This study suggests that robotic single-port inguinal hernia surgery is feasible, safe, and efficient.
Keywords: Inguinal hernia, Total extraperitoneal, Robotic Freehand®, Tri-port™
Abstract
Background and Objectives:
Since the introduction of single-incision laparoscopic surgery in 2009, an increasing number of surgical procedures including hernia repair are being performed using this technique. However, its large-scale adoption awaits results of prospective randomized controlled studies confirming its potential benefits. Parallel with single-port surgery development, the issue of the chronic lack of good camera assistants is being addressed by the robotic Freehand® camera controller, which has the potential to replace camera assistants in a large percentage of routine laparoscopic surgery. Although the robotic Freehand has been used in certain operations in urology and gynecology, there have been no published reports in robotic (single-port) hernia surgery.
Methods:
This study reports the first case and a series of 16 patients who underwent robotic single-port total extraperitoneal inguinal hernia repair compared to 16 consecutive cases of conventional single-port inguinal hernia repair. Patients were matched for age, sex, body mass index, American Society of Anesthesiologists classification, and types of hernia.
Results:
Although operation time was comparable in both, the time wasted for scope cleaning was 8.5 minutes for conventional compared to 1.5 minutes for robotic surgery.
Conclusion:
Robotic single-port inguinal hernia repair is feasible and efficient. This represents a further milestone in laparoscopic surgery.
INTRODUCTION
The introduction of laparoscopic surgery for treating many common surgical conditions, such as gallbladder disease1 and abdominal wall hernias, has transformed the landscape of surgery. Since its first introduction in the early 1990s, laparoscopic inguinal hernia repair has now become the gold standard operation in Australia. Indeed, in 2010, it represented 44% of all inguinal hernia repairs (www.medicareaustralia.gov.au) with the rest being repaired by various open techniques including the Lichtenstein, Bassini, and Shouldice repair. However, this is not the case for most other countries including the United States where the anterior repair predominates.
The techniques of laparoscopic repair, whether it is total extraperitoneal (TEP) or transabdominal preperitoneal (TAPP), have been standardized over the last 20 years such that as far as the technical aspect of the operation is concerned, there has been no new innovation. Instead, many of the new “innovations” have been concentrated on the vast array of mesh prosthetics with many hundreds being available for just one operation. This industry-driven “progress” has resulted in the blunting of real innovations in the art of hernia surgery.
Single-incision laparoscopic surgery, first reported in 20092 probably represents the single most exciting innovation in laparoscopic surgery of the last 2 decades. The main premise of single-port surgery is the use of completely blunt ports, which will negate the risks of bowel and vascular injuries related to the use of sharp secondary trocars in the traditional 3-port surgery; the latter has been shown to cause 0.16% incidence of bowel injuries in a series of 37,000 laparoscopic procedures.3,4 Although the experience in single-port hernia surgery is still in its infancy, the published series to date5–7 have shown it to be safe and efficient. Admittedly, such good results have been obtained by highly committed hernia surgeons in dedicated hernia centers. Its proliferation must be carefully supported by workshops and proctorships so that the past errors experienced with the introduction of laparoscopic cholecystectomy and hernia surgery will not be repeated.
Parallel with the introduction of single-port surgery is the introduction of robotic Freehand® (Prosurgics, Blacknell, UK) surgery, which has the potential to revolutionize the way laparoscopic surgery is performed, especially after-hours when camera assistants (residents) are hard to come by. In Australia, much elective surgery is performed in private hospitals where training residents, to date, are usually not available. Instead, most surgical assistants are local medical officers with varying degrees of competency in camera holding. The robotic Freehand®, which won the 2008 prize for the best surgical innovation by the Society of Laparoendoscopic Surgeons, has increasingly been integrated into laparoscopic procedures. Its use has so far been limited to small case series in urology and gynecology,8–11 but none in hernia surgery.
This study aims to report the first case and first series of robotic single-port TEP inguinal hernia repair compared to “traditional” Laparo-Endoscopic Single Site (LESS™) TEP hernia repair. The study met with the approval of the Independent Review Board of the Holroyd Private Hospitals for the purpose of data collection from patients.
