Abstract
Cell-to-cell signal transduction is vital for orchestrating the whole-body physiology of multi-cellular organisms, and many endogenous macromolecules, proteins, and nucleic acids function as such transported signals. In plants, many of these molecules are transported through plasmodesmata (Pd), the cell wall-spanning channel structures that interconnect plant cells. Furthermore, Pd also act as conduits for cell-to-cell movement of most plant viruses that have evolved to pirate these channels to spread the infection. Pd transport is presumed to be highly selective, and only a limited repertoire of molecules is transported through these channels. Recent studies have begun to unravel mechanisms that actively regulate the opening of the Pd channel to allow traffic. This macromolecular transport between cells comprises two consecutive steps: intracellular targeting to Pd and translocation through the channel to the adjacent cell. Here, we review the current knowledge of molecular species that are transported though Pd and the mechanisms that control this traffic. Generally, Pd traffic can occur by passive diffusion through the trans-Pd cytoplasm or through the membrane/lumen of the trans-Pd ER, or by active transport that includes protein–protein interactions. It is this latter mode of Pd transport that is involved in intercellular traffic of most signal molecules and is regulated by distinct and sometimes interdependent mechanisms, which represent the focus of this article.
Keywords: Plasmodesmata, cell-to-cell transport, plant viruses, transcription factors
INTRODUCTION
Plasmodesmata (Pd) are unique channel structures plants have evolved to allow exchange of signals and other macromolecules between cells otherwise separated by the rigid cell wall. The ultrastructure of Pd is relatively well characterized at the electron microscopic level; they represent membrane-lined cytoplasmic bridges, the central region of which is occupied by the trans-Pd ER, termed desmotubule, that forms a continuum between the adjacent cells. The space between the ER and the plasma membrane contains cytoplasmic channels, through which soluble molecules move from cell to cell (for review, see Benitez-Alfonso et al., 2006; Bell and Oparka, 2011). Non-vascular cells in a plant are connected by Pd to each other and to the plant vascular system, the phloem, which acts as a long-distance molecular conduit. Thus, Pd provide a whole-body macromolecular transport network, which is collectively termed the symplastic transport pathway.
In addition to the endogenous macromolecules, Pd serve as gateways for most plant viruses, which pirate this route using their movement proteins (MP) to modify the Pd channel and spread from cell to cell (Figure 1) (Lazarowitz and Beachy, 1999; Waigmann et al., 2004; Boevink and Oparka, 2005; Lucas, 2006; Epel, 2009; Niehl and Heinlein, 2011). Linking their structure to function, Pd have been demonstrated to transport soluble proteins through their cytoplasmic sleeves and membrane proteins through the trans-Pd ER and plasma membrane (Lazarowitz and Beachy, 1999; Waigmann et al., 2004; Boevink and Oparka, 2005; Lucas, 2006; Epel, 2009; Niehl and Heinlein, 2011). The ER-associated Pd transport mode is especially relevant for cell-to-cell transport of plant viruses, many of which encode MPs that associate with the ER (Lazarowitz and Beachy, 1999; Waigmann et al., 2004; Boevink and Oparka, 2005; Lucas, 2006; Epel, 2009; Niehl and Heinlein, 2011).
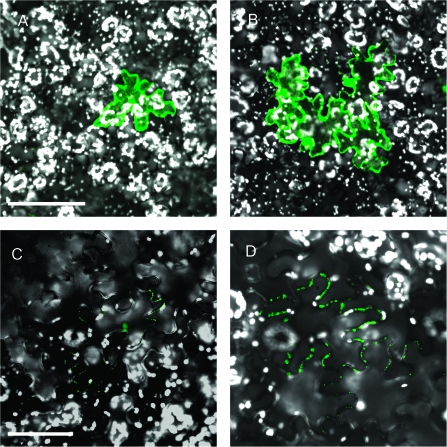
Figure 1.
Cell-to-Cell Transport of Viral MPs.
Constructs encoding CaLCuV MP-GFP (A, B) or TMV MP–YFP (C, D) were transiently expressed in N. benthamiana epidermal cells by biolistic bombardment (Ueki et al., 2009, 2010a). In some cases, the expressed proteins remained confined in single cells (A, C), indicating the lack of movement. In other cases, the proteins moved from cell to cell, resulting in signal clusters composed of several cells (B, D). These differences in the movement capacity most likely reflect the host mechanisms that restrict viral movement as described in the text. Bars are 100 and 50μm in panels (A) and (C), respectively. All images represent single confocal sections.
Trafficking through Pd can be classified into two distinctive types: passive transport by diffusion and active transport by dilating the channel. Probably the most important determinant for the passive molecular transport is the size exclusion limit (SEL) of Pd, which defines the largest size of the molecule that can fit through the undilated channel. In many cases, the SEL is described in terms of the molecular mass of a transported molecule, but the shape of the molecule, namely its hydrodynamic or Stokes radius, is more important as the transport determinant. Passive Pd transport is exemplified by translocation of such marker molecules as dextrans and green fluorescence protein (GFP), often used to monitor Pd permeability (Kim et al., 2005a, 2005b). This type of transport functions for cargoes that are below the SEL, and it most likely occurs directly, without involvement of other cellular components. In contrast, active Pd transport target molecules that are larger than SEL, and it presumably involves specific interactions between the cargo and the Pd transport machinery, which lead to the dilation of Pd. The example of such active Pd traffic is plant viral movement. Plant viruses are equipped with the movement factors, namely MPs that are capable of modifying Pd at the molecular level to aid traffic of viral genomes. Most viral MPs dilate Pd without causing major morphological changes in these channels, presumably by activating the cellular pathway for the active Pd transport. Some MPs, however, permanently modify Pd, forming trans-Pd tubules and eliminating the innate Pd structure. For detailed information about MPs from a wide spectrum of plant virus species, the reader is referred to many excellent and comprehensive reviews (Lazarowitz and Beachy, 1999; Waigmann et al., 2004; Boevink and Oparka, 2005; Scholthof, 2005; Lucas, 2006; Epel, 2009; Niehl and Heinlein, 2011).
One critical aspect of the Pd transport, the understanding of which has advanced dramatically in recent years, is its regulation. Thus, we focus here on the regulatory mechanisms of cell-to-cell transport of non-cell-autonomous cellular proteins and plant viruses (Figure 2). For intercellular traffic of RNA molecules, including mRNA, siRNA, and miRNA, the reader is directed to a recent review (Hyun et al., 2011).
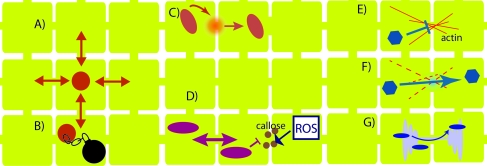
Figure 2.
Summary of Major Regulatory Mechanisms of Pd Transport of Non-Cell-Autonomous Cellular Proteins and Plant Viruses.
(A) Pd transport of some of the MADS domain transcription factors, such as AP3 and PI, occurs by diffusion.
(B) Having been transported through Pd, SHR can be anchored in a cell by binding to SCR.
(C) Numerous proteins that actively move through Pd, such as KN1 and its homologs, phloem non-cell-autonomous proteins, and viral MPs, have the capacity to increase Pd permeability.
(D) Pd transport of proteins is restricted by callose sphincters, which can be regulated by ROS. ROS can also up-regulate Pd permeability by an as-yet unknown pathway.
(E) Actin is found in and around Pd.
(F) Depolymerization of actin leads to elevated Pd permeability.
(G) The traffic of some viral proteins through Pd is mediated by the ER and secretion pathways.
