 in response to glucose starvation and influences the localization of Ent5 in the prolonged phase.
To further investigate the role of PKA in regulating Ent5, we examined the effect of hyperactivation of PKA on the localization and phosphorylation of Ent5. Expression of the Ras2Val19 allele under the MET3 promoter results in constitutively elevated levels of PKA activity, decreased viability during glucose starvation, and increased sensitivity to environmental stresses (Toda et al., 1985; Xue et al., 1998). Expression of Ras2Val19 was induced by transferring cells to media without methionine for 5 h. In these cells, Ent5 still became delocalized after glucose starvation, although some Ent5 remained in small puncta (Figure 8C). Furthermore, the distribution of Ent5 to puncta after prolonged starvation was similar between control and Ras2Val19-expressing cells, suggesting hyperactivation of PKA does not significantly affect the ability of Ent5 to relocalize during glucose starvation. Similarly, expression of Ras2Val19 did not prevent Ent5 hyperphosphorylation during glucose starvation (unpublished data). These data suggest that PKA activity during glucose starvation cannot prevent Ent5 responses to glucose withdrawal.
DISCUSSION
Adaptors are regulated by glucose availability
Glucose starvation stimulates massive remodeling of many cellular functions and has long-term transcriptional consequences. In the absence of glucose, the cell still transcribes and translates some genes, and new metabolic enzymes take over cellular metabolism. However, previous studies suggest rapid and transient responses to glucose starvation also occur (Ashe et al., 2000; Uesono et al., 2004). In this study, we show that the clathrin adaptors, Gga2, Ent5, Ent3, and AP-1 are also subject to immediate, transient responses to glucose starvation. The immediate response of adaptors occurs at the same time that cells arrest translation and depolarize their actin cytoskeleton. All three immediate responses require the activity of PKA, thus the three activities likely respond to one or more factors whose activity is influenced by PKA. These coordinated, immediate responses may prevent the cell from utilizing precious resources in synthesizing and trafficking proteins needed prior to starvation but not needed in the starved cell.
Translation, actin polarity, and adaptor localization all recover to some extent after prolonged starvation. Recovery occurs slowly over the course of hours. During this time frame, the cell is transcribing new genes (Martinez-Pastor and Estruch, 1996). Restoration of translation and adaptor localization is likely essential for the proper functioning of these gene products during glucose starvation. Restoration of translation allows the cell to produce the proteins required for cell survival in the absence of glucose. Resumption of traffic at the TGN and endosomes allows the cell to correctly route proteins to the cell surface or vacuole.
In the absence of glucose, traffic at the TGN and endosomes may be qualitatively different from traffic that occurs in the presence of glucose. In prolonged glucose starvation, AP-1 recruitment to membranes is far weaker than Gga2 recruitment. This differential recruitment may alter the net flow of traffic at the TGN and endosomes. The exact roles of the different adaptors have not been determined. AP-1 and Gga1 and Gga2 perform at least one common essential function; however, they have some unique functions as well (Hirst et al., 2001). Multiple studies suggest that AP-1 reroutes proteins back to the TGN from the endosomes whereas Gga1 and Gga2 route proteins toward the vacuole (Black and Pelham, 2000; Costaguta et al., 2001; Valdivia et al., 2002; Foote and Nothwehr, 2006). By preventing retrieval via the AP-1 pathway but maintaining traffic toward the vacuole, the differential re-recruitment of adaptors observed in glucose starvation may promote increased degradation of proteins that transit via the TGN and endosomes. In support of this observation, Gga1 and Gga2, but not AP-1, are required for the routing of Pma1 to the vacuole in response to glucose starvation (Huang and Chang, 2011).
