INTRODUCTION
In many patients with inflammatory bowel disease (IBD), the symptoms of abdominal pain, bloating and diarrhoea are out of proportion with the demonstrated degree of inflammation; some symptomatic patients may have complete mucosal healing. Such patients often may undergo invasive, costly and potentially dangerous tests in search of residual inflammation. What are the diagnostic considerations in such patients? How should they be managed?
SYMPTOMS OF IRRITABLE BOWEL SYNDROME IN PATIENTS WITH IBD IN REMISSION
Irritable bowel syndrome (IBS) is a commonly encountered disorder, with point prevalence of ~10%1; symptom-based criteria facilitate its diagnosis.2 Although IBD affects ~0.4% of the population in the United States and Europe,3,4 IBD and IBS may concur in a patient, purely by chance. How often do patients with IBD and inflammation in remission manifest symptoms that suggest IBS, usually IBS with diarrhoea (IBS-D)?
From a database of over 1500 patients with IBD, Zaman et al5 identified 30 with ileal Crohn’s disease and 25 with ulcerative colitis involving only the recto-sigmoid, and stable symptoms, no changes in medications or use of chronic steroids for 3 months, and no prior bowel surgery. Rome II IBS criteria were met by 67% of patients with Crohn’s disease and 60% of ulcerative colitis, with similar symptoms in the two diseases. Thus, symptoms of IBS are common in IBD patients whose inflammation appears to be stable or in remission.
CLINICAL STRATEGY IN SYMPTOMATIC PATIENTS WITH IBD WHO APPEAR TO BE IN REMISSION
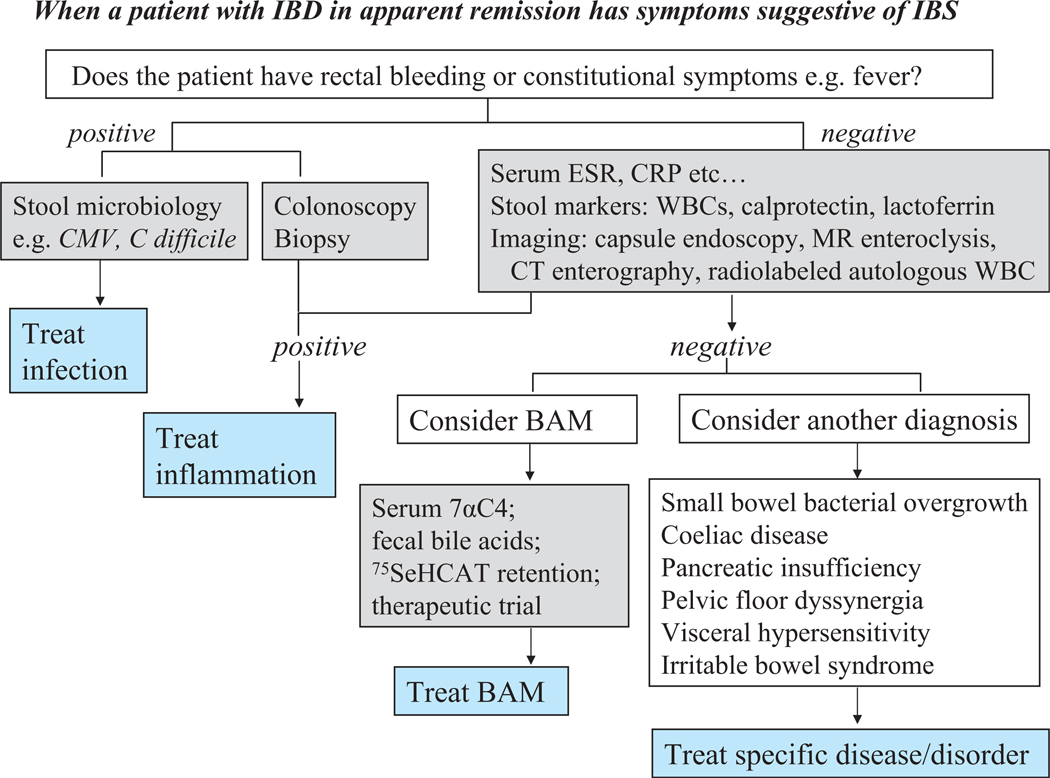
In patients who do not suffer IBD, the symptoms of IBS are not sufficiently specific to differentiate, in the absence of objective tests, other conditions such as pelvic floor dyssynergia or coeliac disease that respectively mimic constipation-predominant IBS (IBS-C) and IBS-D.6 Similarly, in patients with IBD, the presence of diarrhoea, urgency and pain in the absence of significant inflammation typically occur without constitutional symptoms or rectal bleeding, and may result from specific diseases that require specific treatment (figure 1) rather than symptomatic treatment. The first steps are to exclude infection (eg, cytomegalovirus or Clostridium difficile infection), and to confirm that the inflammatory process is sufficiently controlled and unlikely to be the cause of symptoms.