CALLOSE AS A UNIVERSAL Pd REGULATOR
Callose, a beta-1,3-glucan, is the best-studied regulatory component of Pd. When callose accumulates at high levels at the neck region of Pd, it acts as a molecular sphincter that constricts the channel and limits macromolecular traffic (Zavaliev et al., 2011). Callose levels are determined by the balance between its biosynthesis by callose synthases, degradation by beta-1,3-glucanases, and possible retention by other factors (Sagi et al., 2005; Simpson et al., 2009; Zavaliev et al., 2010). Also, elevated levels of reactive oxygen species (ROS) are known to increase callose deposits and, in turn, restrict cell-to-cell transport in the root (Benitez-Alfonso et al., 2009).
Perhaps the most extensively characterized example of cell-to-cell transport of macromolecules regulated by callose is the movement of Tobacco mosaic virus (TMV). During this process, TMV MP presumably associates with the viral genomic RNA to form a movement ribonucleocomplex (Citovsky et al., 1990), targets this complex to Pd (Meshi et al., 1987; Tomenius et al., 1987; Ding et al., 1992; Heinlein et al., 1995), and increases the Pd size exclusion limit to translocate the movement complex through the Pd channel (Wolf et al., 1989; Waigmann et al., 1994). TMV MP has been shown to interact with several very diverse host factors, including cytoskeletal elements, calreticulin, pectin methylesterases, and DnaJ chaperones; these interactions have been suggested to participate in MP targeting to Pd (Heinlein et al., 1995; McLean et al., 1995; Dorokhov et al., 1999; Chen et al., 2000; von Bargen et al., 2001; Chen et al., 2005; Shimizu et al., 2009). For Pd gating, TMV MP likely interacts with a plant ankyrin repeat-containing protein ANK; although ANK itself is a cytoplasmic protein, its complexes with TMV MP localize at Pd (Ueki et al., 2010b). Importantly, the TMV MP–ANK interaction leads to a decrease in Pd callose levels and enhances cell-to-cell movement of TMV MP (Ueki et al., 2010b), potentially via recruitment and/or activation of cellular beta-1,3-glucanases. Consistent with this idea, TMV MP itself has been suggested to recruit beta-1,3-glucanases for Pd movement (Epel, 2009).
Besides viral transport, callose Pd sphincters may be essential to confine SPEACHLESS (SPCH), a bHLH protein that specifies the initiation of stomatal lineage, to the expressing cell for regulation of stomatal patterning (Guseman et al., 2010). An Arabidopsis double mutant in the ERECTA (ER) and ERECTA-LIKE1 (ERL1) receptor-like kinases, er erl1, with a weak phenotype of elevated stomatal density was used in a sensitized genetic screen for more severe stomatal patterning. This approach isolated a mutant, designated chorus (chor), with clustered stomata and incomplete development of precursor cells (Guseman et al., 2010). CHOR was identified as a callose synthase, GSL8, and the chor mutant showed incomplete cytokinesis and was defective in callose formation at the cell plate and Pd in leaf epidermal cells (Guseman et al., 2010). This lack of callose was accompanied by increased Pd transport of a trimetric GFP marker; more importantly, the normally cell-autonomous SPCH moved from cell to cell in the chor plants (Guseman et al., 2010), demonstrating the role of callose and callose-synthesizing enzymes in controlling Pd transport during stomatal development.
REGULATION OF Pd BY ROS
Increasing evidence points to an important role of the intracellular levels of reactive oxygen species (ROS), which represent a by-product of photosynthesis and photorespiration, in Pd regulation (Kim et al., 2002a; Benitez-Alfonso and Jackson, 2009; Benitez-Alfonso et al., 2009; Stonebloom et al., 2009). In the gfp arrested trafficking 1 (gat1) mutant in the gene coding for thioredoxin-m3 (TRX-m3), the diffusion of GFP out of the companion cells in the root phloem was blocked (Benitez-Alfonso and Jackson, 2009; Benitez-Alfonso et al., 2009). GAT1/TRX-m3 is likely involved in the regulation of redox homeostasis because gat1 mutants accumulated high levels of ROS; importantly, these mutant plants also exhibited higher levels of callose in their root tips (Benitez-Alfonso and Jackson, 2009; Benitez-Alfonso et al., 2009), functionally linking ROS to callose formation. Conversely, overexpression of GAT1/TRX-m3 enhanced Pd transport in mature epidermal cells (Benitez-Alfonso and Jackson, 2009; Benitez-Alfonso et al., 2009). Thus, GAT1/TRX-m3 and, by implication, ROS appears to have both positive and negative effects on Pd transport, most likely depending on the type of plant tissue that expresses this protein.
Additional support for the role of ROS in regulation of Pd transport is lent by the studies of an Arabidopsis DEAD-box RNA helicase INCREASED SIZE EXCLUSION1 (ISE1). Mutations in the ISE1 gene produced higher ROS levels and caused increased Pd traffic of 10-kDa fluorescent dextrans in embryos at the mid-torpedo stage (Kim et al., 2002a; Stonebloom et al., 2009). The homozygous ise1 mutant was embryo-lethal, suggesting an essential function for this gene. ISE1 contains an N-terminal mitochondrial targeting signal and the fluorescently tagged protein exhibited mitochondrial localization. Consistently with its expected defective mitochondrial function, the ise1 mutant accumulated elevated levels of ROS (Stonebloom et al., 2009). Interestingly, the homozygous ise1 mid-torpedo embryos showed high content of morphologically altered Pd, suggesting that these ultrastructural alterations of the channel changed its conductivity (Stonebloom et al., 2009). Silencing of the ISE1 gene in mature leaves reproduced the increased macromolecular trafficking, confirming the involvement of ISE1 and, by implication, ROS in Pd regulation.
How can ROS affect Pd transport both positively and negatively? One possibility is that different degrees of increase in ROS content may exert opposing effects on the regulatory machinery of Pd; this is by analogy to the inverted ‘bell-shaped’ dose response of tobamoviral systemic movement to the heavy metal cadmium (Ghoshroy et al., 1998).
ACTIN MICROFILAMENTS AND MYOSIN MOTORS AS Pd REGULATORS
Several immunolocalization studies suggested that actin and myosin localize at Pd (White et al., 1994; Blackman and Overall, 1998; Radford and White, 1998; Overall et al., 2000; Volkmann et al., 2003). Furthermore, actin microfilaments, alone or together with myosin and/or ER elements, are likely involved in Pd targeting of viral proteins (Kawakami et al., 2004; Sheahan et al., 2004; Liu et al., 2005; Prokhnevsky et al., 2005; Wright et al., 2007; Avisar et al., 2008; Hofmann et al., 2009). Consistent with these observations, actin is emerging as a central player in regulation of Pd trafficking. For example, microinjection of actin-depolymerazing agents into plant cells increases Pd permeability (Ding and Kwon, 1996). But perhaps the most compelling evidence for the role of actin in Pd transport was provided by the recent study showing that Cucumber mosaic virus (CMV) MP and TMV MP, which are known to gate Pd, can sever actin microfilaments in vitro (Su et al., 2010). When the cells were treated with phalloidin, which stabilizes actin filaments and inhibits depolymerization of the cytoskeleton, these two viral MPs lost their effect on Pd permeability, linking between Pd gating and actin depolymerization (Su et al., 2010). Both TMV MP and CMV MP bind actin in vitro (McLean et al., 1995; Su et al., 2010), and CMV MP may cap the plus end of the actin filament (Su et al., 2010). It is tempting to speculate that the cellular actin-binding proteins capable of actin filament depolymerization and/or capping (Higaki et al., 2007; Yoneda et al., 2007) may act as endogenous regulators of Pd transport.
