Abstract
Theory suggests that maternally inherited endosymbionts can promote their spread and persistence in host populations by enhancing the production of daughters by infected hosts, either by improving overall host fitness, or through reproductive manipulation. In the doubly infected parasitoid wasp Encarsia inaron, Wolbachia manipulates host reproduction through cytoplasmic incompatibility (CI), but Cardinium does not. We investigated the fitness costs and/or benefits of infection by each bacterium in differentially cured E. inaron as a potential explanation for persistence of Cardinium in this population. We introgressed lines infected with Wolbachia, Cardinium or both with the cured line to create a similar genetic background, and evaluated several parasitoid fitness parameters. We found that symbiont infection resulted in both fitness costs and benefits for E. inaron. The cost was lower initial egg load for all infected wasps. The benefit was increased survivorship, which in turn increased male production for wasps infected with only Cardinium. Female production was unaffected by symbiont infection; we therefore have not yet identified a causal fitness effect that can explain the persistence of Cardinium in the population. Interestingly, the Cardinium survivorship benefit was not evident when Wolbachia was also present in the host, and the reproduction of doubly infected individuals did not differ significantly from uninfected wasps. Therefore, the results of our study show that even when multiple infections seem to have no effect on a host, there may be a complex interaction of costs and benefits among symbionts.
Keywords: facultative symbiont, multiple infection, reproductive parasite, secondary symbiont, sex ratio, sperm depletion
Introduction
Maternally inherited bacterial endosymbionts are common among arthropods (Douglas, 1989; Werren et al., 1995; Hurst and Jiggins, 2000; Russell et al., 2003; Duron et al., 2008), with the most widespread bacterial endosymbiont, Wolbachia, recently estimated to infect 66% of arthropod species, although often at low prevalence within a species (Hilgenboecker et al., 2008). Many symbionts are obligate and mutualistic, performing essential nutritive or reproductive functions for their hosts (Douglas, 1989). Other symbionts are facultative, often infecting only a portion of a population, and without which hosts can survive and potentially thrive (O'Neill et al., 1997). Because such facultative symbionts usually cannot be cultivated outside their hosts, their roles remained obscure for many years (Moran, 2006). With the advent of molecular techniques, however, interest in these bacteria has escalated and it has become increasingly evident that facultative symbionts can have major effects on their host's biology, ecological relationships and evolutionary dynamics (Moran, 2006).
The influence of facultative symbionts on their hosts can range from beneficial to detrimental, and may be conditional on extrinsic factors (Russell and Moran, 2006; Haine, 2008). Theory suggests that maternally inherited endosymbionts can promote their spread and persistence in host populations by enhancing the production of daughters relative to uninfected individuals in the population (Caspari and Watson, 1959; Turelli, 1994; Werren, 1997). This can be accomplished by providing a net fitness benefit to infected individuals, increasing their survival and/or fecundity, and thereby increasing female offspring production (Weeks and Stouthamer, 2004). Other symbionts are only beneficial under some conditions. For example, Hamiltonella defensa increases survival of its host, the pea aphid, by providing protection from parasitism (Oliver et al., 2003). In populations under strong parasitism pressure, the frequency of H. defensa-infected individuals increases, but in populations without parasitism the frequency of infection decreases (Oliver et al., 2008), suggesting that there is also a cost for carrying the symbiont.
Other studies have also shown that symbionts impose costs on their hosts yet persist in populations. In most cases, these symbionts are reproductive manipulators that promote their own spread in a population by encouraging the production of female progeny, or reducing the reproduction of uninfected females (reviewed in Werren et al., 2008). As long as infected females produce more daughters on average than uninfected females, the symbiont can persist and spread in the population, even if total progeny production is reduced (Caspari and Watson, 1959; Turelli, 1994; Werren, 1997). Over time, one would expect evolution toward reduced cost in such systems, because of the strictly vertical transmission and the shared fate between host and symbiont (Weeks et al., 2007). However, there are situations in which detrimental effects may persist. In particular, multiple symbiont infection within a shared host may tend to foster competitive interactions, higher symbiont titer and increased cost to the host (Oliver et al., 2006; but see Engelstadter et al., 2007). It has even been theorized that under some conditions coinfection may allow cost-inflicting symbionts to ‘hitchhike' with more beneficial partners, and invade a host population that could not be invaded by the cost-inflicting symbiont alone (Vautrin et al., 2007).
Infection by multiple symbionts is reasonably common (Duron et al., 2008), yet there has been relatively little work on fitness consequences of multiple infections for hosts, nor the individual cost or benefit derived from each symbiont. Most studies have focused on multiple coinfecting strains of a single symbiont (the most common symbiont, Wolbachia; Mouton et al., 2004), and/or comparing populations of hosts that are naturally infected with different symbiont combinations (Narita et al., 2007; Ros and Breeuwer, 2009). The latter type of study is often the only type possible for some relatively intractable systems, but does not control for host genotype differences among populations, nor does it allow dissection of each symbiont's contribution to the phenotype of multiply infected hosts, as symbionts in singly infected hosts have a different evolutionary context from their coinfecting counterparts and may differ substantially in their effects on the host.