PKA activity is a prerequisite for immediate changes in adaptor localization
The activity of PKA in the presence of glucose is a prerequisite for adaptor, translation, and cell polarity responses to glucose starvation. PKA is active in the presence of glucose, and it is therefore somewhat unexpected that it is required for three unrelated responses to glucose starvation. Our results, combined with previous studies, offer an explanation for the PKA requirement. The immediate responses of actin and translation to glucose starvation can be restored by deletion of the transcription factors inhibited by PKA (Uesono et al., 2004). This suggests that a transcriptional target of PKA may mediate these immediate responses. Order-of-addition experiments in this study show PKA activity is required prior to glucose starvation for adaptor delocalization. Furthermore, PKA must be inhibited for prolonged periods to prevent delocalization (Figure 5A). The requirement for prolonged inhibition is consistent with a transcriptional target of PKA mediating the immediate response of adaptors to glucose starvation, although we cannot rule out PKA regulation of the activity or localization of a protein.
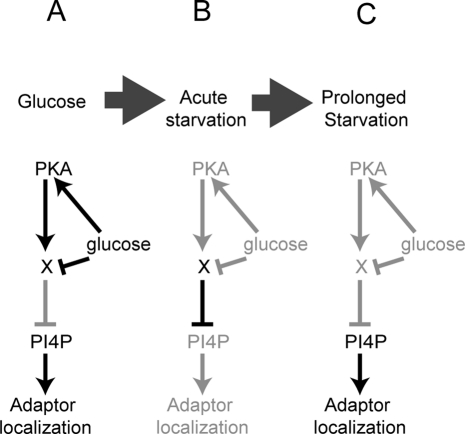
The unknown target could thus be a protein whose expression, localization, or activity requires PKA in the presence of glucose (Figure 9). In this scenario, PKA allows the accumulation of the target activity in the presence of glucose. Although this activity is capable of displacing adaptors from membranes, it is held in check by the presence of glucose. On the removal of glucose, the PKA target is activated and causes adaptor delocalization. When PKA is inhibited, either by the removal of glucose or by direct inhibition of PKA, the target activity dissipates. During prolonged starvation, dissipation allows recovery of adaptors. When PKA is inhibited chemically or by mutation of GPR1 or RAS2 in the presence of glucose, the target activity levels drop. Loss of the PKA target activity does not alter adaptor localization in the presence of glucose; however, without the target activity, adaptors do not delocalize during glucose starvation. It is unclear whether the same PKA target regulates translation, actin polarity, and adaptors, or whether multiple targets coordinately regulate these processes.
FIGURE 9:
Possible model of the role of PKA in the acute phase of glucose starvation and the effect on adaptor localization. (A) In the presence of glucose, PKA directs the activation of an unknown factor (X). This factor is kept inactive by the presence of glucose. (B) On glucose starvation, the unknown factor becomes active and reduces levels of PI4P at the TGN and endosomes. (C) When PKA is inhibited, the unknown factor is not active, and the immediate response does not occur upon glucose starvation. Depletion of activity of the unknown factor levels after prolonged starvation also allows recovery of PI4P levels and adaptor re-recruitment.
Although the factor that communicates the immediate response is unknown, the downstream effectors of this factor are known in the case of translation (Pop2) and actin polarity (Rom2; Uesono et al., 2004). In the case of adaptor localization, the target may be PI4P levels. On glucose starvation, PI4P levels are significantly reduced due to redistribution of the PI4P-synthesizing enzyme Pik1 and -degrading enzyme Sac1 (Faulhammer et al., 2007; Demmel et al., 2008a). PI4P recruits Gga2 to the TGN and endosomes in yeast (Demmel et al., 2008b). AP-1 in humans also binds PI4P (Wang et al., 2003). Because Ent3 and Ent5 bind Gga1 and Gga2 and AP-1, PI4P likely influences Ent3 and Ent5 recruitment as well (Costaguta et al., 2006). Thus reduction of PI4P levels at the TGN and endosomes in response to glucose starvation is expected to dramatically reduce recruitment of all adaptors. It remains to be determined whether PI4P levels change fast enough to mediate the immediate responses of adaptors to glucose starvation, and if so, how PKA ensures this rapid change.
