Abstract
The activation of innate immune response is initiated by engagement of pattern-recognition receptors (PPRs), such as Toll-like receptors (TLRs). These receptors are expressed in peripheral leukocytes and in many cell types in the central nervous system (CNS). The expression of TLRs in CNS was mainly studied in astrocytes and microglial cells. However, new evidence indicates that these receptors may play an important role in neuronal homeostasis. The expression of TLRs in the CNS is variable and can be modulated by multiple factors, including pro-inflammatory molecules, which are elevated in neurodegenerative diseases and can increase the expression of TLRs in CNS cells. Moreover, activation of TLRs induces the release of pro-inflammatory cytokines. Therefore, TLRs have been shown to play a role in several aspects of neurodegenerative diseases. Here we will discuss results reported in the recent literature concerning the participation of TLRs in neurodegenerative diseases.
Keywords: Toll-like receptors, central nervous system, Inflammation, Alzheimer’s disease, multiple sclerosis
1. Introduction
Inflammation is an important and fast host response to tissue injury, autoimmune reactions and infectious agents. In peripheral tissues, outside the blood brain barrier (BBB), the classical inflammatory signs are swelling, redness, heat, pain and loss of function. Key events driving inflammation include migration and invasion of leukocytes (neutrophils, lymphocytes and macrophages) and the release of soluble mediators such as kinins, prostaglandins, classical cytokines and chemokines. Several of these mediators produced at the site of the inflammatory response generate local and systemic effects therefore are key targets for therapeutic intervention in many diseases [1].
The ‘immune privilege’ of the central nervous system (CNS) is indispensable for damage limitation during inflammation in a sensitive organ with poor regenerative capacity. It is now clear that while peripheral immune components access to the CNS is restricted and tightly controlled, the CNS is capable of mounting dynamic immunologic and inflammatory responses to a variety of insults [2–4]. Several stimuli such as trauma, infections, toxins and systemic pro-inflammatory cytokines are capable of eliciting an immediate and short lived activation of the innate immune system within the CNS [3, 5]. This acute neuroinflammatory response includes activation of microglia (resident immune cells) resulting in their morphological and phenotypical changes, and the release of inflammatory mediators such as cytokines and chemokines by these cells [4]. Under physiological conditions, microglia exhibit a quiescent phenotype which is associated with the production of anti-inflammatory and neurotrophic factors [6]. Activated microglia, however, promote an inflammatory response that serves to further engage the immune system and initiate tissue repair [7].
This response is frequently self-limiting, resolving once infection has been eradicated or the tissue damage has been repaired. However, persistence of inflammatory stimulation, by exogenous or endogenous factors, or a failure in normal resolution mechanisms caused by overwhelming inflammatory cycles, may result in pathological consequences [7]. Activated microglial cells and macrophages are essential for the clearance of invading microorganisms and injured tissue. They can be stimulated to express a variety of pro-inflammatory cytokines such as IL-1, TNF-α and IL-6, as well as superoxide and nitric oxide, which are neurotoxic and may amplify underlying disease states [8].
Although the causes of several pathological conditions in the CNS, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and multiple sclerosis (MS), are complex and may involve multiple factors, an active role of the innate host defense mediated by mononuclear phagocytes has been clearly demonstrated [9].
Despite the fact that inflammation may not typically represent an initiating factor in neurodegenerative diseases, it is clear that a balance between pro- and anti-inflammatory signals, determining sustained inflammatory responses in the CNS, is important in the disease progression [7, 9].
Activation of pro-inflammatory innate immune responses can be initiated by engagement of germline-encoded pattern-recognition receptors (PRRs), such as Toll-like receptors (TLRs), which are expressed by many cell types in the CNS cells. The final outcome of the diseases will depend on regulatory pathways driven by PRRs and mediators associated with PRRs activation [9–10].
2. Overview of TLR activation and signaling
The surface of pathogens typically bear repeating patterns of molecular structure referred to as pathogen-associated molecular patterns (PAMPs) [11]. The innate immune system recognizes such pathogens by means of PRRs that bind features of these regular patterns [12]. The TLR family is the best characterized group of innate immune receptors in terms of known ligands, downstream signaling pathways and functional relevance [13].