PRELIMINARY WORK
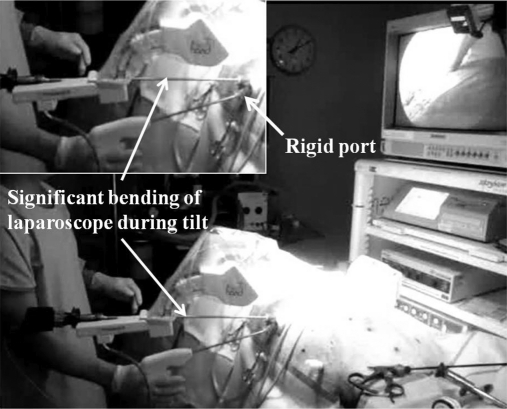
A review of Medline, GoogleScholar, and Prosurgics Web site (maker of robotic Freehand) in October 2010 revealed no reports of the use of the robotic Freehand in laparoscopic inguinal hernia repair let alone single-port hernia repair. The author, having performed over 150 cases of single incision laparoscopic TEP inguinal hernia repair in the past 12 months, embarked on a project to assess the feasibility of integrating robotic Freehand into single-port hernia surgery. Following an office setup of the robotic Freehand and a trial of controlling the robotic arm with head movements and the use of a foot pedal, an animal model (swine) of a laparoscopic ventral hernia repair (LVHR) procedure was simulated. This opportunity arose from a laparoscopic ventral hernia repair workshop (run by the author), which had received approval by the Western Sydney Area Health and Animal Ethics Committee, Westmead Australia. The animal was euthanized before the simulation with the robotic Freehand. The robotic Freehand was set up (Figure 1), and 2 different ports (SILS [Covidien, Norwalk, Connecticut, USA] and Tri-port [Olympus Winter & Ibe GmbH, Hamburg, Germany]) were used to assess their applicability in robotic hernia surgery. This trial was positive in that the dissecting instruments could be manipulated in the same way as a conventional single-port LVHR and that rock steady image was obtained with precise control of the camera directed to the area where the surgeon is operating. However, it was found that the relative rigidity of the SILS port resulted in significant and potentially damaging bending of the 5.5-mm/52-cm/30° -angled laparoscope (Karl-Storz, Hamburg, Germany) that is used routinely for single-port hernia surgery. Such bending was noted to be especially prominent during vertical movement. This was not experienced to any extent with the floppy Tri-port. Hence, robotic single-port hernia surgery with the 5-mm and 52-cm laparoscope is only possible with floppy single ports.
Figure 1.
Simulated robotic Freehand® laparoscopic ventral hernia repair in a swine model.
MATERIALS AND METHODS
The study period was from October to December 2010. Patients on routine lists for LESS TEP inguinal hernia repair were selected for robotic single-port surgery based on time availability. It was anticipated that the initial introduction would not permit the smooth running of the operating lists and that only 1 or 2 cases per list could be performed robotically. Typically, these patients would be first on the list. Our patients are placed on the operating lists on a first-come-first-serve basis unless they have medical conditions like diabetes that would necessitate being placed first on the list. These robotic cases were compared with a similar number of consecutive “conventional” LESS TEP repair in the same period.