When adaptors eventually relocalize back to membranes, their localization is different from that of unstarved cells: all adaptors are found in fewer structures, a significant fraction of each of the adaptors is diffusely cytosolic, and a smaller fraction of AP-1 is membrane-associated, as compared with the other adaptors. Because these changes still occur in cells with inhibited PKA, other signaling pathways likely affect TGN–endosome traffic during this prolonged phase. Such pathways may include the Snf1/AMP-activated protein kinase pathway, which is activated in low glucose and other stresses; the target of rapamycin (TOR) pathway, which is activated in nutrient-rich conditions; and the Sch9/Akt pathway, which is also activated in nutrient-rich conditions (Yorimitsu et al., 2007; Hedbacker and Carlson, 2008; Stephan et al., 2009; Ramachandran and Herman, 2011). Further studies are needed to determine whether any of these pathways act downstream of PKA in establishing the immediate response, or whether they are involved in the prolonged response to starvation.
Posttranslational modifications of adaptors change in response to glucose
We observed changes in posttranslational modification of Gga2 immediately upon glucose starvation, and changes in Ent3 and Ent5 upon prolonged starvation. Although the change in Gga2 is coincident with the immediate response, and the changes in Ent3 and Ent5 are coincident with the recovery phase, the functional significance of these modifications is unclear. It is intriguing that changes in adaptor modifications require the prerequisite PKA function in high glucose. In Tpk1-as cells pretreated with 1NM-PP1, Ent5 does not become hyperphosphorylated in response to glucose starvation. Similarly in cells lacking Gpr1, changes in posttranslational modifications of Gga2, Ent3, and Ent5 in response to starvation are blocked.
Further studies are needed to address whether the modifications themselves alter adaptor localization, or whether they reflect changes in accessibility of adaptors to modifying enzymes secondary to changes in adaptor localization, or if they are caused by a mechanism unrelated to adaptor localization.
In summary, we have shown that adaptors are subject to global, biphasic modulation upon glucose starvation. The first phase is rapid, and adaptors are largely removed from membranes, which likely severely reduces traffic. During the second, slow phase, adaptors are partially re-recruited to membranes. Differential recruitment of adaptors during the slow phase may substantially alter the itinerary of numerous proteins. In the presence of glucose, PKA performs one or more as yet unknown activities required for the localization changes that occur in the immediate phase and the posttranslational modifications that occur in the prolonged phase.
MATERIALS AND METHODS
Yeast strains
Yeast strains are listed in Table 1. Fluorescent tags and gene deletions were introduced by standard PCR-based methods (Longtine et al., 1998). Strains containing multiple mutations were generated by standard yeast genetics. Ent5 mutations were generated using QuikChange mutagenesis (Stratagene, Agilent, Santa Clara, CA) with a bacterial cloning vector containing the genomic region of Ent5 (pMD173). Deletion replacement was used to introduce the mutations into the endogenous genomic locus. The yeast centromeric plasmid pPHY795, containing the Ras2Val19 allele under the MET3 promoter, was generously provided by Paul Herman (Howard et al., 2003). Expression of Ras2Val19 was induced by transferring cells to medium lacking methionine for 5 h.
TABLE 1:
Strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| BY4742 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Invitrogen |
| DLY3 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ENT5-GFP(S65T)::His3MX | This study |
| DLY4 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 GGA2-GFP(S65T)::KanMX6 | This study |
| DLY36 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 APL4-GFP(S65T)::KanMX6 | This study |
| DLY6 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ENT3-GFP(S65T)::KanMX6 | This study |
| DLY35 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 SEC7-GFP(S65T)::KanMX | This study |