Figure 1.
When a patient with inflammatory bowel disease (IBD) in apparent remission has symptoms suggestive of irritable bowel syndrome (IBS). 7αC4, 7α-hydroxy-4-cholesten-3-one; BAM, bile acid malabsorption; CMV, cytomegalovirus; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; 75Se-HCAT (23-selena-25-homotaurocholate; WBC, white blood cells.
BLOOD AND STOOL TESTS IN DISCRIMINATING IBD FROM IBS
The number of bowel movements, presence of blood in the stool, diverse activity indices, and simple blood tests such as serum C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are used to assess inflammatory activity in IBD. However, activity indices may not necessarily reflect inflammation. In the Crohn’s disease activity index (CDAI), abdominal pain, loose stools and general well-being, and the use of diphenoxylate or loperamide for diarrhoea are all manifestations of IBS with diarrhoea and may not reflect inflammation. ESR and CRP are less useful in more subtle IBD; they did not perform as well as stool calprotectin in differentiating ‘organic’ from ‘non-organic’ diseases.7 Table 18–15 summarises the ability of other blood and stool markers to discriminate IBD from IBS: faecal calprotectin, lactoferrin and S100A12 (a calcium-binding proinflammatory protein secreted by granulocytes) appear good screening tests to identify residual inflammation.
Table 1.
Biomarkers in the discrimination of irritable bowel syndrome (IBS) from inflammatory bowel disease (IBD)
| Biomarker (ref) | Patients studied | Discrimination of IBD from IBS |
|---|---|---|
| Faecal calprotectin7 | 102 CD, 87 UC, 339 IBS | Abnormal calprotectin had an OR for disease of 27.8 (95% CI, 17.6 to 43.7) |
| Faecal calprotectin8 | Organic diarrhoea 65 (incl. 9 CD), 55 IBS | 64% sensitivity and 80% specificity with 70% positive and 74% negative predictive values for organic causes |
| Faecal lactoferrin9 | 104 CD, 80 UC, 31 IBS, and 56 HCs | 90% specific for identifying inflammation in active IBD; elevated faecal lactoferrin 100% specific in ruling out IBS |
| Faecal calprotectin and lactoferrin10 | 23 IBD, 91 IBS | Sensitivity and negative predictive value of calprotectin both 100%, and 78% and 95%, respectively, for lactoferrin; specificity and positive predictive value slightly higher for lactoferrin |
| Faecal human β-defensin-211 | 30 active UC, 46 IBS, and 24 HCs | HBD-2 levels highest in active UC, almost as high in IBS, and lowest for HCs; accuracy for IBS versus IBD low |
| Faecal lactoferrin, calprotectin; blood leucocytes, CRP, and IBD antibodies12 | 36 CD, 28 UC, 30 IBS, and 42 HCs | Overall accuracy: lactoferrin 90%, calprotectin 89%, lactoferrin latex-agglutination 78%, OBTI 74%, CRP 73%, blood leucocytes 63%, CD antibodies (ASCA+/pANCA− or ASCA+/pANCA+) 55%, UC antibodies (pANCA+/ASCA−) 49%. |
| Serum anti-flagellin antibodies13 | 61 CD, 50 UC 112 IBS, and 43 HCs | Few patients with IBS have elevated anti-flagellin titres, at much lower titre than in CD, and slightly higher than in UC; overall diagnostic accuracy not reported |
| Faecal S100A1214 | 64 UC, 64 CD, and 73 IBS | Serum level of 54.4 ng/ml could predict UC and CD with 66.7% sensitivity and 64.4% specificity |
| Serum S100A1215 | 32 CD, 27 UC, 24 IBS, 88 infectious enteritis, 24 HCs | Sensitivity of 86% and specificity of 96% for distinguishing IBD from IBS |
Overall accuracy is calculated by addition of the true-positive and true-negative test results.