TLRs are type I transmembrane proteins with ectodomains containing leucine-rich repeats that mediate the recognition of PAMPs; transmembrane domains; and intracellular Toll-interleukin 1 receptor (TIR) domains required for downstream signal transduction. A total of 10 and 12 functional TLRs have been identified in humans and mice, respectively, with TLR1-TLR9 being conserved in both species. Mouse TLR10 is not functional because of a retrovirus insertion, and TLR11, TLR12 and TLR13 have been lost from the human genome. Moreover, studies that used using mice deficient in different TLR, have demonstrated that each individual TLR has a distinct function in terms of PAMP recognition and induction of immune responses [11, 14].
TLR activation initiates signal transduction pathways that lead to diverse transcriptional responses. The first major pathway triggered by TLRs activates the transcription factor NF-κB, which regulates the transcription of many genes that encode proteins involved in immunity and inflammation. The second one activates the Jun amino-terminal kinase (JNK) and p38 MAP kinases, which also induce increased transcription and regulate the stability of mRNAs that contain AU repeats [15]. TLRs 2, 4, 5, 7 and 9 activate NF-κB and MAP kinases through a pathway that involves the IL-1 receptor associated kinase (IRAK) 4 and 1. An interesting issue is that, although all TLRs can activate NF-κB and MAP kinases, there are differences in the final gene expression pattern resulting from the activation of individual TLRs. The specificity of these effects can be understood at least in part by a set of adaptor proteins that are differentially recruited to TLRs, such as myeloid differentiation primary response protein88 (MyD88), MyD88-adapter-like (Mal, also known as TIRAP), TIR-related adaptor protein inducing interferon (Trif) and Trif-related adaptor molecule (Tram) [16]. Similar to TLRs, MyD88 has a Toll-IL-1 receptor (TIR) domain that participates in the ligand-induced assembly of a TIR-TIR platform by the dimerization of two TLRs, with the exception of TLR4 and TLR2, which links to MyD88 indirectly via the bridging Mal protein, and TLR3, which signals exclusively through Trif [17].
Recently, it was determined that the death domains of human MyD88 and IRAK-4 assemble into closed complexes having unusual stoichiometries of 7:4 and 8:4, the Myddosome [18]. IRAK-4 is recruited to the receptor complex and interacts with MyD88 via homotypic death domain interactions; it becomes activated and phosphorylates the recruited IRAK-1. Once IRAK-1 is active, Traf-6 become activated resulting in phosphorylation of I-κB which leads to NF-κB activation, and activation of JNK and p38 MAP kinase pathways. The NF-κB pathway is activated by all TLRs, with the exception of TLR3 [15].
The finding that Mal/TIRAP-deficient mice responded normally to the TLR5, TLR7 and TLR9 ligands, as well as to IL-1 and IL-18, but have defects in cytokine production and in activation of NF-κB and MAPKs in response to ligands for TLR2 and TLR4, confirmed that Mal is not necessary involved in the signaling pathways of all members of the TLR family, and therefore this may account for the specificity in the downstream signaling of individual TLRs [19]. Interestingly, the induction of IFN-β and activation of the transcription factor IRF3 (which regulates IFN-β production) in both MyD88 and Mal/TIRAP single and double knockout mice were normal, suggesting a pathway independent of MyD88. In addition, induction of IFN-β production due to activation of TLR3 was also independent of the MyD88 pathway [15, 20].
The biggest divergence in signaling among TLRs is therefore exemplified by TLR3 and 4 that activate IRF3 and induce IFN-β production, followed by a phase of IFN-dependent gene expression; and the other TLRs that do not activate IRF3 pathway [21]. In this case, Trif, which is recruited by both TLR4 and TLR3, is responsible for the activation of IRF3. Moreover, Tram, participates only in TLR4 signaling, interacts with Trif and appear to serve similar functions than Mal, acting as adapters to recruit Trif and MyD88, respectively, in TLR4 signaling [15].
A fifth TIR adaptor named sterile alpha- and armadillo-motif-containing protein (SARM) seems to function as an inhibitor of both TRIF- and MyD88-mediated AP-1 activation [22].
As mentioned before, the outcome of TLR signaling is determined, in part, by the cells in which they are expressed and by the selective use of signaling adaptors. In addition, both the ligand recognition by TLRs and the functional outcome of ligand binding may be governed by the compartmentalization (subcellular location) of the TLRs and their signaling adaptors [13, 23]. Briefly, the TLRs involved in the recognition of nucleic acids (TLR3, TLR7, TLR8 and TLR9) are localized within intracellular endolysosomal compartments, whereas TLR1, TLR2, TLR4, TLR5 and TLR6 that recognize PAMPs of extracellular microbes are expressed on the cell surface [13, 23]. Moreover, TLRs that are normally located on the cell surface can also be internalized to the endocytic pathway once they are activated. For instance, upon recognition of LPS on the cell surface, TLR4 first induces Mal/TIRAP-MyD88 signaling on the plasma membrane and is endocytosed and activates TRAM-TRIF in early endosomes leading to the induction of type-I interferons [24].