REGULATION OF Pd TRANSPORT AND SECRETION
Possible involvement of the secretion pathways in Pd transport is indicated by the presence of desmotubule, a trans-Pd strand of the ER that connects the endomembrane systems of the adjacent cells. Indeed, the ER/Golgi secretion pathway has been implicated in Pd targeting of viral proteins (Lazarowitz and Beachy, 1999; Oparka, 2004; Waigmann et al., 2004; Verchot-Lubicz, 2005; Epel, 2009; Niehl and Heinlein, 2011) as well as endogenous proteins (Lee et al., 2003; Sagi et al., 2005; Levy et al., 2007; Wright et al., 2007; Thomas et al., 2008; Simpson et al., 2009). Notably, TMV MP associates with the ER (Brill et al., 2000; Fujiki et al., 2006), and its cell-to-cell transport may involve diffusion along the trans-Pd ER membrane down the MP concentration gradient (Guenoune-Gelbart et al., 2008; Barton et al., 2011). In contrast, cell-to-cell movement of Tomato mosaic virus (ToMV) MP was not affected by treatment of plant tissues with the ER/Golgi secretion pathway inhibitor, brefeldin A (Tagami and Watanabe, 2007); the reasons for this difference in Pd transport of these two closely related MPs remain unclear.
A more intricate link between the host secretory system and Pd transport of viral MPs was uncovered in a recent study that reported specific interaction between synaptotagmins and MPs of several plant viruses (Lewis and Lazarowitz, 2010). Synaptotagmins, a family of proteins predominantly found in animal cells, function as calcium sensors that regulate exo- and endocytosis of synaptic vesicles. The function of plant synaptotagmins is just beginning to emerge. Arabidopsis synaptotagmin A (SYTA) was reported to interact with MPs of three plant viruses, two begomoviruses Cabbage leaf curl begomovirus (CaLCuV) and Squash leaf curl virus (SqLCV), and an unrelated tobamovirus, TMV (Lewis and Lazarowitz, 2010). Importantly, cell-to-cell movement of TMV MP and CaLCuV MP was significantly suppressed in the syta-1 line background compared to wild-type (WT), demonstrating that SYTA is essential for the Pd transport of these viral MPs. Interestingly, a SYTA deletion mutant that lacks the C2B domain interfered with the MP movement and inhibited the formation of plasma membrane-derived endosomes. That this SYTA mutant did not affect the Pd localization of the viral MPs suggests that SYTA potentiates Pd transport of MPs via an endocytic recycling pathway (Lewis and Lazarowitz, 2010), possibly by a mechanism similar to budding of retroviruses (Morita and Sundquist, 2004). Thus, vesicular endocytosis and Pd transport may share at least some regulatory mechanisms.
REGULATION OF Pd BETWEEN COMPANION CELLS AND SIEVE ELEMENTS IN THE PHLOEM
The plant phloem consists of companion cells (CC) and sieve elements (SE); because SE is enucleated, its proteins are encoded by the CC genome and transported from CC to SE through Pd connecting these two cell types. Furthermore, some proteins and RNA molecules that arrive to SE from CC continue their travel through the vascular system, spreading throughout the plant systemically and functioning in a non-cell-autonomous fashion (Ishiwatari et al., 1998; Ruiz-Medrano et al., 1999). Thus, the CC/SE system represents a unique tool to study both cell-to-cell and systemic transport of macromolecules. For example, the leaf sucrose transporter SUT1 is essential for phloem loading and long-distance transport of photoassimilates. Both SUT1 and its mRNA are found in SE, and the transcript preferentially associates with Pd between SE and CC, suggesting that the SUT1 mRNA as well as the SUT1 protein are transported into SE through Pd (Kühn et al., 1997).
Protein transport from CC to SE most likely involves up-regulation of Pd permeability because several proteins in the phloem sap of pumpkin (Cucurbita maxima) and castorbean (Ricinus communis), which are presumed to have moved there from CC, increased Pd SEL when microinjected to mesophyll cells (Balachandran et al., 1997). For example, CmPP16 from pumpkin phloem exudate, which is functionally similar to MP of Red clover necrotic mosaic virus (RCNMV), gates Pd, binds its mRNA and the genomic RNA of RCNMV, possibly transporting these molecules to distant tissues (Xoconostle-Cázares et al., 1999).
An glimpse into the regulatory mechanisms that govern Pd transport within the CC/EE system was afforded by the study of non-cell-autonomous heat shock cognate 70 (Hsc70) chaperones isolated from pumpkin sap (Aoki et al., 2002). Functional analysis of mutant forms of these Hsc70s identified a C-terminal motif that, when engineered into a human Hsp70, potentiated Pd transport of this normally cell-autonomous protein (Aoki et al., 2002). Although this Hsc70 domain was necessary for transport through Pd, it was not sufficient for this process because fusing it to GFP did not promote the GFP movement between cells, suggesting that another domain within Hsc70/Hsp70 is also required to induce Pd transport (Aoki et al., 2002).
The ability of the plant Hsc70/Hsp70 protein family members to up-regulate Pd may have been adopted during the evolution by some plant viruses for their movement between cells. Specifically, closteroviruses, such as Beet yellow virus (BYV), encode proteins with homology to the Hsp superfamily (Karasev, 2000). In the case of BYV, its Hsp70-related p65 protein binds microtubules (Karasev et al., 1992), localizes to Pd (Medina et al., 1999), and forms Pd transport-competent complex with the viral genome (Alzhanova et al., 2001).
DEVELOPMENTAL REGULATION OF Pd TRANSPORT
Pd permeability and, hence, traffic through these channels, are tightly regulated, depending on the developmental stage of the plant, tissue type, and biotic and/or abiotic stress conditions (Kim et al., 2002a, 2005a, 2005b). One critical developmental point at which Pd SEL is altered is during structural changes in Pd as cells elongate and tissues mature (Burch-Smith et al., 2011). Initially, Pd are generated during cytokinesis, and then continue to form as tissue develops; the former structures are termed primary Pd, while the latter are termed secondary Pd (Ehlers and Kollmann, 2001). In addition to this developmental classification, the Pd can be classified based on their structural features as simple and branched. Branched Pd are formed by interconnecting the existing simple Pd, and they often have central cavities that simple Pd lack and that are formed by the intersecting channels (Burch-Smith et al., 2011).
Historically, traffic through primary Pd has been presumed less selective as compared to transport through branched Pd (Oparka et al., 1999). On the other hand, Arabidopsis shoot apical meristem, which contains predominantly simple Pd, exhibits higher SEL than other tissues (Sessions et al., 2000; Wu et al., 2003). However, recent studies revealed more complex relationships between the origin, structure, and regulation of Pd permeability (for review, see Burch-Smith et al., 2011). This notion is illustrated by the recently discovered Arabidopsis protein INCREASED SIZE EXCLUSION2 (ISE2), a putative DEVH box RNA helicase that localizes at stress-induced granule-like structures in the cytoplasm. ISE2 was suggested to play a crucial role in Pd permeability control because ise2 mutant mid-torpedo embryos traffic 10-kDa fluorescent dextrans whereas the WT embryos at the same developmental stage do not (Kobayashi et al., 2007). Importantly, the ise2 embryos contain both simple and branched Pd while WT embryos contain only simple Pd, suggesting that branched Pd may in fact be more permeable than simple Pd (Kobayashi et al., 2007; Burch-Smith et al., 2011) and challenging the existing dogma of branched Pd being more selective (Oparka et al., 1999).