ASCA, anti-Saccharomyces cerevisiae antibodies; CD, Crohn’s disease; CRP, C-reactive protein; HCs, healthy controls; OBTI, immunochromatographic test for detection of human haemoglobin; pANCA, perinuclear antineutrophil cytoplasmic antibodies; UC, ulcerative colitis.
RADIOLOGICAL IMAGING
When the question of IBS in IBD arises, the patient is symptomatic and colonoscopy has usually excluded active colitis or terminal ileitis. What about small bowel inflammatory disease? Approaches to image the small bowel include barium follow through (or enteroclysis), CT enterography, MR enteroclysis, or capsule endoscopy.16 Radiolabelled autologous leucocyte scintigraphy is used in few centres.
In general, MR and CT enterography have similar sensitivities for detecting active small bowel inflammation17: MR enteroclysis may be preferred because of the absence of radiation exposure and better characterisation of stenotic lesions, whereas CT provides better temporal resolution, mesenteric imaging and shorter length of examination.18 MR imaging is applied for quantifying IBD severity, though further clinical validation is desirable.19
IS DIARRHOEA IN IBD IN REMISSION DUE TO BILE ACID MALABSORPTION?
Hofmann and Poley showed that steatorrhoea occurs when the extent of combined ileal Crohn’s disease and resection exceeds 100 cm20; with less than 100 cm of Crohn’s disease or resection, diarrhoea results from bile acid malabsorption (BAM). There are three general approaches to diagnose BAM: serum 7α-hydroxy-4-cholesten-3-one (7αC4), an indirect measurement of hepatic bile acid synthesis which is closely related to the faecal loss of bile acids21,22; 48 h faecal bile acid excretion; and 75Se-HCAT (23-selena-25-homotaurocholate, a synthetic bile acid) retention23 on scintigraphy, which is based on whole-body retention at 7 days of the radiolabelled bile acid and retention of <12% reflects BAM. A surrogate test is fasting serum fibroblast growth factor (FGF)19, which is reciprocally related to fasting serum 7αC4.24 Where the tests are not available, therapeutic trials (eg, with cholestyramine 4 g three times a day (tid); oral colesevelam 625 mg tablets, up to two tablets tid) are indicated.
IS THERE ANOTHER CAUSE OF PERSISTENT SYMPTOMS WHEN THE IBD IS IN REMISSION?
As clinicians, we tend to apply Occam’s razor, or the law of parsimony (‘entities must not be multiplied beyond necessity’), and apply a single diagnosis when the symptoms can all fit that diagnosis. However, just as it is possible for patients with paranoid schizophrenia to have real enemies, this principle is not irrefutable.
Symptomatic patients with IBD but without overt inflammation should be further assessed to exclude conditions that are discussed briefly here.
Small bowel bacterial overgrowth and abnormal motility in IBD
Small bowel bacterial overgrowth (SBO) is more likely to occur in Crohn’s disease, especially in the presence of strictures, fistulae or by-pass surgery with a blind loop. There is also evidence of impaired propulsive function25 resulting in stasis and SBO in inactive Crohn’s disease.
The diagnosis of SBO is challenging because of poor specificity of the breath hydrogen (H2) excretion tests.26 For example, in 150 patients with active Crohn’s disease,27 25.3% had positive glucose-H2 breath test, and these patients reported a higher rate of abdominal complaints, increased stool frequency and lower body weight. There was no correlation with the CDAI; it is unclear why positive tests were more common (26.8%) with exclusive colitis, than with exclusive ileitis (18.8%). Ileocaecal valve resection may be a factor in breath test positivity.28 Cultures of small bowel aspirates are more specific, but may be less sensitive. Ultimately, a therapeutic trial with a poorly absorbed antibiotic (eg, neomycin, metronidazole, quinolone, rifaximin) may be the most practical clinical approach in suspected SBO.
Coeliac disease
About 1 in 80 of the population has coeliac disease,29 the incidence being generally proportional to the prevalence of HLA DQ2 or DQ8 haplotypes. Less than 1% have IBD;3,4 these two relatively rare diseases may occur in the same patient. A database of laboratory tests for coeliac disease and ICD-9 codes for IBD (N=6908) and coeliac disease (N=512) over ~5 years identified 18 patients with both IBD and coeliac disease. These patients were more often female, commonly had extra-intestinal manifestations and more aggressive disease.30 Although the concurrence of IBD and coeliac disease appears rare, it is relatively simple to screen for coeliac disease (serum tissue transglutaminase antibodies).