The goal of this study was to investigate the fitness costs and/or benefits exerted by multiple symbionts in a coinfected host. Encarsia inaron (Hymenoptera: Aphelinidae) is an introduced parasitic wasp that has both Wolbachia and Cardinium endosymbionts (Perlman et al., 2006). Wolbachia is an α-Proteobacteria that has been shown to maintain its prevalence within many arthropod taxa through a variety of reproductive manipulations (Werren et al., 2008). Cardinium is a more recently described symbiont that is not closely related to Wolbachia (Cardinium is a member of the Bacteroidetes), yet is capable of a similar array of reproductive manipulations (Zchori-Fein et al., 2001, 2004; Hunter et al., 2003). In E. inaron, Wolbachia was shown to cause CI, whereby matings between infected males and uninfected females do not produce viable female offspring (White et al., 2009). Cardinium, however, did not cause CI, nor did it interact with the Wolbachia-induced CI phenotype. We therefore investigated symbiont influence on host fitness as a potential explanation for the persistence of Cardinium in the wasp population.
Materials and methods
Cultures
E. inaron is a minute parasitoid (<1 mm) that develops as a primary parasitoid in a variety of whitefly species (family Aleyrodidae, Manzari et al., 2002). The doubly infected E. inaron culture (‘both') originated from Siphoninus phillyreae pupae collected in Tucson, Arizona in 2002, and has been propagated on sweet potato whitefly (Bemisia tabaci) in the laboratory as described in Perlman et al. (2006). This population of B. tabaci does not contain either Cardinium or Wolbachia (Zchori-Fein and Brown, 2002), and therefore does not have the potential to interfere with transmission or detection of these symbionts in E. inaron. The differentially infected cultures (‘Cardinium,' ‘Wolbachia' and ‘cured') were previously generated using low doses of antibiotics, as described in White et al. (2009). To ensure similar genetic backgrounds among the cultures, we carried out an introgression series such that the genetic background of the Cured culture replaced the background of the Wolbachia, Cardinium and doubly infected cultures. A total of 50 females of each infected line were mated with cured males. Progeny of these crosses was backcrossed to cured males for an additional seven generations, to create four differentially infected cultures that were genetically homogeneous (sharing 99.5% genes). We propagated these cultures from generation to generation using 100 mated females until the fitness assays were conducted seven (experiment 1) or nine (experiment 2) generations later. The infection status of each culture was verified in the generation before the experiments and the generation after the experiments using diagnostic PCR as described in White et al. (2009). In both instances, all individuals possessed the correct symbionts (24 out of 24 wasps in the preexperimental generation, 80 out of 80 wasps in the postexperimental generation).
Fitness assays
We collected fitness data in two experiments. For each experiment, we put eight pots of cowpea plants (Vigna ungulata) that had been previously infested with B. tabaci into individual rearing jars and randomly assigned each jar to receive parasitoids from one of the four wasp cultures (two jars per culture). Each jar received 25 E. inaron females. After 14 days we collected all leaf material with developing wasp pupae from each plant, and placed it in an emergence jar for each culture. We inspected each jar daily, removing all newly emerged females. On the first day that at least 30 females emerged, we collected these females and immediately froze them for later determination of egg load by dissection under × 50 magnification. As egg load in this species is correlated to size (White J, unpublished data) we also measured the hind tibia length for each wasp using a micrometer in a compound microscope at × 200 magnification.
For statistical comparisons of egg load among the cultures, we used two-way analysis of variance (ANOVA) with infection status and experiment as fixed and random factors, respectively, followed by Fisher's protected least significant difference for separation of means. We used this approach, rather than a blocked ANOVA, because a powdery mildew was present on the plants during the second experiment causing potential interactive effects between infection status and experiment. To determine whether significant differences in egg load arose from underlying differences in wasp size or differential egg load for a given size, we conducted post hoc two-way ANOVA on hind tibia length, as well as analysis of covariance of egg load with hind tibia length included as a covariate. Hind tibia length was significantly correlated with egg load and did not interact significantly with treatment: it was therefore an appropriate covariate for this analysis.
