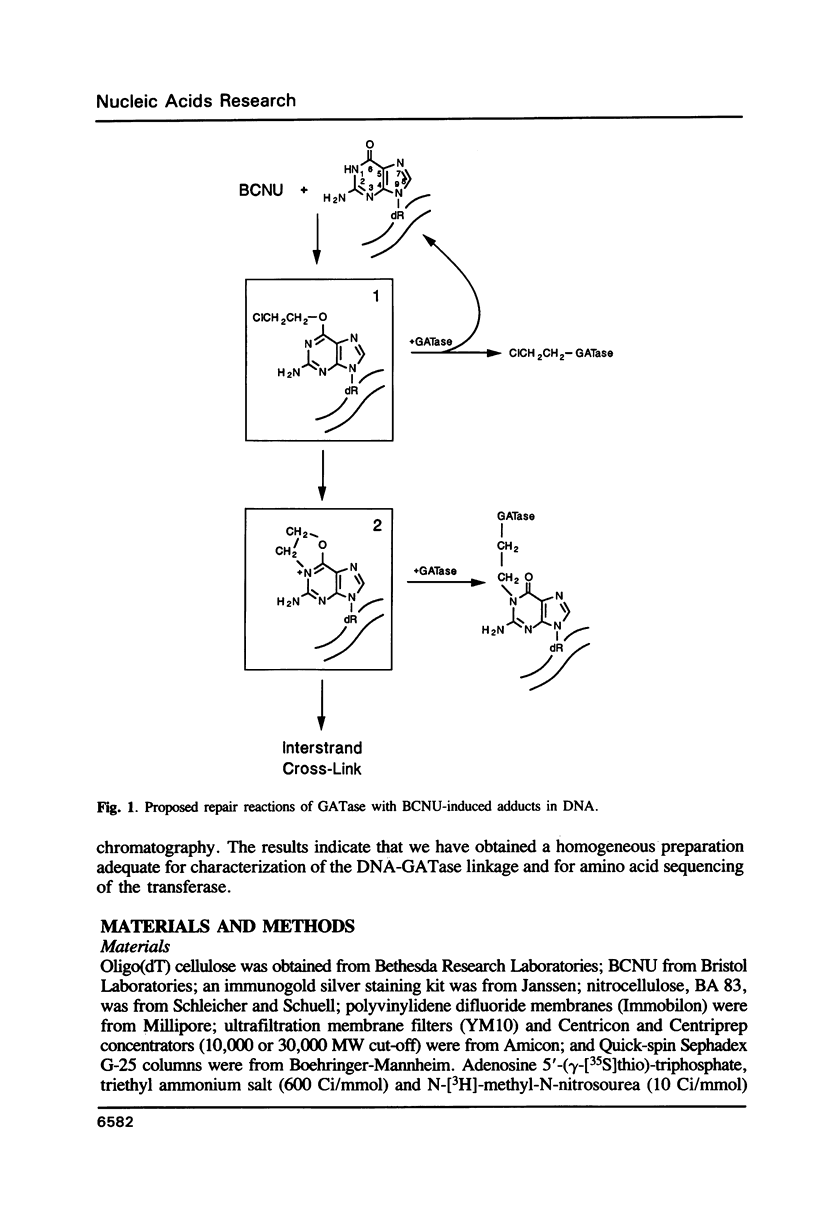
Abstract
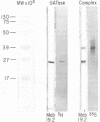
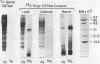
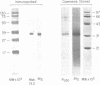
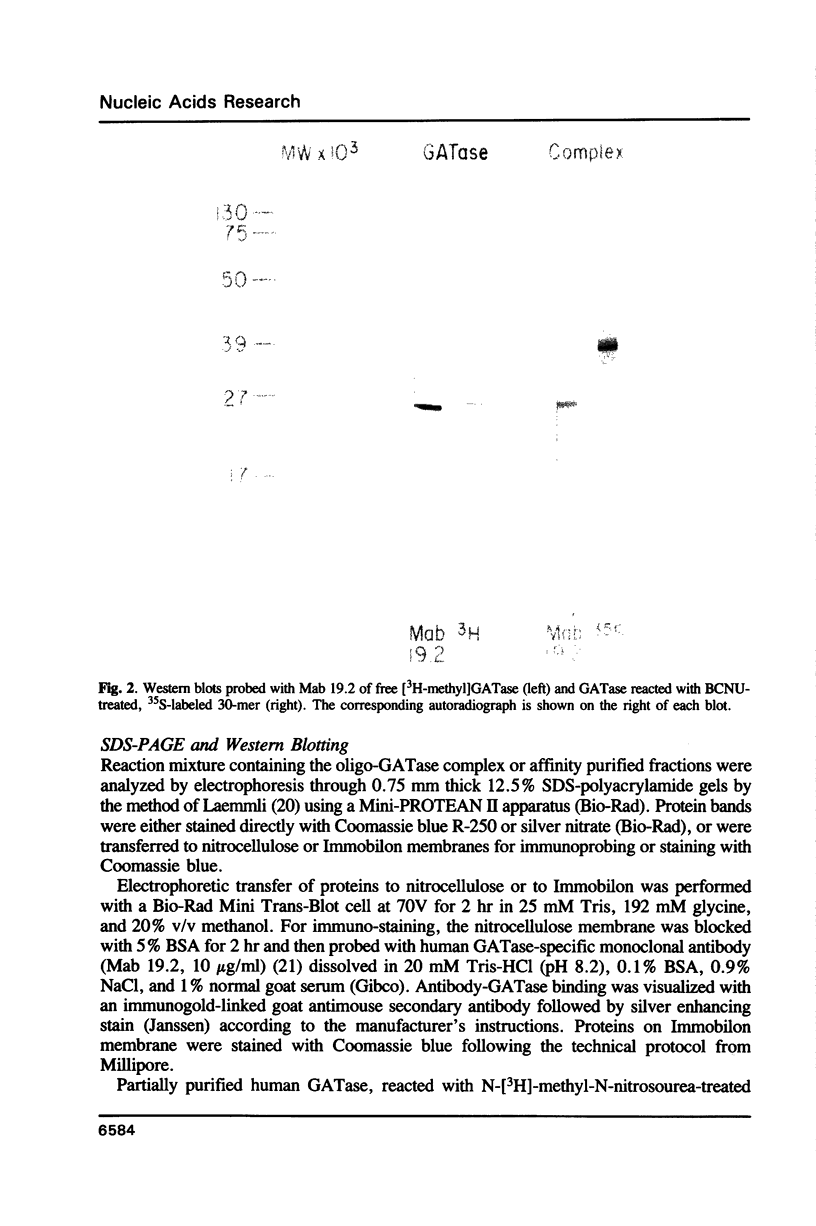
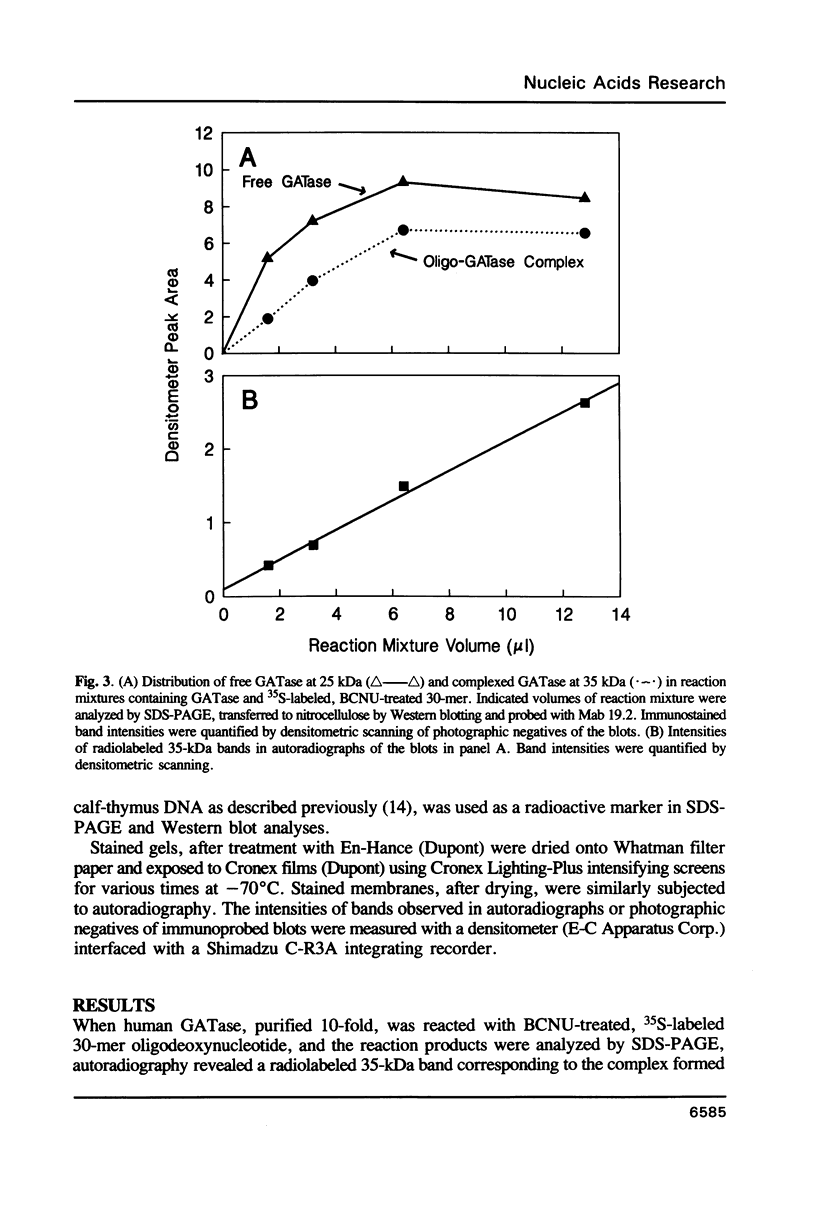
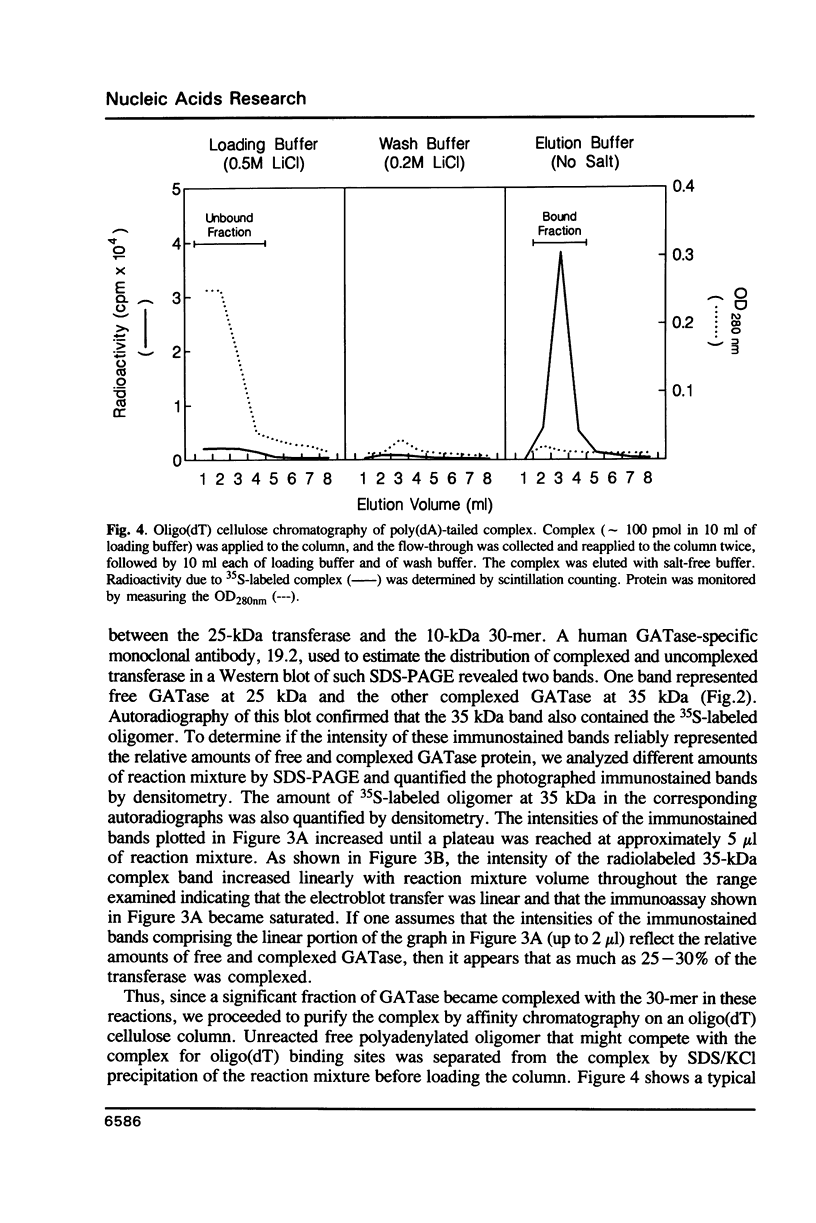
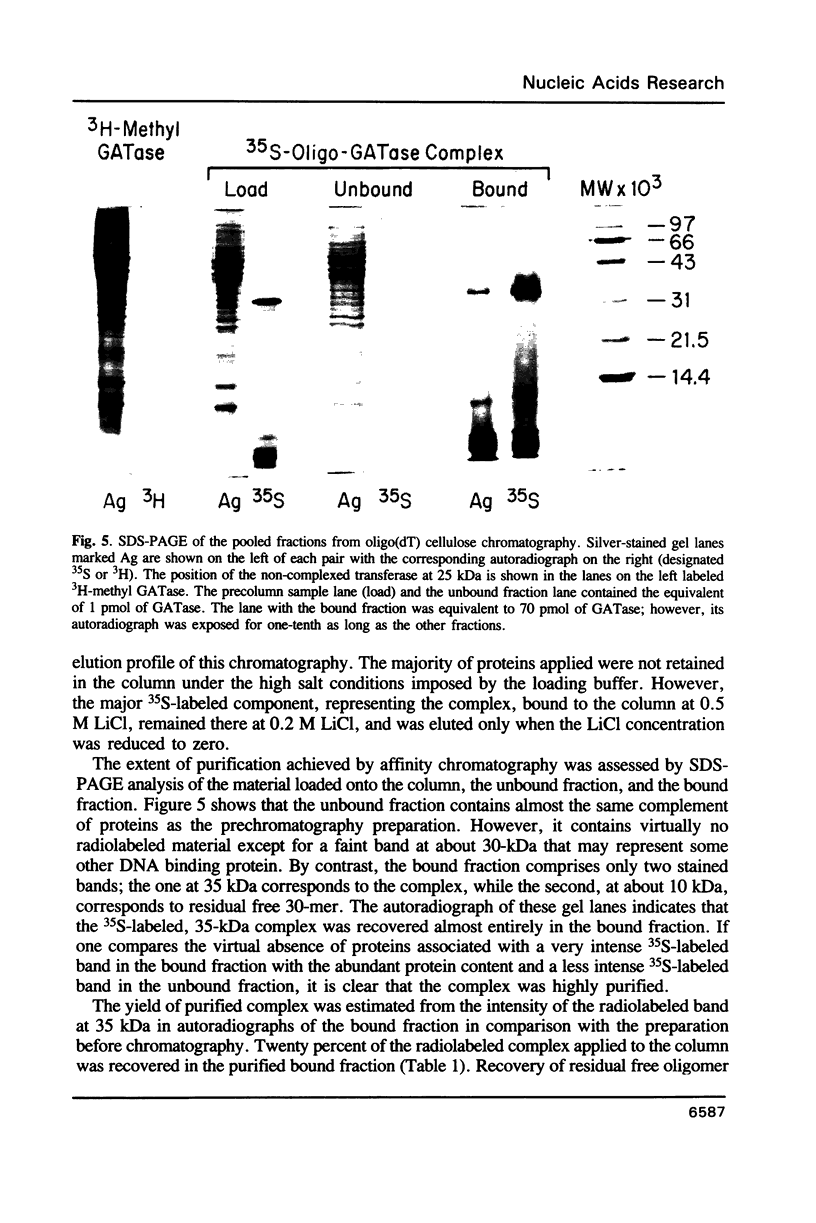
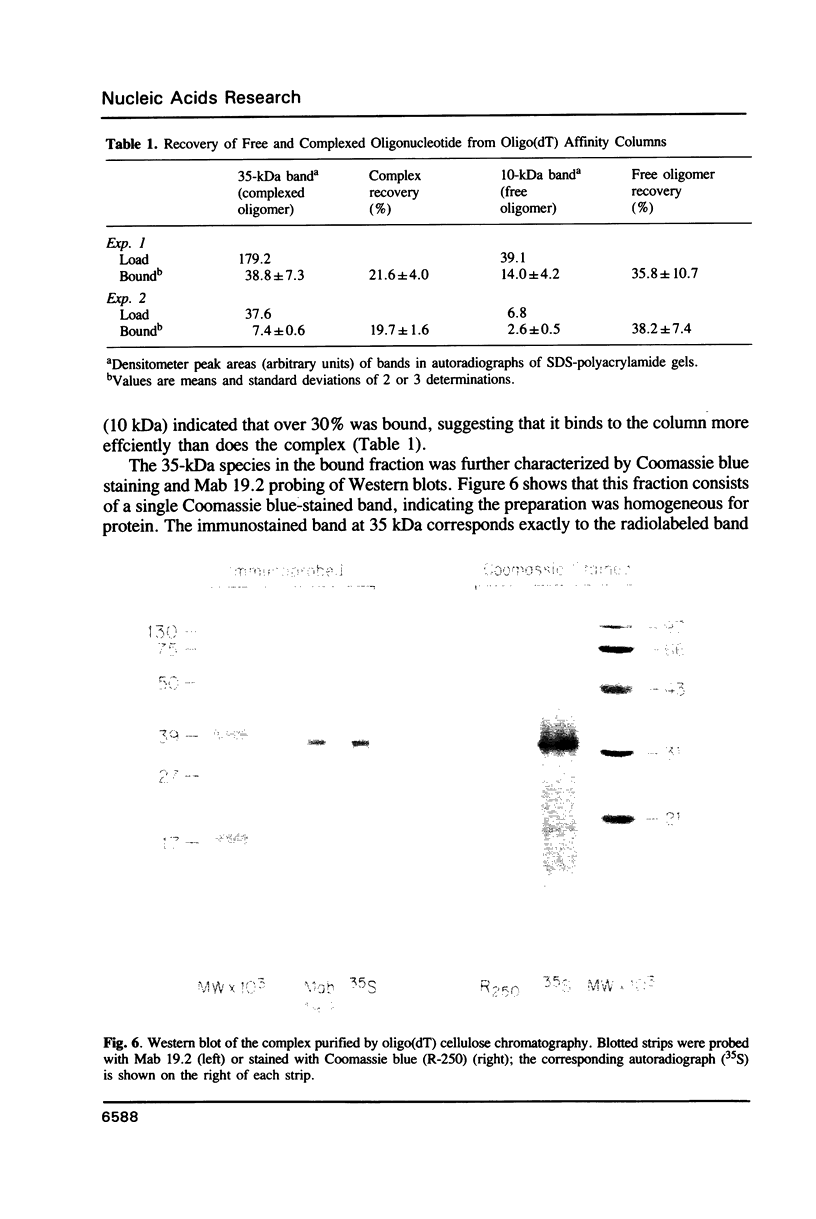
Tumor cells resistant to chloroethylnitrosourea (CENU) therapy contain high levels of O6-alkylguanine DNA-alkyltransferase (GATase), a DNA repair enzyme that aborts DNA interstrand cross-linking by removing CENU-induced O6-alkylguanine adducts. Because the transferase binds covalently to CENU-treated oligonucleotides, we reacted partially purified GATase from cultured human lymphoblasts with a BCNU-treated, 35S-5'-end-labeled, synthetic oligonucleotide designed to have a polyadenylated 3' terminus. Immunoprobing Western blots of this reaction mixture