Pancreatic insufficiency
There is ‘functional evidence’ of pancreatic insufficiency in IBD. In 100 patients with Crohn’s disease and 100 patients with ulcerative colitis, and 100 controls, pancreatic insufficiency and its clinical course over 6-month follow-up were tested using faecal elastase-1 (FE-1). FE-1 was ≤200 µg/g stool in 22 patients with ulcerative colitis and 14 patients with Crohn’s disease. However, at 6-month follow-up, FE-1 test was normal in 24/36 patients. Persistently low stool FE-1 was associated with ≥3 bowel movements per day, loose stools, previous surgery and longer duration of disease, but not with clinically active disease.31
Rarely, structural idiopathic chronic pancreatitis may occur in IBD. A 16-year experience of three hospitals in France showed eight cases with pancreatic duct stenosis, inter- and intra-lobular fibrosis, or acinar regression in addition to exocrine insufficiency.32 In that 1999 article, the literature review identified six patients with ulcerative colitis and 14 with Crohn’s disease with structural chronic pancreatitis.32 Painless chronic pancreatitis with exocrine insufficiency and normal or minimally altered pancreatic ducts on endoscopic retrograde cholangiopancreatography (ERCP) is also described.33
In summary, pancreatic insufficiency should be considered when patients with IBD without significant ileal resection appear to be in remission, and have features suggesting steatorrhoea.
Are pelvic floor dyssynergia and visceral hypersensitivity factors in IBD?
Evacuation disorders are usually confused with IBS-C;6 however, evacuation disorders may cause urgency and overflow diarrhoea34, abdominal or pelvic pain, and bloating, which may mimic IBS-D. Involvement of the rectum and perianal structures, sometimes with fistulation in IBD, may lead to pelvic floor dysfunction (PFD) in patients with IBD. PFD has not been formally assessed in patients with active or inactive IBD. Disorders of ileal pouch evacuation may conceivably cause pouch stasis and predispose to pouchitis. Reduced pouch evacuation is usually attributed to impaired pouch contractility,35 though PFD has not been investigated. As in non-IBD patients, PFD should be excluded in patients with IBD with abdominal pain, bloating or constipation.
It had been postulated that IBD might predispose to afferent hypersensitivity and, therefore, to the potential for ‘IBSlike’ symptoms in patients with IBD. However, the data are conflicting, and the main papers in the literature have evaluated sensitivity in pouch patients. Shen et al36 suggested that some patients have ‘irritable pouch’ because scores of sensation of gas, urge to defaecate, and pain sensation ratings at various distension pressures were significantly higher than in pouchitis and normal pouch patients. However, changes in pouch compliance may influence sensation and afferent hypersensitivity may not be the only cause of higher sensation. Similarly, Bernstein et al37 reported a lower volume threshold for stool sensation in patients with ileal pouches; however, there was also reduced pouch compliance. Conversely, in patients with unoperated ulcerative colitis, discomfort thresholds were higher (suggesting reduced afferent sensitivity) compared with patients with IBS and controls.38 Increased rectal sensitivity and decreased rectal compliance associated with active ulcerative colitis tended to normalise when colitis went into remission suggesting that inflammation itself does not permanently sensitise afferent nerves to cause hypersensitivity.39
In summary, there is no convincing evidence for visceral hypersensitivity in patients with unoperated IBD. Further studies of afferent and pelvic floor functions in patients with IBD presenting with symptoms suggestive of IBS are required.
Irritable bowel syndrome: a real association or reflection of occult inflammation?