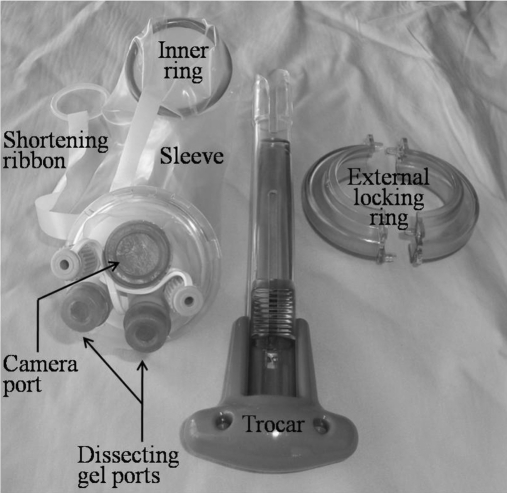
The LESS TEP inguinal hernia repair is performed in a standard fashion. Briefly, the patient is prepped from epigastrium to mid thighs and draped, exposing a 3-cm window of prepped skin from just above the umbilicus to the pubic symphysis. This ensures minimal skin exposure. After filtration with 20mL of Marcaine with 1:200,000 Adrenaline inferior to the umbilicus and on the rectus sheath on the side of port entry, a 2-cm crescentic incision is made within the confines of the umbilicus. The rectus sheath on the side of the hernia is dissected and incised to no more than 2cm. With the patient being placed in the Trendelenburg position, the extraperitoneal space on the side of the hernia is dissected with a balloon (Covidien, Norwalk, Connecticut, USA). The inner ring of the Tri-port (Figure 2) is then placed in the blunt trocar, and after lubrication with a lubricant, the trocar is then inserted into the extraperitoneal space and the inner ring is deployed. The excess sheath and ribbon is then alternately shortened until the inner ring is firmly pulled up against the deep surface of the anterior rectus sheath. The outer ring is then applied to the port and is additionally locked in position with towel clips to prevent dislodgement of the outer rings during the operation. After small stab incisions are made into the gel ports, a blunt 10-mm trocar is then inserted into the 10-mm gel port into the extraperitoneal space past the inner ring. After insufflations with CO2, the position of the trocar is confirmed with insertion of the laparoscope. Because the skin and fascial incisions are no more than 2cm, the available space within the plastic sheath is no more than 20mm and hence with the 12-mm actual external diameter of the 10-mm port would not permit insertion of 2-mm x 5-mm dissecting instruments easily. The 10-mm port is pulled back along the laparoscope at the same time as the dissecting instruments are inserted into the immediately available space in line with the scope (Figure 3). If the scope needs to be cleaned, then one of the dissecting instruments is withdrawn, and the 10-mm port is re-inserted into the extraperitoneal space past the inner ring before the scope is withdrawn. Once cleaned, the scope is inserted into the extraperitoneal space, and the dissecting instrument is inserted at the same time as the 10-mm port is withdrawn as before.
Figure 2.
Components of Tri-port™.
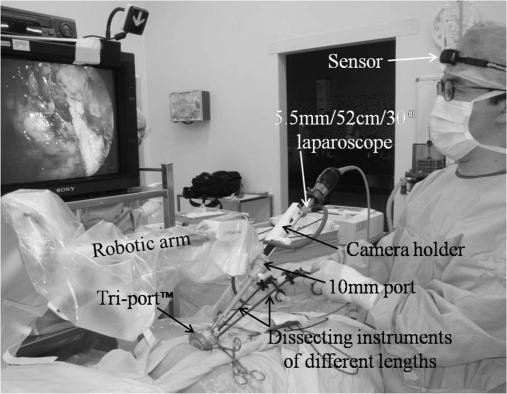
Figure 3.
Patient setup for robotic Freehand® single-port TEP inguinal hernia repair.
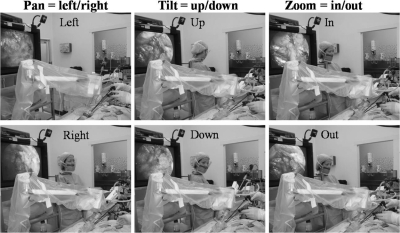
Patient setup for single-port robotic Freehand TEP inguinal hernia repair is as for conventional single-port repair, except with the placement of the robotic arm positioned over the Tri-port after its placement (Figure 3). This usually requires some adjustments to ensure maximal range of movements with minimal clashing with dissecting instruments. Once the laparoscope has been inserted into the camera holder and secured, the movements of the robotic arm are then precisely controlled by the surgeon's head movements with the sensor being worn on his forehead. Once the direction of movement is achieved, the precise and incremental movement of the robotic arm is controlled with the foot pedal. The basic movements of the robotic arm are Pan=left/right, Tilt=up/down, and Zoom=in/out (Figure 4). In this way, any combination of robotic arm movements can be achieved, allowing for precise dissection and a rock steady image.