Developmental regulation of Pd is necessitated by their critical role in transport of signal molecules, such as those involved in positional signaling during morphogenesis. The first such signal protein discovered is the maize homeodomain protein KNOTTED1 (KN1) (Lucas et al., 1995). The initial discovery of KN1 cell-to-cell movement was based on the observations that KN1 mRNA is expressed in the inner meristem cell layers, but not in the surface L1 layer, whereas the protein is found all cell layers, including L1 (Lucas et al., 1995). KN1 was also shown to bind its mRNA in a sequence-specific manner and increase Pd SEL, suggesting that this protein can transport its own mRNA through Pd (Lucas et al., 1995; Jackson, 2002; Kim et al., 2002b). Importantly, the Pd-mediated traffic of KN1 is directional. For example, when GFP–KN1 is expressed in leaf mesophyll cells, the fusion protein moves into the epidermal layer, but it does not move into the mesophyll when expressed in the epidermis (Kim et al., 2002b). KNOTTED1-like homeodomain protein 1/BREVIPEDICELLUS (KNAT1/BP) and SHOOTMERISTEMLESS (STM) represent the most closely related Arabidopsis orthologs of maize KN1. While STM, similarly to KN1, functions in initiation and maintenance of shoot apical meristem, KNAT1/BP is involved in flower development (Long et al., 1996; Bharathan et al., 1999; Reiser et al., 2000; Douglas et al., 2002; Venglat et al., 2002). Similarly to GFP–KN1, GFP–KNAT1/BP and GFP–STM traffic from the L1 to the L2/L3 layers of the shoot apical meristem (Kim et al., 2003). Furthermore, the L1-specific expression of non-cell-autonomous KN1 and KNAT1 partially complements the stm-11 mutant, but the cell-autonomous GUS–KN1, which does not traffic out of the L1 layer, does not complement stm-11 when expressed in L1, demonstrating the non-cell-autonomous function of KNAT1 (Kim et al., 2003). The homeodomain of KN1 was found to interact with the movement protein binding protein 2C (MPB2C), and it was essential for cell-to-cell transport of KN1 (Bolduc et al., 2008). Thus, KN1 most likely is specifically recognized by the Pd transport machinery through its homeodomain, although the molecular mechanism by which this recognition leads to Pd gating and intercellular transport remains unknown.
Another important example of developmentally regulated Pd transport is the cell-to-cell trafficking of some MADS box transcription factors. Specifically, an Arabidopsis type-C MADS box factor, AGAMOUS (AG), expressed in meristem L1 layer, moves from cell to cell and functions non-cell-autonomously (Urbanus et al., 2010). Type-B MADS box proteins DEF and GLO of Antirrhinum majus also traffic between cell layers and likely function non-cell-autonomously (Perbal et al., 1996), while their Arabidopsis orthologs APETALA3 (AP3) and PISTILLATA (PI) do not seem to have this capacity (Irish and Jenik, 2001; Jenik and Irish, 2001). That a GFP dimer similar in size to AP3 and PI can diffuse between several cell layers of the meristem indicates that the Pd SEL in this tissue is large enough to allow also diffusion of AP3 and PI and suggests that the inability of AP3 and PI to function non-cell-autonomously may be due to their intracellular anchoring (Irish and Jenik, 2001; Jenik and Irish, 2001). Similarly, a plant-specific floral transcription factor LEAFY, also known to function non-cell-autonomously, has been suggested to move through Pd passively (Kim et al., 2003; Bolduc et al., 2008). Collectively, these data raise an interesting possibility that passive diffusion within meristem may be the default cell-to-cell transport pathway for proteins smaller than the SEL of Pd in this tissue, and that it is the inability of such proteins to move through Pd that is actively effected by an as-yet unknown cell-anchoring mechanism.
The molecular basis for the developmental regulation of Pd permeability remains unknown. Potentially, it may involve a specific subset of the recently described eight members of the Pd-located proteins, PDLPs, two of which are expressed in the shoot apical meristem (Bayer et al., 2008). One of them, At2g33330, is expressed predominantly in the L1 layer, where the directional transport of KN1 was observed (Bayer et al., 2008), and the other, At1g04520, is expressed throughout the tissue (Bayer et al., 2008). Whereas the role for PDLPs in Pd transport of the plant endogenous proteins remains to be elucidated, their involvement in plant viral movement has been demonstrated. Specifically, PDLPs likely participate in movement of Grape fan leaf virus (GFLV) and Cauliflower mosaic virus (CaMV), the MPs of which form tubular structures that span Pd and destroy their structure (Ritzenthaler and Hofmann, 2007). A recent study revealed that all eight PDLPs interact with GFLV MP and serve as anchoring points for initiating the tubule formation (Amari et al., 2010). In the triple pdlp1/2/3 mutant, which does not show obvious developmental phenotypes, both GFLV and an evolutionally distant CaMV showed delayed local and systemic spread, demonstrating the important role of PDLPs in cell-to-cell and long-distance movement of these viruses (Amari et al., 2010).
The developmental stage of the tissue may also regulate the ability of some viral MPs to gate Pd. For example, TMV MP is phosphorylated at its C-terminal by a Pd-associated protein kinase (PAPK) (Lee et al., 2005). MP phosphorylation at the C-terminal Ser-258, Thr-261, and Ser-265 residues negatively regulates its Pd gating activity (Waigmann et al., 2000), and, in tobacco leaves, this phosphorylation has been shown to occur in a developmentally controlled basipetal fashion (Citovsky et al., 1993), namely from tip to base, with the tip being the most mature and the base the least developed part of the leaf (Turgeon, 1989).
REGULATION OF DEVELOPMENT BY Pd TRANSPORT
The relationship between Pd transport and plant development is bidirectional: while Pd transport is regulated by plant development (see previous section), plant development also is regulated by Pd transport. The best-studied examples of such regulation are the effects of Pd transport of transcription factors on root and trichome development in Arabidopsis.
Root hair positioning is determined by an intricate mechanism that defines two-dimensional patterning of root hair cells (H cells) and hairless cells (N cells) in the initially uniform root epidermal cells. N cell differentiation is regulated by an activator-inhibitor system, which includes WEREWOLF (WER), GLABRA2 (GL2), basic helix-loop-helix (bHLH) factors GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3), CAPRICE (CPC), and a WD40 repeat protein TRANSPARENT TESTA GLABRA1 (TTG1) (Masucci et al., 1996; Wada et al., 1997; Walker et al., 1999; Payne et al., 2000; Lee and Schiefelbein, 2002; Bernhardt et al., 2003; Kurata et al., 2003; Zhang et al., 2003; Kang et al., 2009). WER, forming the trimetric complex with TTG1 and GL3 or EGL3, promotes expression of GL2 and CPC (Payne et al., 2000; Bernhardt et al., 2003; Zhang et al., 2003; Bernhardt et al., 2005). The cells that express GL2 differentiate into N cells, because GL2 suppresses root hair formation (Lee and Schiefelbein, 1999; Koshino-Kimura et al., 2005). The CPC gene is predominantly expressed in the N cells, but not in the adjacent H cells, whereas the CPC protein moves to the H cells and represses the GL2 expression (Wada et al., 2002). CPC contains a distinctive amino acid motif required for its Pd transport, suggesting that this transport occurs by an active mechanism, rather than via passive diffusion (Wada et al., 2002). Thus, Pd transport of CPC plays a critical role in root hair development.