3. TLR expression in the CNS
Microglia and astrocytes are the main cells responsible for innate immunity in the CNS for the control of pathogen invasion and colonization. These cells also generate signals for recruitment and activation of cells that participate in adaptive immunity to finally eradicate the infection [25]. As revealed by in vivo studies in rodents, mRNA of TLRs 1–9 are expressed in the CNS [26]. The expression of TLRs in CNS cells can be up-regulated by infection, inflammation or TLR stimulation [27–29], therefore amplifying the innate immune response.
Different cell types express TLRs in the brain of both rodents and humans and different expression patterns have been described in these species [30]. For instance, astrocytes in the CNS of healthy humans barely express TLRs. But once inflammation develops, TLR expression emerges on the cell surface of astrocytes at low levels detectable by immunohistochemistry [31]. Real-time PCR for TLRs 1–10 in cultured human astrocytes revealed a basal TLR3 expression that could be rapidly enhanced by exposure to inflammatory cytokines IFN-γ, IL-1β, and IFN-β [32]. Likewise, Bowman et al., demonstrated low constitutive expression of the messenger RNA encoding TLR2, TLR4, TLR5, and TLR9 in resting cultured murine astrocytes, which was up-regulated following exposure to specific bacteria-derived ligands [33].
TLR3 in CNS is particularly interesting as it is highly expressed in both murine and human astrocytes and is also highly expressed in the resting CNS suggesting that it may have vital immune or homeostatic roles in the brain [30, 34]. For instance, TLR3-mediated activation of astrocytes leads to a marked induction of the enzyme indoleamine 2,3-dioxygenase, which acts as a local immune-suppressive factor [35].
It is important to note that when TLR expression is analyzed in primary cells of CNS, caution must be used concerning the purity of astroglial cultures and the exclusion of the presence of microglia [36–37], which may cause erroneous attribution of TLRs expression to astrocytes.
Microglia represent the first line of innate defense against viral and bacterial infection of the CNS. These cells respond to TLR stimulation by producing cytokines and other inflammatory mediators and by enhancing phagocytosis of microorganisms and aggregated extracellular proteins [29, 38–39]. Microglial cells express a wide array of TLRs (TLRs 1–9) [40]. Interestingly, microglial cells expressing TLRs are located in many areas of the brain but with some preference to regions close to the circulation such as meninges and circumventricular organs (CVOs) [41]. These findings highlight that the presence of TLRs in resident microglia of the mouse brain may be critical for shaping CNS response to circulating endotoxin and other TLR ligands.
The expression of TLRs in neuronal cells
The study of TLR expression and function in the CNS was mainly focused on glial cells. However, new evidence indicates that TLRs may also play a role in neuronal homeostasis. In fact, neurons express different TLRs, including TLRs 1–9 [42] and TLRs 11–13 [43]. Okun et al., have shown the expression of mRNAs for TLRs 1–9 as well as TLRs 2, 3 and 4 proteins in rat primary neuronal cultures [44]. Another report indicated that TLRs 1–8 mRNA was expressed in neurons in mice [45]. In addition, TLR2 and 6 proteins were shown to be expressed in murine neurons in vivo under physiological conditions. As described for other cell types, neuronal expression of TLRs is variable and can be induced and modulated by several factors [46–47]. TLR3 expression was detected in cultured human neurons following viral infection [48], and in neurons of patients with rabies or herpes simplex virus infection [49].
In a murine model of neurocysticercosis, Mishra et al., found that in the normal brain, mRNAs of TLRs 11–13 were constitutively expressed [43]. Parasite infection caused an increase of both mRNAs and proteins of TLRs 11–13 by several fold. All three TLR proteins were present in both CNS and immune cells. TLR13 expression level was the highest, followed by TLR11 and TLR12. In brain cells, TLR 11–13 proteins appeared to be the highest in neurons [43]. However, TLR13 was also present in ependymal cells, endothelial cells of pial blood vessels, and astrocytes [43].