Figure 4.
Basic robotic Freehand® movements: Pan, tilt and zoom.
The principles of dissection for TEP inguinal hernia repair are the same whether it is the traditional 3 ports, single-port, or robotic single-port repair; namely, identification and dissection of the suprapubic space (for orientation and to avoid bladder injury), dissection of the lateral space, and then complete reduction of any indirect sac together with any associated lipoma of the cord and adequate proximal dissection of the peritoneum for placement of a 12-cm to 15-cm light weight mesh. This is fixed with 2 Protack staples (Covedien, Norwalk, USA) in the midline and 1 laterally. As the CO2 is released and the patient moved into reversed Trendelenburg position, the descent of the peritoneum over the mesh is observed carefully. The fascial defect is closed with 1 Nylon suture continuously, and the skin wound is closed with subcutaneous and subcuticular dissolvable sutures.
The relative lack of triangulation with single-port surgery can be overcome with the use of a smaller and longer 30° angled laparoscope, dissecting instruments of different lengths and modified dissection techniques, such as “vertical” and “inline” dissection.7 Although angulated instruments and flexible laparoscope could have been used, the costs and complexity of the single-port procedure would have increased, and therefore these were not used in this study.
The following data were prospectively collected: age and sex of the patients, ASA grading, body mass index, side of the hernia, type of hernia (direct/indirect), number of times the scope was cleaned, duration of each cleaning episode, duration of surgery (from skin incision to skin closure time), wound infection, length of hospital stay, patient satisfaction, and recurrence (early failure) on follow-up. Patients were asked to rank their satisfaction with the operation 1-month postoperatively: highly satisfied, satisfied, or dissatisfied.
RESULTS
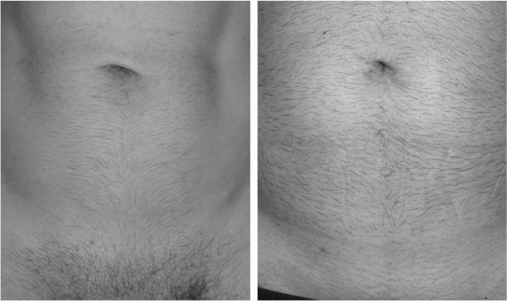
From October to December 2010, 16 patients underwent robotic Freehand TEP inguinal hernia repair, and these were compared with 16 consecutive “conventional” unilateral LESS TEP inguinal hernia repairs in the same period. Patients were matched in terms of age, sex, ASA, BMI, and type of hernia (Table 1). The number of times the scope needed to be cleaned was significantly lower with the robotic group (1.5 times) compared with the “conventional” single-port group (8 times) (P=.01). This resulted in the total amount of time spent on cleaning the scope from 8 minutes during the “conventional” single-port hernia repair to 1.5 minutes during robotic surgery (P=.01). The duration of surgery was similar in both groups: 48 minutes for robotic compared to 52 minutes for “conventional” single-port hernia repair. Two patients in each group also had concomitant umbilical hernia repair. There were no wound infections in either group. Virtually all patients in both groups were highly satisfied with the operation: 14 highly satisfied and 2 satisfied in both groups. All patients were impressed with their virtually scarless incisions (Figure 5). There was no early failure on follow-up with all patients having been seen at least 6 months after their operation.
Table 1.
Comparison of Robotic vs Conventional Single-port Total Extraperitoneal Inguinal Hernia Repaira
| Robotic | Conventional | |
|---|---|---|
| Age | 46 | 48 |
| ASA | 1 | 1 |
| BMI (Kg/m2) | 28.4 | 29.2 |
| Type of hernia (direct/indirect) | 6/10 | 6/10 |
| Operation time (min) | 48 (range 35-95) | 52 (range 40-125) |
Patients were matched for age, body mass index (ASA), American Society of Anesthesiologists (ASA), type of hernia and operation time.
Figure 5.
Virtually scarless incisions after robotic Freehand® TEP inguinal hernia repair.