Some patients with IBD who are in inflammatory remission with persistent abdominal discomfort and diarrhoea may indeed have IBS! Minderhoud et al identified Rome II IBS-like symptoms in patients with IBD in remission in a third of 73 patients with ulcerative colitis and 42% of 34 patients with Crohn’s disease who were in remission. The presence of IBS-like symptoms impaired quality of life (QOL).40 Simren et al documented lower levels of global well-being (PGWB) and QOL and higher levels of anxiety and depression in patients with IBD in remission who have IBS-like symptoms. Anxiety and duration of disease were significant predictors of developing IBS-like symptoms whereas age, use of chronic therapy for IBD or for BAM, and disease extent were not significant risk factors.41
In a study of patients with IBD in remission (CDAI ≤150 and UCDAI ≤3, and serum CRP <10, while off corticosteroids or biologics), IBS-like symptoms were common; however, abnormal faecal calprotectin levels suggest that the mechanism in most cases was occult inflammation rather than coexistent IBS. Faecal calprotectin levels and HAD scores were higher and QOL scores lower in patients with Crohn’s disease and those with ulcerative colitis with than in those without IBS-type symptoms.42
Long and Drossman43 proposed that peripheral and central factors influence symptom generation in both IBD and IBS. However, it is still unclear what factors determine the presentation of overt inflammatory changes of IBD rather than pauci-inflammatory bowel disturbance of IBS.
CONCLUSION
In patients with IBD in remission in whom residual inflammation or enteral infection are excluded, there may be another disease to which symptoms may be attributed. Effective and relatively inexpensive screening serological tests are available to exclude caeliac disease and BAM. SBO can be suspected based on the anatomical derangements resulting from Crohn’s disease or the effects of bypass surgery. There is no easy, sensitive and specific test for SBO, and a therapeutic trial with a poorly absorbed antibiotic may be the most effective approach. Significant chronic pancreatic insufficiency appears to be extremely rare. Ultimately, some patients may have IBS too, and this may be a reflection of a common diathesis for IBD and IBS. In these patients, the IBS can be managed symptomatically. However, it is recommended that anti-motility agents be used judiciously to avoid potential complications of IBD. Measuring gastrointestinal and colonic transit44,45 may individualise and optimise therapy for the patients with significantly accelerated transit.
Acknowledgement
The author thanks Mrs Cindy Stanislav for excellent secretarial assistance.
Funding NIH grants R01-DK079866 and 1RC1-DK086182.
Footnotes
Competing interests None.
Provenance and peer review Commissioned; externally peer reviewed.
REFERENCES
- 1.Halder SL, Locke GR, 3rd, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Loftus CG, Loftus EV, Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 4.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographical distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Zaman MS, Robson KM, Lembo AJ. Overlap of irritable bowel syndrome (IBS) symptoms in patients with inflammatory bowel disease (IBD) Am J Gastroenterol. 2002;97:S284. [Google Scholar]
- 6.Camilleri M. Do the symptom-based, Rome criteria of irritable bowel syndrome lead to better diagnosis and treatment outcomes? The con argument. Clin Gastroenterol Hepatol. 2009;8:129. doi: 10.1016/j.cgh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 8.Carroccio A, Iacono G, Cottone M, et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhoea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49(6 Pt 1):861–867. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 9.Kane SV, Sandborn WJ, Rufo PA, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 10.Otten CM, Kok L, Witteman BJ, et al. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–1280. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 11.Langhorst J, Junge A, Rueffer A, et al. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:404–410. doi: 10.1038/ajg.2008.86. [DOI] [PubMed] [Google Scholar]
- 12.Schoepfer AM, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32–39. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 13.Schoepfer AM, Schaffer T, Seibold-Schmid B, et al. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil. 2008;20:1110–1118. doi: 10.1111/j.1365-2982.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 14.Manolakis AC, Kapsoritakis AN, Georgoulias P, et al. Moderate performance of serum S100A12, in distinguishing inflammatory bowel disease from irritable bowel syndrome. BMC Gastroenterol. 2010;10:118. doi: 10.1186/1471-230X-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–1713. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara AK, Leighton JA, Heigh RI, et al. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128–134. doi: 10.1148/radiol.2381050296. [DOI] [PubMed] [Google Scholar]