New evidence indicates that neuronal TLR activation may play a role in the development [44]. TLR3 is expressed in the mouse central and peripheral nervous systems and is concentrated in the growth cones of the neurons [50]. As shown by Cameron et al., activation of TLR3 by its specific ligands, polyinosine:polycytidylic acid (poly I:C) or by mRNA, rapidly caused growth cone collapse and inhibited neurite extension independent of NF-κB [50]. Moreover, mice lacking functional TLR3 were resistant to the neurodegenerative effects of poly I:C. Neonatal mice injected with poly I:C were found to have fewer axon exiting dorsal root ganglia and displayed sensorimotor defects. Therefore, TLR3 functions autonomously in neurons to regulate axonal growth [50]. In the study by Lathia et al., TLR3 was shown as a negative regulator of embryonic neural progenitor cell (NPC) proliferation [51]. TLR3 protein was detected in brain cells in early embryonic stages of development, and in cultured NPC [51]. NPC from TLR3-deficient embryos formed greater numbers of neurospheres compared with neurospheres from wild-type embryos. The number of proliferating cells was also increased in the developing cortex of TLR3-deficient mice compared with wild-type mice [51]. In addition, treatment of cultured embryonic cortical neurospheres with poly I:C significantly reduced the number of proliferating cells and neurosphere formation in wild type but not TLR3 deficient NPCs [51].
4. TLRs in neurodegenerative diseases
Pro-inflammatory molecules, which are elevated in neurodegenerative diseases, can increase the expression of TLRs in CNS cells [29, 40, 52]. Activation of these TLRs in turn may also increase the local release of pro-inflammatory cytokines [40, 53]. Therefore, the role for TLRs in several aspects of neurodegenerative diseases has been proposed, for instance, in Alzheimer’s disease (AD) where persistent glial activation without acute inflammation is observed and in MS where an adaptive immune response is elicited subsequent to glial activation and inflammation [5].
TLRs in Alzheimer’s disease(AD)
AD is the most common cause of dementia in human characterized by progressive neurodegeneration [54]. Pathological features in the brain parenchyma of AD patients include focal deposits of fibrillar amyloid β (Aβ) peptides in blood vessel wall and in neuritic plaques, accumulation of abnormal tau filaments as neurofibrillary tangles, reactive gliosis and inflammation [55–56]. Neuritic plaques consisting of Aβ deposits, in particular those formed by the 42 amino acid peptide (Aβ42) [57], are considered the foci of regional inflammatory responses in AD brain, as evidenced by increased levels of acute phase proteins, pro-inflammatory cytokines, complement components and proteases [9, 58–59]. Of note is the association of activated microglia with neuritic plaques containing high levels of Aβ42 [59–60], which have been shown to be directly toxic to neurons and also act as a pro-inflammatory stimulant of microglial cells. Aβ42 has been reported to interact with microglial cells through several reported cell surface receptors [9]. Among those, the G protein coupled formyl peptide receptor like 1 (FPRL1, now termed FPR2) and its mouse homolog mFPR2, have been shown to mediate the chemotactic activity of Aβ42 for microglia suggesting its involvement in the recruitment of microglial cells in AD brain [61]. FPR2 also acts as a port for the up-take of Aβ42 by microglia cells [29, 52, 62–63]. Microglial cells coordinate inflammatory responses in the CNS and express a wide array of TLRs (TLRs 1–9) at varying levels in humans [31] and mice [40, 64]. TLRs may participate in AD pathology through at least three mechanisms. First, TLRs have been reported to increase the recognition of fibrillar Aβ by microglial cells through the interaction with other cell surface receptors, including CD36, α6β1 integrin, CD47 and scavenger receptor A that bind fibrillar Aβ to initiate the activation of intracellular signaling pathways [65–66]. Second, TLRs could be activated in the Aβ receptor complex to elicit the production of pro-inflammatory cytokines and reactive oxygen and nitrogen radicals, which may contribute to the inflammatory response and neuronal toxicity [66]. Third, TLRs and CD14 have been implicated in fibrillar Aβ internalization by microglia [67], which required CD14 for the uptake of fibrillar Aβ and both TLR2 and 4 are necessary for fibrillar Aβ-stimulated phagocytosis (Fig. 1) [66].
Figure 1.