| DLY22 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 SNF7-GFP(S65T)::KanMX6 | This study |
| DLY7 | Mat α his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 ENT5-GFP(S65T)::KanMX6 CHC-RFP::KanMX6 | This study |
| DLY8 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 gpr1Δ::KanMX4 | Invitrogen |
| DLY9 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ras2Δ::KanMX4 | Invitrogen |
| DLY10 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ras1Δ::KanMX4 | Invitrogen |
| DLY11 | Mat α his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 lys2Δ0 ENT5-GFP(S65T)::KanMX6 gpr1Δ::KanMX4 | This study |
| DLY12 | Mat a his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 ENT5-GFP(S65T)::KanMX6 ras2Δ::KanMX4 | This study |
| Y3175 | Mat A ade2–1 can1–100 his3–11,15 leu2–3112 trp1–1 ura3–1 tpk2::KAN tpk3::TRP1 tpk1-M164G | Stephan et al., 2009 |
| DLY13 | Mat a ade2–1 can1–100 his3–11,15 leu2–3112 trp1–1 ura3–1 tpk2::KAN tpk3::TRP1 tpk1-M164G ENT5-GFP(S65T)::His3MX | This study |
| DLY14 | Mat a ade2–1 can1–100 his3–11,15 leu2–3112 trp1–1 ura3–1 tpk2::KAN tpk3::TRP1 tpk1-M164G GGA2-GFP(S65T)::His3MX | This study |
| DLY15 | Mat a ade2–1 can1–100 his3–11,15 leu2–3112 trp1–1 ura3–1 tpk2::KAN tpk3::TRP1 tpk1-M164G GGA2-GFP(S65T)::His3MX | This study |
| DLY16 | Mat a ade2–1 can1–100 his3–11,15 leu2–3112 trp1–1 ura3–1 tpk2::KAN tpk3::TRP1 tpk1-M164G APL4-GFP(S65T)::His3MX | This study |
| DLY17 | Mat a ade2–1 can1–100 his3–11,15 leu2–3112 trp1–1 ura3–1 tpk2::KAN tpk3::TRP1 tpk1-M164G ENT3-GFP(S65T)::His3MX | This study |
| GPY2731 | Mat α ura3-52, his3Δ-200, trp1-Δ901, leu2-3112, lys2-801, suc2-Δ9, GAL, MEL, ent5Δ::TRP1 | Duncan et al., 2003 |
| DLY19 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ENT5 T41I::URA3 | This study |
| DLY20 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ENT5 S257::URA3 | This study |
| DLY21 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ENT5 T41I,S257A::URA3 | This study |
| DLY23 | Mat α his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 ent5Δ::KanMX4 | Invitrogen |
Antibodies and reagents
Polyclonal antibodies to adaptors were generated in rabbits against full-length GST-tagged Ent3, Ent5, and Gga2 in rabbits. Gga2, Ent3 and Ent5 antibodies were affinity-purified by passing serum first over 2 ml of Affigel-10 (Bio-Rad, Hercules, CA) cross-linked to purified GST to deplete GST signal. Depleted serum was bound to 2 ml of Affigel-10 cross-linked to purified GST-tagged full-length protein and eluted from the matrix with glycine according to manufacturer's instructions. Other reagents were obtained as follows: rabbit polyclonal Adh1 and GFP antibodies (Abcam, Cambridge, MA), Alexa Fluor secondary antibodies (Invitrogen, Carlsbad, CA), protein A Sepharose (GE Healthcare, Waukesha, WI), lyticase (Sigma-Aldrich, St. Louis, MO), 1NM-PP1 (Calbiochem, San Diego, CA), Halt phosphatase inhibitors (Pierce, Rockford, IL), [32P]ATP (PerkinElmer, San Jose CA). Calf intestinal alkaline phosphatase, restriction enzymes, and bovine PKA were obtained from New England Biolabs (Ipswich, MA). Protease inhibitors and DMSO were obtained from Sigma-Aldrich. Yeast peptone (YP) media is 1% bacto-yeast extract (Difco, Detroit, MI) and 2% bacto-peptone (Difco) supplemented with 20 μg/ml adenine, uracil, and l-tryptophan. Yeast peptone dextrose (YPD) is YP with 2% dextrose. Synthetic defined (SD) media is 0.67% of yeast nitrogen base without amino acids (Difco) and 2% dextrose. Supplemented SD media contained 100μg/ml adenine, l-leucine, l-lysine, l-tryptophan; 50 μg/ml l-histidine, l-methionine, and 20 μg/ml uracil. Supplemented synthetic media (SM) without dextrose was used for all starvation experiments.
Growth conditions and glucose starvation
Yeast cells were grown in YPD or supplemented SD at 30°C and aerated by rotary shaking. For glucose starvation, cells were grown overnight in SD, diluted the next day, and grown to mid-log phase (OD600 ∼0.2–0.5) prior to glucose withdrawal. To remove glucose, cells were pelleted and washed three times with SM, and then incubated for times indicated or for 3–5 min for acute starvations and 2–3 h for prolonged starvations. For PKA inhibitions, cells were incubated with 2 μM 1NM-PP1 or an equivalent amount of DMSO following the growth to mid-log phase.