DISCUSSION
This study reports not only the first known case but also the first series of robotic Freehand TEP inguinal hernia repair compared to “conventional” single-port repair. The concept of SILS has been attributed to Wittmoser,12 who performed thoracoscopic sympathectomy in the early 1970s via a single intercostal incision through which all the instruments were inserted into a multifunctional port. Although Pelosi13 first described the technique of single umbilical puncture for laparoscopic appendicectomy in 1992, the first case of single-port TEP inguinal hernia repair was only performed in 2008.2 There have, to date, been only a handful of reports of single-port inguinal hernia repair with the largest series reporting 100 cases.5–7 These reports have shown that single-port hernia repair is safe and efficient. While the number of inguinal hernia repairs being performed with the single-port is increasing exponentially, it only comes from a very low base, which means that large-scale adoption by laparoscopic hernia surgeons will take time. This is only likely to occur once sufficient evidence-based benefits (in the form of prospective randomized controlled studies) have been carried out and that additional benefits can be demonstrated. One such trial has received approval by the Human Ethics Committee of the Holroyd Private Hospital, Sydney, Australia, and the author will commence this trial in early 2011 with a total of 100 patients being enrolled into 2 arms of the study. This study is expected to complete enrollment in the same year with the final results being available in mid 2012. It is expected that other hernia centers will follow suit so that the results from different centers can be pooled to obtain even more statistically powerful results.
At the start of the robotic single-port study, the author had performed over 150 cases of single-port TEP inguinal hernia repair and had in fact been performing single-port hernia surgery for inguinal (and ventral) hernia repair since September 2009. It seemed a natural and logical progression to take single-port hernia surgery one step further into the realm of robotic surgery. The latter is obviously feasible, and although the number of cases of robotic Freehand TEP inguinal hernia repair having been performed is still small (16 cases), it has been shown to be safe and efficient with the time taken for robotic surgery to be the same as that of “conventional” single-port TEP repair. Although the robotic arm will never be as instantaneous as a highly competent and intuitive camera assistant, the latter cannot predict accurately 100% of the time the movements of the dissecting instruments, and this results in significant time wasting in having to clean the scope. On average, 8.5 minutes was spent on cleaning the scope for the “conventional” compared to 1.5 minutes for the robotic Freehand TEP hernia repair. This seems to have more than made up for the additional time spent on remotely moving the robotic arm. It should be added that although each scope-cleaning episode wastes 1 minute, the actual time wasted will be longer, because the surgeon will need to re-orientate himself to the area where he was working before. This further increases the time wasted during conventional single-port hernia surgery and adds to the time saved during robotic surgery.
Although a good camera assistant can follow the dissecting instruments and ensure that the area of dissection is in the center of the image, the robotic Freehand procedure can achieve the same by the retracting instrument moving the area of dissection into view. In this way, there is less need to constantly moving the robotic arm. This adaptation of dissection only comes from the experience of the surgeon in being able to perform the dissection whatever the environment by the 2 hands working in collaboration. This is innate surgical innovation.
There are however drawbacks in terms of costs with robotic surgery. The single-use camera holder currently costs US $160, and currently its use in privately insured patients is not covered by the medical insurance companies even though the equivalent cost for the camera assistant is $280 for an inguinal hernia repair. This would mean that such patients would be liable for the additional costs. However, in Workers Compensation cases the disposables are fully covered. For patients who are self-funding for the procedure, the additional cost of the disposable is balanced out by not having to use the assistant, ie, it is cost neutral.
The second case that was attempted was a left inguinal hernia repair, and it quickly became apparent that, due to the design of the robotic arm and the position of the camera holder, it was not possible to perform left-sided single-port inguinal hernia repair robotically. This case underwent the “conventional” single-port TEP hernia repair and was not included in the study. Therefore, all cases of robotic single-port hernia repair in this study were for right-sided inguinal hernias. It is however possible to perform left-sided single-port hernia repair robotically but only with the flexible laparoscope such as Endo-Eye (Olympus, Hamburg, Germany), because the robotic arm could be moved away from the dissecting instruments where the tip of the laparoscope is angulated.