with GATase-specific monoclonal antibody indicated that 25-30% of the transferase became complexed. We purified this complex by affinity chromatography with oligo(dT) cellulose, recovering homogenous material that appeared as a discrete 35-kDa Coomassie blue or silver-stained band after SDS-polyacrylamide gel electrophoresis. Autoradiography and Western immunoblotting confirmed that this band contained both the radiolabeled oligonucleotide and the GATase protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P., Houghton P. J., Houghton J. A. O6-Alkylguanine-DNA alkyltransferase activity correlates with the therapeutic response of human rhabdomyosarcoma xenografts to 1-(2-chloroethyl)-3-(trans-4-methylcyclohexyl)-1-nitrosourea. Proc Natl Acad Sci U S A. 1985 May;82(9):2985–2989. doi: 10.1073/pnas.82.9.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P. Isolation and purification of O6-alkylguanine-DNA alkyltransferase from human leukemic cells. Prevention of chloroethylnitrosourea-induced cross-links by purified enzyme. Pharmacol Ther. 1985;31(1-2):121–140. doi: 10.1016/0163-7258(85)90040-3. [DOI] [PubMed] [Google Scholar]
- Brent T. P., Remack J. S. Formation of covalent complexes between human O6-alkylguanine-DNA alkyltransferase and BCNU-treated defined length synthetic oligodeoxynucleotides. Nucleic Acids Res. 1988 Jul 25;16(14B):6779–6788. doi: 10.1093/nar/16.14.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P., Smith D. G., Remack J. S. Evidence that O6-alkylguanine-DNA alkyltransferase becomes covalently bound to DNA containing 1,3-bis(2-chloroethyl)-1-nitrosourea-induced precursors of interstrand cross-links. Biochem Biophys Res Commun. 1987 Jan 30;142(2):341–347. doi: 10.1016/0006-291x(87)90279-8. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Suppression of cross-link formation in chloroethylnitrosourea-treated DNA by an activity in extracts of human leukemic lymphoblasts. Cancer Res. 1984 May;44(5):1887–1892. [PubMed] [Google Scholar]