- 17.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113–121. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher JG, Huprich J, Loftus EV, Jr, et al. Computerized tomography enterography and its role in small-bowel imaging. Clin Gastroenterol Hepatol. 2008;6:283–289. doi: 10.1016/j.cgh.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Rimola J, Ordás I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: Validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. doi: 10.1002/ibd.21551. Published Online First: 8 Nov 2010. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2009.01288.x. 734–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenicek M, Duricova D, Komarek V, et al. Bile acid malabsorption in inflammatory bowel disease: Assessment by serum markers. Inflamm Bowel Dis. doi: 10.1002/ibd.21502. Published Online First: 5 Nov 2010. [DOI] [PubMed] [Google Scholar]
- 23.Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn’s disease and indications for its assessment using SeHCAT. Gut. 1994;35:90–93. doi: 10.1136/gut.35.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walters JR, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Annese V, Bassotti G, Napolitano G, et al. Gastrointestinal motility disorders in patients with inactive Crohn’s disease. Scand J Gastroenterol. 1997;32:1107–1117. doi: 10.3109/00365529709002989. [DOI] [PubMed] [Google Scholar]
- 26.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klaus J, Spaniol U, Adler G, et al. Small intestinal bacterial overgrowth mimicking acute flare as a pitfall in patients with Crohn’s Disease. BMC Gastroenterol. 2009;9:61. doi: 10.1186/1471-230X-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutgeerts P, Ghoos Y, Vantrappen G, et al. Ileal dysfunction and bacterial overgrowth in patients with Crohn’s disease. J Clin Invest. 1981;11:199–206. doi: 10.1111/j.1365-2362.1981.tb01841.x. [DOI] [PubMed] [Google Scholar]
- 29.Logan I, Bowlus CL. The geoepidemiology of autoimmune intestinal diseases. Autoimmun Rev. 2010;9:A372–A378. doi: 10.1016/j.autrev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Alam M, Shen B, Achkar JP, et al. Concurrent inflammatory bowel disease (IBD) and celiac disease (CeD): an aggressive form of IBD? Am J Gastroenterol. 2003;98:S80–S81. [Google Scholar]
- 31.Maconi G, Dominici R, Molteni M, et al. Prevalence of pancreatic insufficiency in inflammatory bowel diseases. Assessment by fecal elastase-1. Dig Dis Sci. 2008;53:262–270. doi: 10.1007/s10620-007-9852-y. [DOI] [PubMed] [Google Scholar]
- 32.Barthet M, Hastier P, Bernard JP, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94:2141–2148. doi: 10.1111/j.1572-0241.1999.01287.x. [DOI] [PubMed] [Google Scholar]
- 33.Seyrig JA, Jian R, Modigliani R, et al. Idiopathic pancreatitis associated with inflammatory bowel disease. Dig Dis Sci. 1985;30:1121–1126. doi: 10.1007/BF01314044. [DOI] [PubMed] [Google Scholar]
- 34.Crowell MD, Lacy BE, Schettler VA, et al. Subtypes of anal incontinence associated with bowel dysfunction: clinical, physiologic, and psychosocial characterization. Dis Colon Rectum. 2004;47:1627–1635. doi: 10.1007/s10350-004-0646-4. [DOI] [PubMed] [Google Scholar]
- 35.Shen B, Achkar JP, Lashner BA, et al. Irritable pouch syndrome: a new category of diagnosis for symptomatic patients with ileal pouch-anal anastomosis. Am J Gastroenterol. 2002;97:972–977. doi: 10.1111/j.1572-0241.2002.05617.x. [DOI] [PubMed] [Google Scholar]
- 36.Shen B, Sanmiguel C, Bennett AE, et al. Irritable pouch syndrome is characterized by visceral hypersensitivity. Inflamm Bowel Dis. doi: 10.1002/ibd.21412. Published Online First 3 Aug 2010. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein CN, Rollandelli R, Niazi N, et al. Characterization of afferent mechanisms in ileoanal pouches. Am J Gastroenterol. 1997;92:103–108. [PubMed] [Google Scholar]
- 38.Chang L, Munakata J, Mayer EA, et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao SS, Read NW, Davison PA, et al. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology. 1987;93:1270–1275. doi: 10.1016/0016-5085(87)90255-1. [DOI] [PubMed] [Google Scholar]
- 40.Minderhoud IM, Oldenburg B, Wismeijer JA, et al. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469–474. doi: 10.1023/b:ddas.0000020506.84248.f9. [DOI] [PubMed] [Google Scholar]
- 41.Simrén M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 42.Keohane J, O’Mahony C, O’Mahony L, et al. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788–1794. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- 43.Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what? A challenge to the functional-organic dichotomy. Am J Gastroenterol. 2010;105:1796–1798. doi: 10.1038/ajg.2010.162. [DOI] [PubMed] [Google Scholar]
- 44.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22 doi: 10.1111/j.1365-2982.2009.01442.x. 293–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadik R, Stotzer PO, Simrén M, et al. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008;20:197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]