Preparation of cell lysates, immunoprecipitations, and immunoblotting
For immunoprecipitations (IPs), 50 OD600 of yeast cells were converted to spheroplasts and then subjected to glass-bead lysis in Buffer A containing 100 mM MES (pH 6.5), 0.5 mM MgCl2, 1 mM EDTA, 2 mM NaN3, 0.2 mM dithiothreitol (DTT), and protease and phosphatase inhibitors (Costaguta et al., 2006). Triton X-100 was added to the lysate to a final concentration of 1%, and the lysate was incubated on ice for 10 min. The lysate was then cleared by centrifugation, and the supernatant was incubated overnight with antibodies and protein A Sepharose at 4°C. IPs were washed with three times with Buffer A and one time with 10 mM Tris-HCl, 10 mM NaCl. To demonstrate in vivo phosphorylation of adaptors, IPs were washed with Buffer A without phosphatase inhibitors one time, incubated with calf alkaline phosphatase for 1 h at 37°C and then with Buffer A and 10 mM Tris-HCl, 10 mM NaCl as described above. Samples were resuspended in Laemmli buffer containing 4% SDS and 100 mM β-mercaptoethanol and then boiled. For whole-cell extracts, 2 OD600 of cells were resuspended in Laemmli sample buffer, boiled, and subjected to glass-bead disruption. The extracts were cleared by centrifugation. Following SDS–PAGE, samples were transferred to nitrocellulose, blocked with 4% milk in TBS-T, and then probed with primary and fluorescent secondary antibodies. Adh1 was used as a loading control. Fluorescence signals were detected on a Typhoon imaging system (Amersham Biosciences, Piscataway, NJ). Immunoblots were quantitated using ImageJ software (http://rsb.info.nih.gov/ij/download.html).
Microscopy, image processing, and quantitation
Cells were grown as described above and then briefly pelleted and resuspended in 50–200 μl media prior to imaging. Cells (2–4 μl) were mounted on acid-washed coverslips coated with Concanavalin-A and then imaged. For each field, 12–16 Z-stacks (0.4 μm) were captured using a 100 × oil objective (numerical aperture 1.4) on a Nikon Eclipse TE300 (Melville, NY) using a Hamamatsu Orca-ER digital camera (Hamamatsu, Japan). Z-stacks were then compressed into a single maximum-intensity image in ImageJ. The number of puncta per cell was quantitated by counting the number of foci in a single Z-stack from the middle of the cell (n > 40 cells) for each condition specified. Statistical significance was determined using the Mann-Whitney U test.
Cell fractionations
Mid-log-phase cells were washed and concentrated to 25 OD600/ml in 100 mM Tris-SO4 (pH 9.4), 10 mM DTT, and 2% glucose and then incubated for 10 min at room temperature. To digest the cell wall for nonstarved samples, cells were washed twice in YPD and then resuspended to 10 OD600/ml in 1 M sorbitol, 10 mM Tris-HCl (pH 7.5), 25 μg/ml lyticase in YP in the presence of 0.5% glucose. To digest the cell wall for glucose-starved samples, cells were washed twice in SM and resuspended to 10 OD600/ml in 1 M sorbitol, 10 mM Tris-HCl (pH 7.5), 25 μg/ml lyticase in SM. The cells were then incubated with gentle agitation for 15 min at 30°C. Spheroplasts were pelleted and resuspended to 50 OD600/ml in 0.2 M sorbitol, 50 mM potassium acetate, 2 mM EDTA, 20 mM HEPES (pH 6.0), 1 mM DTT, and protease inhibitor cocktail (Sigma). Spheroplasts were dounced 20 times with a tight-fitting pestle. Large cell fragments were removed by pelleting at 1500 rpm for 5 min in an HB-4 (Sorvall, Thermo Scientific, Lafayette, CO) rotor with a microfuge tube adaptor. High-speed supernatant and pellets were generated by pelleting at 75,000 rpm for 5 min in a TLA100-rotor (Beckman Coulter, Brea, CA). Immunoblots were quantitated with ImageJ software. Fraction of total in pellet was calculated by comparing protein levels in the high-speed pellet to the summed protein levels of the high-speed pellet and supernatant.