- Briscoe W. T., Duarte S. P. Preferential alkylation by 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) of guanines with guanines as neighboring bases in DNA. Biochem Pharmacol. 1988 Mar 15;37(6):1061–1066. doi: 10.1016/0006-2952(88)90511-4. [DOI] [PubMed] [Google Scholar]
- Connors T. A. Alkylating agents, nitrosoureas and alkyltriazenes. Cancer Chemother Biol Response Modif. 1987;9:23–35. [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludlum D. B., Mehta J. R., Tong W. P. Prevention of 1-(3-deoxycytidyl),2-(1-deoxyguanosinyl)ethane cross-link formation in DNA by rat liver O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1986 Jul;46(7):3353–3357. [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res. 1986 Apr 11;14(7):2971–2987. doi: 10.1093/nar/14.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B., Renard A., Verly W. G. Repair of O6-ethylguanine in DNA by a chromatin fraction from rat liver: transfer of the ethyl group to an acceptor protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6766–6770. doi: 10.1073/pnas.78.11.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Scicchitano D., Morimoto K., Dolan M. E. Specificity of O6-alkylguanine-DNA alkyltransferase. IARC Sci Publ. 1987;(84):30–34. [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Renard A., Verly W. G. A chromatin factor in rat liver which destroys O6-ethylguanine in DNA. FEBS Lett. 1980 May 19;114(1):98–102. doi: 10.1016/0014-5793(80)80868-4. [DOI] [PubMed] [Google Scholar]
- Scicchitano D., Jones R. A., Kuzmich S., Gaffney B., Lasko D. D., Essigmann J. M., Pegg A. E. Repair of oligodeoxynucleotides containing O6-methylguanine by O6-alkylguanine-DNA-alkyltransferase. Carcinogenesis. 1986 Aug;7(8):1383–1386. doi: 10.1093/carcin/7.8.1383. [DOI] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Bedell M. A., Billings K. C., Umbenhauer D. R., Pegg A. E. Cell-specific differences in O6-alkylguanine DNA repair activity during continuous exposure to carcinogen. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5499–5502. doi: 10.1073/pnas.79.18.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Mechanism of action of the nitrosoureas--V. Formation of O6-(2-fluoroethyl)guanine and its probable role in the crosslinking of deoxyribonucleic acid. Biochem Pharmacol. 1983 Jul 1;32(13):2011–2015. doi: 10.1016/0006-2952(83)90420-3. [DOI] [PubMed] [Google Scholar]
- Trask D. K., DiDonato J. A., Muller M. T. Rapid detection and isolation of covalent DNA/protein complexes: application to topoisomerase I and II. EMBO J. 1984 Mar;3(3):671–676. doi: 10.1002/j.1460-2075.1984.tb01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]