The role of TLRs in fibrillar Aβ recognition and activation of microglial cells. TLR2 and TLR4 may interact with other cell surface receptors such as CD36, α6β1 integrin, CD47 and scavenger receptor A (SR-A), to recognize fibrillar Aβ on the cell surface. This recognition might lead to the activation of microglial cells, which may lead to enhanced production of pro-inflammatory molecules and increased endocytosis. ROS: reactive oxygen species; NO: nitric oxide.
Recent in vivo studies performed in a double transgenic (APPswe/PSEN1dE9) mouse model of AD demonstrated that the lack of TLR4 in those mice produced increased cortical and hippocampal Aβ load, suggesting the participation of TLR4 in the Aβ clearance by microglial cells [68]. Another study using APPswe/PSEN1dE9 double transgenic mice and deficient in TLR2 has shown delayed Aβ deposition compared to control mice. TLR2 deficiency accelerated spatial and contextual memory impairment, which correlated with increased levels of Aβ42 and transforming growth factor beta1 (TGFβ1) in the brain [69]. Michaud et al., reported that reduction of MyD88 expression by more than 50% in a mouse model of AD accelerated spatial learning and memory deficits. Brains of APPswe/PS1-MyD88+/− mice showed delay in the accumulation of Aβ plaques but increased soluble levels of Aβ oligomers [70]. Furthermore, the number of inflammatory monocytes and the level of IL-1β gene expression were significantly reduced in the brain of IAPPswe/PS1 tg mice with impaired MyD88 signaling [70]. These data suggest that activation of MyD88 signaling pathway linked to TLRs may restrict disease progression in APPswe/PS1 transgenic mice.
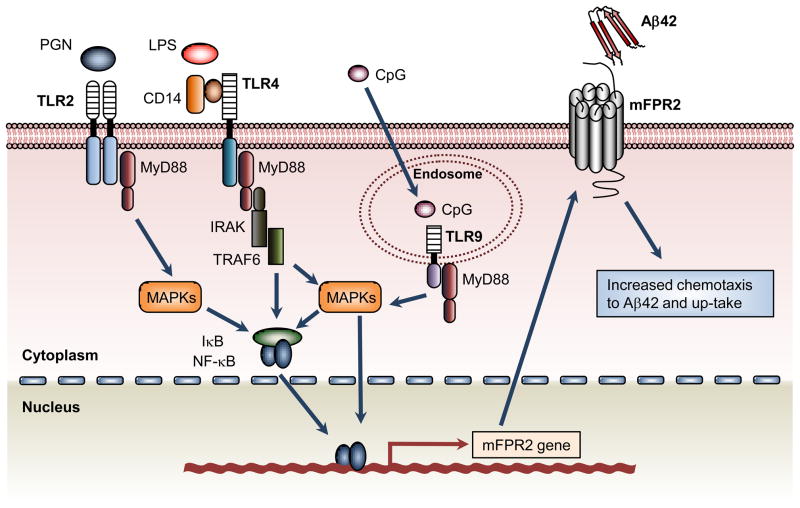
Activation of several TLRs in mouse microglia increases the expression of the G-protein coupled formyl peptide receptor mFPR2, which recognizes bacterial and host-derived chemotactic agonist peptides and Aβ42 [71]. Mouse microglia stimulated with bacterial LPS (a ligand for TLR4) [63], PGN and PamCAG (ligands for TLR2) [52] or CpG (a ligand for TLR9) [29], not only exhibited increased chemotactic responses to Aβ42, but also a markedly enhanced mFPR2-mediated uptake of Aβ42 (Fig. 2). Thus, TLR-activated microglia may participate in the pro-inflammatory responses seen in the AD brain and in the uptake and processing of Aβ42. Further studies have shown that TLRs are essential for microglia up-take and processing of Aβ42, and the up-take of Aβ42 was mainly mediated by a GPCR, presumably mFPR2 [68]. Recently, Scholtzova et al. demonstrated that activation of TLR9 by methyl CpG leads to a 66% and 80% reduction in the cortical and vascular amyloid burden, respectively, in AD mice [72], in association with significant reduction in Aβ42, Aβ40, and Aβ oligomer levels.
Figure 2.
The role of TLRs in the up-regulation of the G-protein coupled receptor mFPR2. TLR2, TLR4 and TLR9 in microglia are activated by LPS, PGN and CpG ODN, respectively. This leads to activation of signaling cascades involving MAPK and NF-κB, which promote the expression of functional mFPR2. This G protein-coupled receptor mediates microglial chemotaxis in response to Aβ42 and also the up-take of Aβ42 by these cells.