To assess survivorship and lifetime progeny production, we collected a second group of female wasps that were <1-day old from each culture (145 total: 10–15 per culture in experiment 1, 24 per culture in experiment 2). E. inaron usually mates shortly after eclosion and does not multiply mate, but to ensure that all females had opportunity to mate, we placed them in small groups in 35 mm ventilated Petri dishes for 24 h at a ratio of one female to two males. All females were mated to males of the same infection status, resulting in four treatments. We chose to mate females and males within each infection status, rather than mate all females to males of a single infection status, because we wanted to maximize the possibility of detecting among-culture differences that could arise through differences in either female or male quality.
We placed individual female wasps on excised cowpea leaf disks on 1% agar medium in a 35 mm Petri dish. Each leaf disk had approximately 50 first to third instar B. tabaci hosts for oviposition (range=30–75 whiteflies per disk). The Petri dishes were covered with modified screen-top lids, and placed in an environmental chamber at 27 °C, 60% relative humidity, 16:8 h photoperiod. Every 24 h, we transferred each wasp to another leaf disk until death. When a wasp died, she was frozen at −20 °C for later analysis. We recorded her cause of death, and calculated total survivorship in days. We maintained each leaf disk on agar for 2 weeks and quantified and sexed the progeny that emerged. If a mother produced no female offspring, we checked her mating status by dissecting out her spermatheca as described in White et al. (2009). Females that were unmated (six wasps) were excluded from the data set.
We compared survivorship, female and male progeny production among the cultures with two-way ANOVA as before, excluding an additional 30 wasps that did not die from natural causes (drowned in agar, damaged during transfer among leaf disks or lost). To incorporate this censored data, we also performed a Kaplan–Meier nonparametric analysis on the survivorship data. The results of the Kaplan–Meier and the standard ANOVA were functionally identical, and the ANOVA met normality and homoscedacity assumptions, so we opted to present the ANOVA for simplicity of interpretation. Male and female progeny data were transformed (ln and sqrt(x+1), respectively) to improve conformity to normality and homoscedacity assumptions. All significant ANOVAs were followed by Fisher's protected least significant difference for separation of means.
We also estimated vertical transmission efficiency of each symbiont in all infected lines, to provide context for observed fitness outcomes. To measure vertical transmission, we randomly selected 14–16 additional wasps from each symbiont-infected culture, gave them 2 consecutive days of host leaf disks (as described above) and reared their progeny to adulthood. We extracted DNA from each mother and all of her progeny individually (mean=12.33±0.45 s.e. progeny per mother) using a modified Chelex bead method (Groot et al., 2005; White et al., 2009). PCR detection for Wolbachia was performed using primers hcpA_F1 (5′-GAAATARCAGTTGCTGCAAA-3′) and hcpA_R1 (5′-GAAAGTYRAGCAAGYTCTG-3′) for the hcpA gene (Baldo et al., 2006). The PCR temperature profile was 94 °C for 2 min, followed by 36 cycles of 94 °C for 30 s, 53 °C for 30 s, 72 °C for 1.5 min, followed by 70 °C for 10 min. PCR detection for Cardinium was performed using primers GyrBF43 (5′-ACTACGGGGCACCACTGTTCATTT-3′) and GyrBR295 (5′-GCGTGGGCATAATAGAAGTTTTGG-3′) for the GyrB gene. The PCR temperature profile was 94C for 3 min, followed by 29 cycles of 94 °C for 30 s, 68 °C for 30 s, 72 °C for 45 s, followed by 72 °C for 10 min. All negative results were checked for DNA quality using either primers ef1-αF139 (5′-TTGCCACACCGCTCATATCGCTTG-3′) and ef1-αR321 (5′-CGCTTTTCACGCTCTTCGGATTTT-3′) for the host nuclear gene ef1-α or H3aF (5′-ATGGCTCGTACCAAGCAGACVGC-3′) and H3bR (5′-ATATCCTTRGGCATRATRGTGAC-3) for histone H3 (Colgan et al., 1998).
Results
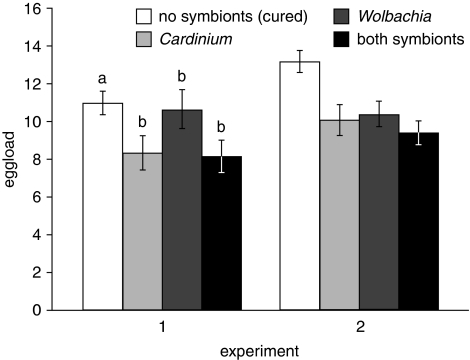
Endosymbiont infection diminished E. inaron egg load. Cured parasitoids had larger egg loads than symbiont-infected wasps in the other three cultures (Finfection=10.23, d.f.=3, 3, P=0.044; Figure 1). There was no difference in egg load between experiments (Fexp.=5.23, d.f.=1, 3.2, P=0.101), nor was there a significant interaction between culture and experiment (Finfection × exp.=0.93, d.f.=3, 413, P=0.426). Cured parasitoids averaged 2.6 (29%) more eggs than infected wasps. There was no evidence for an additive effect of symbiont infection, however; E. inaron in the doubly infected culture did not differ significantly from the singly infected Cardinium and Wolbachia cultures.