Trichome spacing is also determined by an activator–inhibitor mechanism, which involves R2R3 MYB transcription factors GLABRA1 (GL1) or MYB23 (Kirik et al., 2004b, 2005), bHLH factors GLABRA3 (GL3), or ENHANCER OF GLABRA3 (EGL3) (Payne et al., 2000; Bernhardt et al., 2003; Zhang et al., 2003; Bernhardt et al., 2005), and the TRANSPARENT TESTA GLABRA1 (TTG1) (Galway et al., 1994; Walker et al., 1999). These proteins form a ternary transcriptional activation complex composed of a R2R3 MYB factor, TTG1, and a bHLH factor. This complex promotes GL2 transcription, which leads the cell to differentiate into a trichome. On the other hand, formation of trichomes is inhibited by one of R3 repeat MYB-like transcription factors, such as TRIPTYCHON (TRY) (Schellmann et al., 2002), CAPRICE (CPC) (Wada et al., 1997), ENHANCER OF TRY AND CPC1 (ETC1) (Kirik et al., 2004b), ETC2 (Kirik et al., 2004a), TRICHOMELESS1, and CAPRICE LIKE MYB3 (CPL3) (Tominaga et al., 2008), which presumably compete for the binding of R2R3 MYB to bHLH. The formation of the activation complex in the cells undergoing trichome development is accompanied by the inhibition of this process in the surrounding cells. The need to coordinate the correct trichome spacing implies involvement of cell-to-cell transport of the regulatory factors. Indeed, the TTG1 gene is transcribed in most epidermal cells while the TTG1 protein levels are significantly higher in the trichome than in the surrounding epidermis (Bouyer et al., 2008). This discrepancy in the TTG1 transcript and protein levels was explained based on the ability of TTG1 to bind GL3 and on trichome-specific expression of GL3. The TTG1 protein was proposed to move between cells and become trapped within GL3-expressing trichomes yet depleted from the surrounding cells that lack GL3 (Bouyer et al., 2008). Consistent with this hypothesis, TTG1 was shown to increase Pd permeability and move from cell to cell (Bouyer et al., 2008). Thus, as in the case of root epidermal cell differentiation, differentiation of trichomes depends on Pd transport of a transcriptional regulator.
Patterning of root endodermis is also regulated by Pd transport of a transcription factor. SHORTROOT (SHR) is a plant-specific GRAS-type transcription factor, which is expressed in stele, but not in endodermis, where the SHR protein is expected to function. Thus, SHR is expected to function in a non-cell-autonomous manner. Indeed, SHR has been shown to move, presumably through Pd, from the stele to adjacent cells (Nakajima et al., 2001). Importantly, SHR does not diffuse through Pd freely; rather, it accumulates in just one layer of endodermal cells. This precise and limited Pd transport is governed by the SHR interaction with SCARECROW (SCR) that sequesters SHR in the nucleus (Cui et al., 2007). Since SHR activates the SCR promoter (Nakajima et al., 2001), the SCR-dependent SHR nuclear trapping provides positive feedback for SCR transcription as well as prevents Pd transport of SHR into other cell layers (Cui et al., 2007). A similar regulatory mechanism was observed with SHT and SCR homologs in rice, suggesting that the strategy of specifying tissue differentiation via Pd transport of transcription factors is evolutionally conserved among different plant species (Cui et al., 2007).
CONCLUDING REMARKS
Perhaps one of the most intriguing aspects of intercellular communication and transport in most higher eukaryotes, from plants to mammals, is traffic of macromolecular complexes through direct cytoplasmic bridges between the adjacent cells. In plants, these cytoplasmic intercellular connections are represented by Pd, and here we reviewed recent major advances in our understanding of the regulatory mechanisms that govern Pd transport and their involvement in plant development, morphogenesis, and interactions with the invading viruses. Three major points are highlighted by this body of studies. First, the mechanisms for Pd gating, a process that has long been almost a complete enigma, are starting to emerge, and they include relaxation of callose sphincter and severing of actin filaments. It remains unclear, however, whether these pathways are interdependent, able to cross-talk, or they function separately to each other. Second, the regulation of Pd permeability becomes positioned as one of the major mechanisms for determination of tissue patterning. Furthermore, the repertoire of the developmental cues that traverse Pd during these developmental events is becoming ever complex. Third, plant viruses in general and their MPs in particular remain one of the premiere tools for study of Pd transport, and the information gleaned from these studies is often directly applicable to the cellular processes that employ similar transport strategies.
Whereas, in plants, the notion of traffic of cellular macromolecules as well as pathogens through cytoplasmic bridges between cells has been established for decades, it is just emerging in mammalian systems (Gallagher and Benfey, 2005). In mammals, these intercellular connections, termed tunneling nanotubes (TNTs), represent a relatively recent discovery (Onfelt et al., 2005; Davis and Sowinski, 2008; Rustom, 2009). Like Pd, TNTs traffic proteins (Onfelt et al., 2005; Davis and Sowinski, 2008; Rustom, 2009) and serve as conduits for pathogens, such as prions (Gerdes, 2009), and, potentially, human immunodeficiency virus (HIV) particles (Eugenin et al., 2009a, 2009b). Also similarly to Pd transport, TNT transport is regulated by actin filaments (Zhu et al., 2005; Gurke et al., 2008; Veranic et al., 2008) and, possibly, ROS (Zhu et al., 2005). Based on these conceptual and mechanistic similarities, future investigation of TNTs may be informed by the Pd research. Thus, the impact of the studies of Pd transport and regulation reaches beyond the already broad fields of plant biology and plant–pathogen interactions.
FUNDING
The work in our laboratory is supported by grants from USDA NIFA, NIH, NSF, BARD, and BSF to V.C. No conflict of interest declared.
References
- Alzhanova DV, Napuli AJ, Creamer R, Dolja VV. Cell-to-cell movement and assembly of a plant closterovirus: roles for the capsid proteins and Hsp70 homolog. EMBO J. 2001;20:6997–7007. doi: 10.1093/emboj/20.24.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amari K, et al. A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog. 2010;6:e1001119. doi: 10.1371/journal.ppat.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Kragler F, Xoconostle-Cazares B, Lucas WJ. A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc. Natl Acad. Sci. U S A. 2002;99:16342–16347. doi: 10.1073/pnas.252427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avisar D, Prokhnevsky AI, Dolja VV. Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog. J. Virol. 2008;82:2836–2843. doi: 10.1128/JVI.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Xiang Y, Schobert C, Thompson GA, Lucas WJ. Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc. Natl Acad. Sci. U S A. 1997;94:14150–14155. doi: 10.1073/pnas.94.25.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DA, Cole L, Collings DA, Liu DY, Smith PM, Day DA, Overall RL. Cell-to-cell transport via the lumen of the endoplasmic reticulum. Plant J. 2011;66:806–817. doi: 10.1111/j.1365-313X.2011.04545.x. [DOI] [PubMed] [Google Scholar]
- Bayer E, Thomas C, Maule A. Symplastic domains in the Arabidopsis shoot apical meristem correlate with PDLP1 expression patterns. Plant Signal Behav. 2008;3:853–855. doi: 10.4161/psb.3.10.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Oparka K. Imaging plasmodesmata. Protoplasma. 2011;248:9–25. doi: 10.1007/s00709-010-0233-6. [DOI] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Jackson D. Redox homeostasis regulates plasmodesmal communication in Arabidopsis meristems. Plant Signal Behav. 2009;4:655–659. doi: 10.4161/psb.4.7.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Cantrill L, Jackson D. Plasmodesmata: Cell-to-Cell Channels in Plants. New York: Springer; 2006. [Google Scholar]
- Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc. Natl Acad. Sci. U S A. 2009;106:3615–3620. doi: 10.1073/pnas.0808717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development. 2003;130:6431–6439. doi: 10.1242/dev.00880. [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development. 2005;132:291–298. doi: 10.1242/dev.01565. [DOI] [PubMed] [Google Scholar]
- Bharathan G, Janssen BJ, Kellogg EA, Sinha N. Phylogenetic relationships and evolution of the KNOTTED class of plant homeodomain proteins. Mol. Biol. Evol. 1999;16:553–563. doi: 10.1093/oxfordjournals.molbev.a026136. [DOI] [PubMed] [Google Scholar]
- Blackman LM, Overall RL. Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J. 1998;14:733–741. [Google Scholar]
- Boevink P, Oparka KJ. Virus–host interactions during movement processes. Plant Physiol. 2005;138:1815–1821. doi: 10.1104/pp.105.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc N, Hake S, Jackson D. Dual functions of the KNOTTED1 homeodomain: sequence-specific DNA binding and regulation of cell-to-cell transport. Sci. Signal. 2008;1:pe28. doi: 10.1126/scisignal.123pe28. [DOI] [PubMed] [Google Scholar]
- Bouyer D, Geier F, Kragler F, Schnittger A, Pesch M, Wester K, Balkunde R, Timmer J, Fleck C, Hulskamp M. Two-dimensional patterning by a trapping/depletion mechanism: the role of TTG1 and GL3 in Arabidopsis trichome formation. PLoS Biol. 2008;6:e141. doi: 10.1371/journal.pbio.0060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill LM, Nunn RS, Kahn TW, Yeager M, Beachy RN. Recombinant Tobacco mosaic virus movement protein is an RNA-binding, alpha-helical membrane protein. Proc. Natl Acad. Sci. U S A. 2000;97:7112–7117. doi: 10.1073/pnas.130187897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Stonebloom S, Xu M, Zambryski PC. Plasmodesmata during development: re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma. 2011;248:61–74. doi: 10.1007/s00709-010-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Sheng J, Hind G, Handa A, Citovsky V. Interaction between the Tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 2000;19:913–920. doi: 10.1093/emboj/19.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Tian GW, Gafni Y, Citovsky V. Effects of calreticulin on viral cell-to-cell movement. Plant Physiol. 2005;138:1866–1876. doi: 10.1104/pp.105.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Knorr D, Schuster G, Zambryski PC. The P30 movement protein of Tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990;60:637–647. doi: 10.1016/0092-8674(90)90667-4. [DOI] [PubMed] [Google Scholar]
- Citovsky V, McLean BG, Zupan J, Zambryski PC. Phosphorylation of Tobacco mosaic virus cell-to-cell movement protein by a developmentally-regulated plant cell wall-associated protein kinase. Genes Dev. 1993;7:904–910. doi: 10.1101/gad.7.5.904. [DOI] [PubMed] [Google Scholar]
- Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY, Blilou I, Scheres B, Benfey PN. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316:421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- Ding B, Kwon MO. Evidence that actin filaments are involved in controlling the permeability of plasmodesmata in tobacco mesophyll. Plant J. 1996;10:157–164. [Google Scholar]
- Ding B, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ. Secondary plasmodesmata are specific sites of localization of the Tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992;4:915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorokhov YL, Makinen K, Frolova OY, Merits A, Saarinen J, Kalkkinen N, Atabekov JG, Saarma M. A novel function for a ubiquitous plant enzyme pectin methylesterase: the host-cell receptor for the Tobacco mosaic virus movement protein. FEBS Lett. 1999;461:223–228. doi: 10.1016/s0014-5793(99)01447-7. [DOI] [PubMed] [Google Scholar]
- Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. 2002;14:547–558. doi: 10.1105/tpc.010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers K, Kollmann R. Primary and secondary plasmodesmata: structure, origin, and functioning. Protoplasma. 2001;216:1–30. doi: 10.1007/BF02680127. [DOI] [PubMed] [Google Scholar]
- Epel BL. Plant virus spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host β-1,3, glucanases. Semin. Cell Dev. Biol. 2009;20:1074–1081. doi: 10.1016/j.semcdb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009a;254:142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT): a potential mechanism for intercellular HIV trafficking. Commun. Integr. Biol. 2009b;2:243–244. doi: 10.4161/cib.2.3.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki M, Kawakami S, Kim RW, Beachy RN. Domains of Tobacco mosaic virus movement protein essential for its membrane association. J. Gen. Virol. 2006;87:2699–2707. doi: 10.1099/vir.0.81936-0. [DOI] [PubMed] [Google Scholar]
- Gallagher KL, Benfey PN. Not just another hole in the wall: understanding intercellular protein trafficking. Genes Dev. 2005;19:189–195. doi: 10.1101/gad.1271005. [DOI] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev. Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- Gerdes HH. Prions tunnel between cells. Nat. Cell Biol. 2009;11:235–236. doi: 10.1038/ncb0309-235. [DOI] [PubMed] [Google Scholar]
- Ghoshroy S, Freedman K, Lartey R, Citovsky V. Inhibition of plant viral systemic infection by non-toxic concentrations of cadmium. Plant J. 1998;13:591–602. doi: 10.1046/j.1365-313x.1998.00061.x. [DOI] [PubMed] [Google Scholar]
- Guenoune-Gelbart D, Elbaum M, Sagi G, Levy A, Epel BL. Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol. Plant–Microbe Interact. 2008;21:335–345. doi: 10.1094/MPMI-21-3-0335. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp. Cell Res. 2008;314:3669–3683. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Guseman JM, Lee JS, Bogenschutz NL, Peterson KM, Virata RE, Xie B, Kanaoka MM, Hong Z, Torii KU. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis CHORUS (GLUCAN SYNTHASE-LIKE 8) Development. 2010;137:1731–1741. doi: 10.1242/dev.049197. [DOI] [PubMed] [Google Scholar]
- Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- Higaki T, Sano T, Hasezawa S. Actin microfilament dynamics and actin side-binding proteins in plants. Curr. Opin. Plant Biol. 2007;10:549–556. doi: 10.1016/j.pbi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Niehl A, Sambade A, Steinmetz A, Heinlein M. Inhibition of Tobacco mosaic virus movement by expression of an actin-binding protein. Plant Physiol. 2009;149:1810–1823. doi: 10.1104/pp.108.133827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun TK, Uddin MN, Rim Y, Kim JY. Cell-to-cell trafficking of RNA and RNA silencing through plasmodesmata. Protoplasma. 2011;248:101–116. doi: 10.1007/s00709-010-0225-6. [DOI] [PubMed] [Google Scholar]
- Irish VF, Jenik PD. Cell lineage, cell signaling and the control of plant morphogenesis. Curr. Opin. Genet. Dev. 2001;11:424–430. doi: 10.1016/s0959-437x(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Ishiwatari Y, Fujiwara T, McFarland KC, Nemoto K, Hayashi H, Chino M, Lucas WJ. Rice phloem thioredoxin h has the capacity to mediate its own cell-to-cell transport through plasmodesmata. Planta. 1998;205:12–22. doi: 10.1007/s004250050291. [DOI] [PubMed] [Google Scholar]
- Jackson D. Double labeling of KNOTTED1 mRNA and protein reveals multiple potential sites of protein trafficking in the shoot apex. Plant Physiol. 2002;129:1423–1429. doi: 10.1104/pp.006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenik PD, Irish VF. The Arabidopsis floral homeotic gene APETALA3 differentially regulates intercellular signaling required for petal and stamen development. Development. 2001;128:13–23. doi: 10.1242/dev.128.1.13. [DOI] [PubMed] [Google Scholar]
- Kang YH, Kirik V, Hulskamp M, Nam KH, Hagely K, Lee MM, Schiefelbein J. The MYB23 gene provides a positive feedback loop for cell fate specification in the Arabidopsis root epidermis. Plant Cell. 2009;21:1080–1094. doi: 10.1105/tpc.108.063180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasev AV. Genetic diversity and evolution of Closteroviruses. Annu. Rev. Phytopathol. 2000;38:293–324. doi: 10.1146/annurev.phyto.38.1.293. [DOI] [PubMed] [Google Scholar]