In vitro phosphorylation of Ent5
Ent5 was immunoprecipitated as described above and resuspended in 50 μl of Buffer A. The IP (10 μl) was washed two times with kinase buffer (100 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 50 μM ATP, 5 mM β-glycerophosphate, 1 mM Na orthovanadate, 1 mM DTT, 0.5 mM EGTA) and then resuspended in 10 μl kinase buffer with 10 μCi of [32P]ATP. PKA (1250 U) was added, and the sample was incubated for 30 min at room temperature. Laemmli buffer was added to the samples, which were then boiled and subjected to SDS–PAGE. The gel was dried and exposed to a phosphor screen, and radiolabeled signals were detected on a Typhoon imaging system and quantitated using ImageJ. To compare the level of phosphorylation between wild-type and mutant Ent5 proteins, the signal intensity of phosphorylated protein was normalized to the amount of immunoprecipitated protein and expressed as percent of wild-type.
Supplementary Material
Acknowledgments
We are grateful to Ted Salmon and Josh Lawrimore for assistance with microscopy. Thanks to Ajit Joglekar for assistance with quantitation and Paul Herman for reagents and insightful comments on the manuscript. We also thank Mark Peifer and Alan Jones for comments on the manuscript. This study was supported by funds from the State of North Carolina and funding from the March of Dimes.
Abbreviations used:
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- GFP
green fluorescent protein
- IP
immunoprecipitation
- PI4P
phosphatidylinositol 4-phosphate
- PKA
protein kinase A
- RFP
red fluorescent protein
- SD
synthetic defined
- SM
synthetic media
- TGN
trans-Golgi network
- YP
yeast peptone
- YPD
yeast peptone dextrose
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-04-0309) on August 10, 2011.
REFERENCES
- Ashe MP, De Long SK, Sachs AB. Glucose depletion rapidly inhibits translation initiation in yeast. Mol Biol Cell. 2000;11:833–848. doi: 10.1091/mbc.11.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Black MW, Pelham HR. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Zhang C, Zhu X, Kahn RA. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS. The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Costaguta G, Duncan MC, Fernandez GE, Huang GH, Payne GS. Distinct roles for TGN/endosome epsin-like adaptors Ent3p and Ent5p. Mol Biol Cell. 2006;17:3907–3920. doi: 10.1091/mbc.E06-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta G, Stefan CJ, Bensen ES, Emr SD, Payne GS. Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell. 2001;12:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmel L, et al. Nucleocytoplasmic shuttling of the Golgi phosphatidylinositol 4-kinase Pik1 is regulated by 14-3-3 proteins and coordinates Golgi function with cell growth. Mol Biol Cell. 2008a;19:1046–1061. doi: 10.1091/mbc.E07-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmel L, et al. The clathrin adaptor Gga2p is a phosphatidylinositol 4-phosphate effector at the Golgi exit. Mol Biol Cell. 2008b;19:1991–2002. doi: 10.1091/mbc.E06-10-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wever V, Reiter W, Ballarini A, Ammerer G, Brocard C. A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J. 2005;24:4115–4123. doi: 10.1038/sj.emboj.7600871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MC, Costaguta G, Payne GS. Yeast epsin-related proteins required for Golgi-endosome traffic define a γ-adaptin ear-binding motif. Nat Cell Biol. 2003;5:77–81. doi: 10.1038/ncb901. [DOI] [PubMed] [Google Scholar]
- Faulhammer F, Kanjilal-Kolar S, Knodler A, Lo J, Lee Y, Konrad G, Mayinger P. Growth control of Golgi phosphoinositides by reciprocal localization of sac1 lipid phosphatase and pik1 4-kinase. Traffic. 2007;8:1554–1567. doi: 10.1111/j.1600-0854.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- Fernandez GE, Payne GS. Laa1p, a conserved AP-1 accessory protein important for AP-1 localization in yeast. Mol Biol Cell. 2006;17:3304–3317. doi: 10.1091/mbc.E06-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote C, Nothwehr SF. The clathrin adaptor complex 1 directly binds to a sorting signal in Ste13p to reduce the rate of its trafficking to the late endosome of yeast. J Cell Biol. 2006;173:615–626. doi: 10.1083/jcb.200510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Wolf J, Brown EL, Ammerer G, Ruis H, Schuller C. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 2002;21:135–144. doi: 10.1093/emboj/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408–2420. doi: 10.2741/2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Townley R, Carlson M. Cyclic AMP-dependent protein kinase regulates the subcellular localization of Snf1-Sip1 protein kinase. Mol Cell Biol. 2004;24:1836–1843. doi: 10.1128/MCB.24.5.1836-1843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lindsay MR, Robinson MS. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell. 2001;12:3573–3588. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard SC, Hester A, Herman PK. The Ras/PKA signaling pathway may control RNA polymerase II elongation via the Spt4p/Spt5p complex in Saccharomyces cerevisiae. Genetics. 2003;165:1059–1070. doi: 10.1093/genetics/165.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Chang A. pH dependent cargo sorting from the Golgi. J Biol Chem. 2011;25:10058–10065. doi: 10.1074/jbc.M110.197889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Davis C, Broach JR. Efficient transition to growth on fermentable carbon sources in Saccharomyces cerevisiae requires signaling through the Ras pathway. EMBO J. 1998;17:6942–6951. doi: 10.1093/emboj/17.23.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraakman L, Lemaire K, Ma P, Teunissen AW, Donaton MC, Van Dijck P, Winderickx J, de Winde JH, Thevelein JM. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol Microbiol. 1999;32:1002–1012. doi: 10.1046/j.1365-2958.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Martinez-Pastor MT, Estruch F. Sudden depletion of carbon source blocks translation, but not transcription, in the yeast Saccharomyces cerevisiae. FEBS Lett. 1996;390:319–322. doi: 10.1016/0014-5793(96)00683-7. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, Robinson LC. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacek J, et al. Global analysis of protein phosphorylation in yeast. Nature. 2005;438:679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Herman PK. Antagonistic interactions between the cAMP-dependent protein kinase and Tor signaling pathways modulate cell growth in Saccharomyces cerevisiae. Genetics. 2011;187:441–454. doi: 10.1534/genetics.110.123372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger AL, Cain NE, Chen EJ, Kaiser CA. Activity-dependent reversible inactivation of the general amino acid permease. Mol Biol Cell. 2006;17:4411–4419. doi: 10.1091/mbc.E06-06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PM, Bilodeau PS, Zhdankina O, Winistorfer SC, Hauglund MJ, Allaman MM, Kearney WR, Robertson AD, Boman AL, Piper RC. GGA proteins bind ubiquitin to facilitate sorting at the trans-Golgi network. Nat Cell Biol. 2004;6:252–259. doi: 10.1038/ncb1107. [DOI] [PubMed] [Google Scholar]
- Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci USA. 2009;106:17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- Toda T, Cameron S, Sass P, Zoller M, Scott JD, McMullen B, Hurwitz M, Krebs EG, Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1987a;7:1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Cameron S, Sass P, Zoller M, Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987b;50:277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Uesono Y, Ashe MP, Toh EA. Simultaneous yet independent regulation of actin cytoskeletal organization and translation initiation by glucose in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:1544–1556. doi: 10.1091/mbc.E03-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia RH, Baggott D, Chuang JS, Schekman RW. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- Xue Y, Batlle M, Hirsch JP. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Gα subunit and functions in a Ras-independent pathway. EMBO J. 1998;17:1996–2007. doi: 10.1093/emboj/17.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Zaman S, Broach JR, Klionsky DJ. Protein kinase A and Sch9 cooperatively regulate induction of autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:4180–4189. doi: 10.1091/mbc.E07-05-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S, Lippman SI, Schneper L, Slonim N, Broach JR. Glucose regulates transcription in yeast through a network of signaling pathways. Mol Syst Biol. 2009;5:245. doi: 10.1038/msb.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.