It is important to note that activation of TLRs at different stages of the disease may result in different outcomes. The activation of these receptors in early stages of the disease may reduce Aβ burden. However, once the disease progresses to later stages, TLR activation is more likely to contribute to neuroinflammation and neurotoxicity. Therefore, studies on the precise role of TLRs in human AD may yield potential molecular target for controlling the course of the disease.
TLRs in multiple sclerosis (MS)
MS is a chronic, demyelinating disease affecting the white matter of CNS. The etiology of MS is unknown, but several lines of evidence support the hypothesis that the pathogenesis is mediated by autoreactive T lymphocytes [73]. Regardless of its cause, it is clear that the progression of MS is associated with an inflammatory reaction that involves activated lymphocytes, macrophages and resident glial cells (astrocytes and microglia) in the brain [74]. Human MS is similar to murine experimental autoimmune encephalitis (EAE) in terms of the pattern of development and progression as well as the immune components involved.
In EAE and MS, there are two main phases of immunopathological events: an initial priming/activation phase in which self reactive aggressive lymphocytes are activated and a subsequent effector phase in which these cells invade the CNS and cause tissue destruction. Persistent activation of microglial cells has also been observed in the chronic phase of relapsing-remitting EAE and a relationship exists between activated microglial cells and the loss of neuronal synapses [74–76]. Similarly, profound activation of microglial cells was seen in progressive MS patients with changes in the normal-appeareance of white matter in a global scale [76].
A number of observations suggest a role for TLR-activation in EAE and MS (Table 1). In active MS, in perivascular areas and in the center of lesions, the expression of TLRs is increased and is co-localized with microglia and astrocytes [21]. MyD88−/− mice are completely resistant to the development of EAE [77]. Wild type (WT) and TLR2−/− mice develop EAE with a similar time course of onset and severity after immunization with myelin oligodendrocyte glycoprotein (MOG) peptide emulsified in CFA (Complete Freund’s Adjuvant) [77]. However, another study suggests that TLR2 to promote CNS neuroinflammation in progressive EAE [78]. In addition, the exacerbation of EAE observed in mice treated with phosphorylated dihydroceramides from Porphyromonas gingivalis or infected with Streptococcus pneumoniae was dependent of TLR2 expression [79–80]. TLR4 deficient mice develop EAE with greater severity which is associated with increased Th17 function [81]. TLR9−/− mice develop the disease with significant delay in onset and reduced severity. Therefore, both TLR9 and MyD88 are required for EAE induction and progression [77]. However, Marta et al. showed that TLR9−/− mice surprisingly exhibited more severe EAE symptoms and higher IL-6 levels in spleen cells than WT mice [81]. Further work is required in order to unravel the controversial participation of TLR9 in EAE.
Table 1.
The role of TLRs in the development of EAE/MS
| TLRs | Animal models | Function in EAE | References |
|---|---|---|---|
| TLR2 | EAE in TLR2−/− mice | Controversial: | |
| No changes in EAE | [77] | ||
| Lower severity of EAE in TLR2−/− mice | [78] | ||
| TLR2 ligands enhance EAE in wild type mice | [79–80] | ||
| TLR3 | EAE in wild type mice | TLR3 stimulation protects from EAE | [83–84] |
| TLR4 | EAE in TLR4−/− mice | Higher severity of EAE in TLR4−/− mice | [81] |
| TLR9 | EAE in TLR9−/− mice | Controversial: | |
| Delayed onset and severity of EAE in TLR9−/− mice | [77] | ||
| Higher severity of EAE in TLR9−/− mice | [81] |
Several studies suggest that the clinical course of EAE varies depending on the TLR signaling cascade and the transcription factors activated. Although earlier evidence suggested that ligation of TLR3 induced the secretion of the chemokine CXCL10 (IP10) that promoted T cell infiltration in EAE [21, 82], TLR3 was also observed to be protective in EAE as a consequence of increased expression of IFN-β [83]. These results suggest that in EAE, signaling through a Myd88-independent pathway, which promotes the expression of type I IFNs, may have a protective role, whereas signaling through the Myd88-dependent pathway, which activates NF-κB and leads to the secretion of pro-inflammatory cytokines, may exacerbate the disease [84]. Thus, TLRs are involved in different aspects of MS (Table 1) and additional studies are required to further determine the precise role of each TLR family member in the disease in order to exploit their beneficial effects while avoid the detrimental consequences caused by TLR activation.