It is important to note that in most Western countries efforts are being made to reduce the time residents spend at work during the working week. Implementation of such directives14 means that there is less time available for residents to do menial tasks, such as holding the camera during laparoscopic surgery, and more time in direct patient care. With the vast number of laparoscopic procedures being performed worldwide, this will have a positive impact on alleviating the work load for residents during emergency and routine laparoscopic surgery for certain procedures such as hernia repair.
CONCLUSION
Although there are justifiable debates regarding the potential benefits of single-port surgery, this will only be settled with additional research, in particular well-run prospective randomized controlled trials. Robotic single-port hernia repair is safe, efficient, and available today. These 2 innovations have the potential to catapult laparoscopic surgery into a new stratosphere: safer, more efficient in terms of operation time and replacement of camera assistants, hence alleviating manpower limitations while producing virtually scarless incisions.
References:
- 1. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001; 5(1): 89–94 [PMC free article] [PubMed] [Google Scholar]
- 2. Filipovic-Cugura J, Kirac I, Kulis T, Jankovic J, Benkavac-Beslin M. Single incision laparoscopic surgery (SILS) for totally extraperitoneal (TEP) inguinal hernia repair: first case. Surg Endosc. 2009; 23: 920–921 [DOI] [PubMed] [Google Scholar]
- 3. Lin P, Daniel R, Grow MD. Complications of laparoscopy: strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999; 26(1): 23–38 [DOI] [PubMed] [Google Scholar]
- 4. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001; 192(6): 677–683 [DOI] [PubMed] [Google Scholar]
- 5. Agrawal S, Shaw A, Soon Y. Single-port laparoscopic totally extraperitoneal inguinal hernia repair with Triport system: initial experience (16 patients). Surg Endosc. 2010; 24(4): 952–956 [DOI] [PubMed] [Google Scholar]
- 6. Surgit O. Single-incision laparoscopic surgery for total extraperitoneal repair of inguinal hernias in 23 patients. Surg Laparosc Endosc Percutan Techn. 2010; 20(2): 114–118 [DOI] [PubMed] [Google Scholar]
- 7. Tran H. Demonstrated safety and efficacy of single incision laparoscopic surgery for total extraperitoneal inguinal hernia repair:. JSLS. 2011; 15(1): 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stolzenburg JU, Franz T, Kallidonis P, et al. Comparison of the FreeHand® robotic camera holder with human assistants during endoscopic extraperitoneal radical prostatectomy. BJU Int. 2001. March; 107(6): 970–9740 Epub 2010 Oct 25 [DOI] [PubMed] [Google Scholar]
- 9. Dogangil G, Davies BL, Rodriguez Baena F. A review of medical robotics for minimally invasive soft tissue surgery. Proc Inst Mech Eng H. 2010; 224(5): 653–679 [DOI] [PubMed] [Google Scholar]
- 10. Brown CT, Kooiman G, Sharman DM, Poulsen J, Grange P. Scarless single-port laparoscopic pelvic kidney nephrectomy. J Laparoendosc Adv Surg Tech. 2010; 22(9): 743–746 [DOI] [PubMed] [Google Scholar]
- 11. Albrecht A, Bansagi Freehand robotic camera controller assisting laparoscopic bilateral oophorectomy. Available at: www.righthealth.com/topic/laparoscopic_oophorectomy/video
- 12. Schurr MO, Buess G. Wittmoser's technique of thoracoscopic sympathectomy and vagotomy. Endosc Surg Allied Technol. 1993; 1(5-6): 266–270 [PubMed] [Google Scholar]
- 13. Pelosi MA. Laparoscopic appendicectomy using a single umbilical puncture (minilaparoscopy). J Repro Med. 1992; 37(7): 588–594 [PubMed] [Google Scholar]
- 14. Goddard A, Pounder R, McIntyre A, Newbery N. Implementation of the European working time directive in 2009 – Implications for UK clinical service provision and training for the medical specialties. Medical Workforce Unit, Royal College of Physicians, London, February 2009 [Google Scholar]