- Karasev AV, Kashina AS, Gelfand VI, Dolja VV. HSP70-related 65 kDa protein of Beet yellow closterovirus is a microtubule-binding protein. FEBS Lett. 1992;304:12–14. doi: 10.1016/0014-5793(92)80578-5. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc. Natl Acad. Sci. U S A. 2004;101:6291–6296. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Cho E, Crawford KM, Hempel FD, Zambryski PC. Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc. Natl Acad. Sci. U S A. 2005a;102:2227–2231. doi: 10.1073/pnas.0409193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Hempel FD, Sha K, Pfluger J, Zambryski PC. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development. 2002a;129:1261–1272. doi: 10.1242/dev.129.5.1261. [DOI] [PubMed] [Google Scholar]
- Kim I, Kobayashi K, Cho E, Zambryski PC. Subdomains for transport via plasmodesmata corresponding to the apical–basal axis are established during Arabidopsis embryogenesis. Proc. Natl Acad. Sci. U S A. 2005b;102:11945–11950. doi: 10.1073/pnas.0505622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Yuan Z, Jackson D. Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development. 2003;130:4351–4362. doi: 10.1242/dev.00618. [DOI] [PubMed] [Google Scholar]
- Kim JY, Yuan Z, Cilia M, Khalfan-Jagani Z, Jackson D. Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc. Natl Acad. Sci. U S A. 2002b;99:4103–4108. doi: 10.1073/pnas.052484099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Lee MM, Wester K, Herrmann U, Zheng Z, Oppenheimer D, Schiefelbein J, Hulskamp M. Functional diversification of MYB23 and GL1 genes in trichome morphogenesis and initiation. Development. 2005;132:1477–1485. doi: 10.1242/dev.01708. [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Huelskamp M, Schiefelbein J. The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev. Biol. 2004a;268:506–513. doi: 10.1016/j.ydbio.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Wester K, Schiefelbein J, Hulskamp M. ENHANCER of TRY and CPC 2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis. Plant Mol. Biol. 2004b;55:389–398. doi: 10.1007/s11103-004-0893-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Otegui MS, Krishnakumar S, Mindrinos M, Zambryski P. INCREASED SIZE EXCLUSION LIMIT 2 encodes a putative DEVH box RNA helicase involved in plasmodesmata function during Arabidopsis embryogenesis. Plant Cell. 2007;19:1885–1897. doi: 10.1105/tpc.106.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino-Kimura Y, Wada T, Tachibana T, Tsugeki R, Ishiguro S, Okada K. Regulation of CAPRICE transcription by MYB proteins for root epidermis differentiation in Arabidopsis. Plant Cell Physiol. 2005;46:817–826. doi: 10.1093/pcp/pci096. [DOI] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- Kurata T, Kawabata-Awai C, Sakuradani E, Shimizu S, Okada K, Wada T. The YORE-YORE gene regulates multiple aspects of epidermal cell differentiation in Arabidopsis. Plant J. 2003;36:55–66. doi: 10.1046/j.1365-313x.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- Lazarowitz SG, Beachy RN. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell. 1999;11:535–548. doi: 10.1105/tpc.11.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Taoka K, Yoo BC, Ben-Nissan G, Kim DJ, Lucas WJ. Plasmodesmal-associated protein kinase in tobacco and Arabidopsis recognizes a subset of non-cell-autonomous proteins. Plant Cell. 2005;17:2817–2831. doi: 10.1105/tpc.105.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Yoo BC, Rojas MR, Gomez-Ospina N, Staehelin LA, Lucas WJ. Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science. 2003;299:392–396. doi: 10.1126/science.1077813. [DOI] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell. 1999;99:473–483. doi: 10.1016/s0092-8674(00)81536-6. [DOI] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. Cell pattern in the Arabidopsis root epidermis determined by lateral inhibition with feedback. Plant Cell. 2002;14:611–618. doi: 10.1105/tpc.010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Erlanger M, Rosenthal M, Epel BL. A plasmodesmata-associated β-1,3-glucanase in Arabidopsis. Plant J. 2007;49:669–682. doi: 10.1111/j.1365-313X.2006.02986.x. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Lazarowitz SG. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl Acad. Sci. U S A. 2010;107:2491–2496. doi: 10.1073/pnas.0909080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Blancaflor EB, Nelson RS. The Tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 2005;138:1853–1865. doi: 10.1104/pp.105.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- Lucas WJ. Plant viral movement proteins: agents for cell-to-cell trafficking of viral genomes. Virology. 2006;344:169–184. doi: 10.1016/j.virol.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Bouche-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. 1995;270:1980–1983. doi: 10.1126/science.270.5244.1980. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- McLean BG, Zupan J, Zambryski PC. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995;7:2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina V, Peremyslov VV, Hagiwara Y, Dolja VV. Subcellular localization of the HSP70-homolog encoded by Beet yellows closterovirus. Virology. 1999;260:173–181. doi: 10.1006/viro.1999.9807. [DOI] [PubMed] [Google Scholar]
- Meshi T, Watanabe Y, Saito T, Sugimoto A, Maeda T, Okada Y. Function of the 30 kd protein of Tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987;6:2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Sundquist WI. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- Niehl A, Heinlein M. Cellular pathways for viral transport through plasmodesmata. Protoplasma. 2011;248:75–99. doi: 10.1007/s00709-010-0246-1. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM. Long-distance calls between cells connected by tunneling nanotubules. 2005 doi: 10.1126/stke.3132005pe55. Sci. STKE. 2005, pe55. [DOI] [PubMed] [Google Scholar]
- Oparka KJ. Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci. 2004;9:33–41. doi: 10.1016/j.tplants.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell. 1999;97:743–754. doi: 10.1016/s0092-8674(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Overall RL, White RG, Blackman LM, Radford JE. Actin and myosin in plasmodesmata. In: Staiger CJ, Baluska F, Volkmann D, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht: Kluwer Academic; 2000. pp. 1–19. [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics. 2000;156:1349–1362. doi: 10.1093/genetics/156.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal MC, Haughn G, Saedler H, Schwarz-Sommer Z. Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development. 1996;122:3433–3441. doi: 10.1242/dev.122.11.3433. [DOI] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV. Actin cytoskeleton is involved in targeting of a viral Hsp70 homolog to the cell periphery. J. Virol. 2005;79:14421–14428. doi: 10.1128/JVI.79.22.14421-14428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford JE, White RG. Localization of a myosin-like protein to plasmodesmata. Plant J. 1998;14:743–750. doi: 10.1046/j.1365-313x.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- Reiser L, Sanchez-Baracaldo P, Hake S. Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Mol. Biol. 2000;42:151–166. [PubMed] [Google Scholar]
- Ritzenthaler C, Hofmann C. Tubule-Guided Movement of Plant Viruses. Berlin-Heidelberg: Springer-Verlag; 2007. [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development. 1999;126:4405–4419. doi: 10.1242/dev.126.20.4405. [DOI] [PubMed] [Google Scholar]
- Rustom A. Hen or egg? Some thoughts on tunneling nanotubes. Ann. N Y Acad. Sci. 2009;1178:129–136. doi: 10.1111/j.1749-6632.2009.04997.x. [DOI] [PubMed] [Google Scholar]