5. Conclusions
TLRs are key molecules recognizing foreign and endogenous danger signals, activating and regulating innate immunity and inflammation, and finally inducing adaptive immunity. TLRs have been shown to play essential roles in infections, inflammatory diseases and cancer. Abundant evidence also suggests that TLRs are important players in neurodegenerative diseases, which involve many inflammatory components. In AD, it is particularly important to determine the precise role of distinct TLRs in Aβ recognition and clearance, and the activation of glial cells. This may provide important clues about the mechanisms responsible for AD-associated neurodegeneration and open new avenues in the search of novel molecular targets for AD vaccination and therapy. New findings relating TLRs to MS are growing and interestingly, some divergence for the roles of different TLRs members are suggested. Thus, TLRs that promote disease progression should be the targets for silencing, whereas TLRs that are able to induce regulatory circuits and inhibit the disease should be exploited.
Acknowledgments
This work was supported by Grant Number 1R01TW007621-01A2 from Fogarty International Center, NIH, USA, CONICET and SECyT-UNC, Argentina. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Fogarty International Center (FIC), NIH, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 (Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–8. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–39. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 5.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–93. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 6.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 7.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 9.Iribarren P, Zhou Y, Hu J, Le Y, Wang JM. Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyte responses in Alzheimer disease. Immunol Res. 2005;31:165–76. doi: 10.1385/IR:31:3:165. [DOI] [PubMed] [Google Scholar]
- 10.Iribarren P, Chen K, Hu J, Zhang X, Gong W, Wang JM. IL-4 inhibits the expression of mouse formyl peptide receptor 2, a receptor for amyloid beta1-42, in TNF-alpha-activated microglia. J Immunol. 2005;175:6100–6. doi: 10.4049/jimmunol.175.9.6100. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 13.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–42. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–90. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 17.Gay NJ, Gangloff M, O’Neill LA. What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol. 2011 doi: 10.1016/j.it.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, et al. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284:25404–11. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 21.Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7:1271–85. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Peng J, Yuan Q, Lin B, Panneerselvam P, Wang X, Luan XL, et al. SARM inhibits both TRIF- and MyD88-mediated AP-1 activation. Eur J Immunol. 2010;40:1738–47. doi: 10.1002/eji.200940034. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi A, Pierce SK. How location governs toll-like receptor signaling. Traffic. 2009;10:621–8. doi: 10.1111/j.1600-0854.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iribarren P, Cui YH, Le Y, Wang JM. The role of dendritic cells in neurodegenerative diseases. Arch Immunol Ther Exp (Warsz) 2002;50:187–96. [PubMed] [Google Scholar]
- 26.Trudler D, Farfara D, Frenkel D. Toll-like receptors expression and signaling in glia cells in neuro-amyloidogenic diseases: towards future therapeutic application. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/497987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKimmie CS, Johnson N, Fooks AR, Fazakerley JK. Viruses selectively upregulate Toll-like receptors in the central nervous system. Biochem Biophys Res Commun. 2005;336:925–33. doi: 10.1016/j.bbrc.2005.08.209. [DOI] [PubMed] [Google Scholar]
- 28.Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–19. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iribarren P, Chen K, Hu J, Gong W, Cho EH, Lockett S, et al. CpG-containing oligodeoxynucleotide promotes microglial cell uptake of amyloid beta 1-42 peptide by up-regulating the expression of the G-protein- coupled receptor mFPR2. FASEB J. 2005;19:2032–4. doi: 10.1096/fj.05-4578fje. [DOI] [PubMed] [Google Scholar]
- 30.Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–21. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 32.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–9. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–91. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 34.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–7. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh HS, Zhao ML, Rivieccio M, Choi S, Connolly E, Zhao Y, et al. Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response. J Virol. 2007;81:9838–50. doi: 10.1128/JVI.00792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–37. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 39.Konat GW, Kielian T, Marriott I. The role of Toll-like receptors in CNS response to microbial challenge. J Neurochem. 2006;99:1–12. doi: 10.1111/j.1471-4159.2006.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–96. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra BB, Gundra UM, Teale JM. Expression and distribution of Toll-like receptors 11–13 in the brain during murine neurocysticercosis. J Neuroinflammation. 2008;5:53. doi: 10.1186/1742-2094-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–83. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6:2859–68. doi: 10.4161/cc.6.23.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson AC, Rossiter JP, Lafon M. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol. 2006;12:229–34. doi: 10.1080/13550280600848399. [DOI] [PubMed] [Google Scholar]