- Sagi G, Katz A, Guenoune-Gelbart D, Epel BL. Class 1 reversibly glycosylated polypeptides are plasmodesmal-associated proteins delivered to plasmodesmata via the Golgi apparatus. Plant Cell. 2005;17:1788–1800. doi: 10.1105/tpc.105.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jurgens G, Hulskamp M. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 2002;21:5036–5046. doi: 10.1093/emboj/cdf524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof HB. Plant virus transport: motions of functional equivalence. Trends Plant Sci. 2005;10:376–382. doi: 10.1016/j.tplants.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sessions A, Yanofsky MF, Weigel D. Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science. 2000;289:779–782. doi: 10.1126/science.289.5480.779. [DOI] [PubMed] [Google Scholar]
- Sheahan MB, Staiger CJ, Rose RJ, McCurdy DW. A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells. Plant Physiol. 2004;136:3968–3978. doi: 10.1104/pp.104.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Yoshii A, Sakurai K, Hamada K, Yamaji Y, Suzuki M, Namba S, Hibi T. Identification of a novel tobacco DnaJ-like protein that interacts with the movement protein of Tobacco mosaic virus. Arch. Virol. 2009;154:2959–2067. doi: 10.1007/s00705-009-0397-6. [DOI] [PubMed] [Google Scholar]
- Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell. 2009;21:581–594. doi: 10.1105/tpc.108.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebloom S, Burch-Smith T, Kim I, Meinke D, Mindrinos M, Zambryski P. Loss of the plant DEAD-box protein ISE1 leads to defective mitochondria and increased cell-to-cell transport via plasmodesmata. Proc. Natl Acad. Sci. U S A. 2009;106:17229–17234. doi: 10.1073/pnas.0909229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Liu Z, Chen C, Zhang Y, Wang X, Zhu L, Miao L, Wang XC, Yuan M. Cucumber mosaic virus movement protein severs actin filaments to increase the plasmodesmal size exclusion limit in tobacco. Plant Cell. 2010;22:1373–1387. doi: 10.1105/tpc.108.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami Y, Watanabe Y. Effects of brefeldin A on the localization of Tobamovirus movement protein and cell-to-cell movement of the virus. Virology. 2007;361:133–140. doi: 10.1016/j.virol.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol. 2008;6:e7. doi: 10.1371/journal.pbio.0060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomenius K, Clapham D, Meshi T. Localization by immunogold cytochemistry of the virus coded 30 K protein in plasmodesmata of leaves infected with Tobacco mosaic virus. Virology. 1987;160:363–371. doi: 10.1016/0042-6822(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development. 2008;135:1335–1345. doi: 10.1242/dev.017947. [DOI] [PubMed] [Google Scholar]
- Turgeon R. The sink-source transition in leaves. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:119–138. [Google Scholar]
- Ueki S, Lacroix B, Krichevsky A, Lazarowitz SG, Citovsky V. Functional transient genetic transformation of Arabidopsis leaves by biolistic bombardment. Nature Protocols. 2009;4:71–77. doi: 10.1038/nprot.2008.217. [DOI] [PubMed] [Google Scholar]
- Ueki S, Meyer B, Yasmin F, Citovsky V. A cell-to-cell macromolecular transport assay in planta utilizing biolistic bombardment. J. Visual Exper. 2010a;42:2208. doi: 10.3791/2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki S, Spektor R, Natale DM, Citovsky V. ANK, a host cytoplasmic receptor for the Tobacco mosaic virus cell-to-cell movement protein, facilitates intercellular transport through plasmodesmata. PLoS Pathog. 2010b;6:e1001201. doi: 10.1371/journal.ppat.1001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus SL, Martinelli AP, Dinh QD, Aizza LC, Dornelas MC, Angenent GC, Immink RG. Intercellular transport of epidermis-expressed MADS domain transcription factors and their effect on plant morphology and floral transition. Plant J. 2010;63:60–72. doi: 10.1111/j.1365-313X.2010.04221.x. [DOI] [PubMed] [Google Scholar]
- Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, Keller W, Martienssen R, Selvaraj G, Datla R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl Acad. Sci. U S A. 2002;99:4730–4735. doi: 10.1073/pnas.072626099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranic P, Lokar M, Schutz GJ, Weghuber J, Wieser S, Hagerstrand H, Kralj-Iglic V, Iglic A. Different types of cell-to-cell connections mediated by nanotubular structures. Biophys. J. 2008;95:4416–4425. doi: 10.1529/biophysj.108.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verchot-Lubicz J. A new cell-to-cell transport model for Potexviruses. Mol. Plant–Microbe Interact. 2005;18:283–290. doi: 10.1094/MPMI-18-0283. [DOI] [PubMed] [Google Scholar]
- Volkmann D, Mori T, Tirlapur UK, König K, Fujiwara T, Kendrick-Jones J, Baluska F. Unconventional myosins of the plant-specific class VIII: endocytosis, cytokinesis, plasmodesmata/pit-fields, and cell-to-cell coupling. Cell Biol. Int. 2003;27:289–291. doi: 10.1016/s1065-6995(02)00330-x. [DOI] [PubMed] [Google Scholar]
- von Bargen S, Salchert K, Paape M, Piechulla B, Kellmann J. Interactions between the Tomato spotted wilt virus movement protein and plant proteins showing homologies to myosin, kinesin, and DnaJ-like chaperons. Plant Physiol. Biochem. 2001;39:1083–1093. [Google Scholar]
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development. 2002;129:5409–5419. doi: 10.1242/dev.00111. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science. 1997;277:1113–1116. doi: 10.1126/science.277.5329.1113. [DOI] [PubMed] [Google Scholar]
- Waigmann E, Chen MH, Bachmaier R, Ghoshroy S, Citovsky V. Phosphorylation of Tobacco mosaic virus cell-to-cell movement protein regulates viral movement in a host-specific fashion. EMBO J. 2000;19:4875–4884. doi: 10.1093/emboj/19.18.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigmann E, Lucas WJ, Citovsky V, Zambryski PC. Direct functional assay for Tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc. Natl Acad. Sci. U S A. 1994;91:1433–1437. doi: 10.1073/pnas.91.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigmann E, Ueki S, Trutnyeva K, Citovsky V. The ins and outs of non-destructive cell-to-cell and systemic movement of plant viruses. Crit. Rev. Plant Sci. 2004;23:195–250. [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 1999;11:1337–1350. doi: 10.1105/tpc.11.7.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RG, Badelt K, Overall RL, Vesk M. Actin associated with plasmodesmata. Protoplasma. 1994;180:169–184. [Google Scholar]
- Wolf S, Deom CM, Beachy RN, Lucas WJ. Movement protein of Tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- Wright KM, Wood NT, Roberts AG, Chapman S, Boevink P, MacKenzie KM, Oparka KJ. Targeting of TMV movement protein to plasmodesmata requires the actin/ER network: evidence from FRAP. Traffic. 2007;8:21–31. doi: 10.1111/j.1600-0854.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Dinneny JR, Crawford KM, Rhee Y, Citovsky V, Zambryski PC, Weigel D. Models of intercellular transcription factor movement in the Arabidopsis apex. Development. 2003;130:3735–3745. doi: 10.1242/dev.00577. [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cázares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science. 1999;283:94–98. doi: 10.1126/science.283.5398.94. [DOI] [PubMed] [Google Scholar]
- Yoneda A, Kutsuna N, Higaki T, Oda Y, Sano T, Hasezawa S. Recent progress in living cell imaging of plant cytoskeleton and vacuole using fluorescent-protein transgenic lines and three-dimensional imaging. Protoplasma. 2007;230:129–139. doi: 10.1007/s00709-006-0237-4. [DOI] [PubMed] [Google Scholar]
- Zavaliev R, Sagi G, Gera A, Epel BL. The constitutive expression of Arabidopsis plasmodesmal-associated class 1 reversibly glycosylated polypeptide impairs plant development and virus spread. J. Exp. Bot. 2010;61:131–142. doi: 10.1093/jxb/erp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliev R, Ueki S, Epel BL, Citovsky V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma. 2011;248:117–130. doi: 10.1007/s00709-010-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development. 2003;130:4859–4869. doi: 10.1242/dev.00681. [DOI] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]