- 50.Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–41. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lathia JD, Okun E, Tang SC, Griffioen K, Cheng A, Mughal MR, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci. 2008;28:13978–84. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, et al. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–9. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- 53.Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, et al. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation. 2010;7:46. doi: 10.1186/1742-2094-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadik K, Wilcock G. The increasing burden of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17 (Suppl 3):S75–9. doi: 10.1097/00002093-200307003-00003. [DOI] [PubMed] [Google Scholar]
- 55.Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–39. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 57.Citron M, Diehl TS, Gordon G, Biere AL, Seubert P, Selkoe DJ. Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities. Proc Natl Acad Sci U S A. 1996;93:13170–5. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cotter RL, Burke WJ, Thomas VS, Potter JF, Zheng J, Gendelman HE. Insights into the neurodegenerative process of Alzheimer’s disease: a role for mononuclear phagocyte-associated inflammation and neurotoxicity. J Leukoc Biol. 1999;65:416–27. doi: 10.1002/jlb.65.4.416. [DOI] [PubMed] [Google Scholar]
- 59.Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis. 2010;37:503–9. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Combs CK. Inflammation and microglia actions in Alzheimer’s disease. J Neuroimmune Pharmacol. 2009;4:380–8. doi: 10.1007/s11481-009-9165-3. [DOI] [PubMed] [Google Scholar]
- 61.Tiffany HL, Lavigne MC, Cui YH, Wang JM, Leto TL, Gao JL, et al. Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J Biol Chem. 2001;276:23645–52. doi: 10.1074/jbc.M101031200. [DOI] [PubMed] [Google Scholar]
- 62.Iribarren P, Chen K, Gong W, Cho EH, Lockett S, Uranchimeg B, et al. Interleukin 10 and TNFalpha synergistically enhance the expression of the G protein-coupled formylpeptide receptor 2 in microglia. Neurobiol Dis. 2007;27:90–8. doi: 10.1016/j.nbd.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui YH, Le Y, Gong W, Proost P, Van Damme J, Murphy WJ, et al. Bacterial lipopolysaccharide selectively up-regulates the function of the chemotactic peptide receptor formyl peptide receptor 2 in murine microglial cells. J Immunol. 2002;168:434–42. doi: 10.4049/jimmunol.168.1.434. [DOI] [PubMed] [Google Scholar]
- 64.McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–25. doi: 10.1016/j.jneuroim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–74. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–92. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, et al. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain. 2005;128:1778–89. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- 68.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–19. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5784–93. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaud JP, Richard KL, Rivest S. MyD88-adaptor protein acts as a preventive mechanism for memory deficits in a mouse model of Alzheimer’s disease. Mol Neurodegener. 2011;6:5. doi: 10.1186/1750-1326-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, et al. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001;21:RC123. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, et al. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer’s disease-related pathology. J Neurosci. 2009;29:1846–54. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 2004;61:1613–5. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 74.Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221:7–14. doi: 10.1016/j.jneuroim.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rasmussen S, Wang Y, Kivisakk P, Bronson RT, Meyer M, Imitola J, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing--remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–29. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 76.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 77.Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–64. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10:958–64. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nichols FC, Housley WJ, O’Conor CA, Manning T, Wu S, Clark RB. Unique lipids from a common human bacterium represent a new class of Toll-like receptor 2 ligands capable of enhancing autoimmunity. Am J Pathol. 2009;175:2430–8. doi: 10.2353/ajpath.2009.090544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herrmann I, Kellert M, Schmidt H, Mildner A, Hanisch UK, Bruck W, et al. Streptococcus pneumoniae Infection aggravates experimental autoimmune encephalomyelitis via Toll-like receptor 2. Infect Immun. 2006;74:4841–8. doi: 10.1128/IAI.00026-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marta M, Andersson A, Isaksson M, Kampe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–75. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 82.Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 83.Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol. 2006;177:7505–9. doi: 10.4049/jimmunol.177.11.7505. [DOI] [PubMed] [Google Scholar]
- 84.Fernandez M, Montalban X, Comabella M. Orchestrating innate immune responses in multiple sclerosis: molecular players. J Neuroimmunol. 2010;225:5–12. doi: 10.1016/j.jneuroim.2010.05.014. [DOI] [PubMed] [Google Scholar]