Figure 1.
Mean±s.e. egg load of differentially infected E. inaron. Lowercase letters above columns in experiment 1 denote treatments that differed significantly at α=0.05 in a two-way ANOVA that included both experiments.
Greater egg load in the cured culture is not solely attributable to either larger parasitoid size or greater egg load for a given size. Wasp size, as measured by hind tibia length, is an important correlate of egg load (R2=0.55, P<0.001) but did not vary significantly among cultures (Finfection=1.30, d.f.=3, 3, P=0.417). There were nonsignificant trends toward larger size in experiment 2 (Fexp.=6.77, d.f.=1, 3.07, P=0.078) and interaction between culture and experiment (Finfection × exp.=2.58, d.f.=3, 413, P=0.053). When hind tibia length was included as a covariate in the analysis of egg load, there was a nonsignificant trend for cured E. inaron to have larger egg load than parasitoids in the infected cultures (Finfection=7.98, d.f.=3, 3, P=0.061) with no difference between experiments (Fexp.=1.11, d.f.=1, 3.3, P=0.363) or interaction between culture and experiment (Finfection × exp.=1.35, d.f.=3, 412, P=0.258).
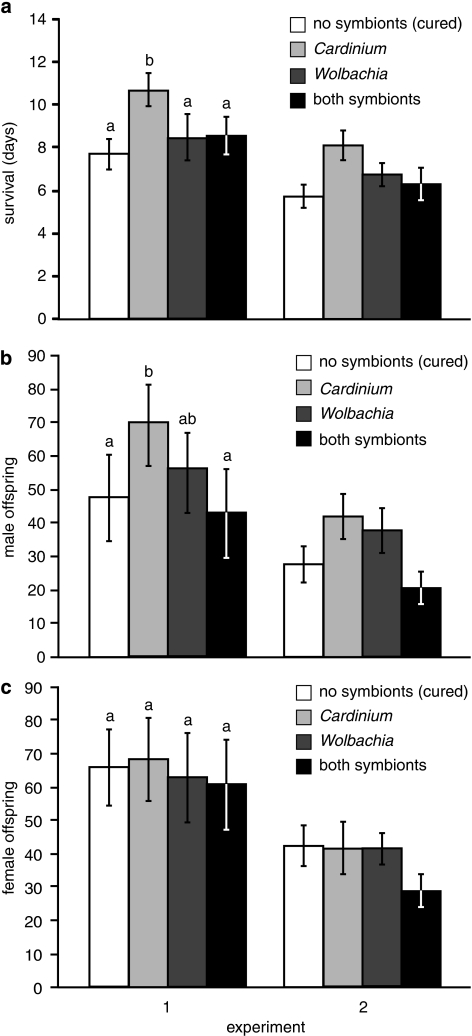
Survivorship was greatest in Cardinium-infected E. inaron (Finfection=27.23, d.f.=3, 3, P=0.011; Figure 2a). Survivorship was also greater in experiment 1 than experiment 2 (Fexp.=101.1, d.f.=1, 3.3, P <0.001), but there was no interaction between culture and experiment (Finfection × exp.=0.16, d.f.=3, 101, P=0.921). Averaged between experiments, wasps that had only Cardinium lived 2.7 days (approximately 40%) longer than cured wasps, 1.7 days (22%) longer than Wolbachia-infected wasps and 2.0 days (28%) longer than doubly infected wasps. Therefore, the survivorship benefit conferred by Cardinium was not evident in individuals coinfected with both Cardinium and Wolbachia.
Figure 2.
Mean±s.e. survival (a), male (b) and female (c) offspring produced by differentially infected E. inaron over their lifetime. For each panel, lowercase letters above columns in experiment 1 denote treatments that differed significantly at α=0.05 in a two-way ANOVA that included both experiments.
Increased survivorship in Cardinium-infected wasps corresponded to increased male offspring production (Finfection=22.94, d.f.=3, 3, P=0.014; Figure 2b), but not increased female offspring production (Finfection=4.12, d.f.=3, 3, P=0.15; Figure 2c). Both male and female offspring production were greater in experiment 1 than in 2 (male Fexp.=43.84, d.f.=1, 3.2, P=0.006; female Fexp.=64.16, d.f.=1, 3.2, P=0.003), but again, there were no interactions between culture and experiment (male Finfection × exp.=0.20, d.f.=3, 101, P=0.89; female Finfection × exp.=0.19, d.f.=3, 101, P=0.91). Averaged between the experiments, Cardinium-infected wasps produced 24 more male offspring (80% more) than doubly infected wasps and 18 more male offspring (50% more) than cured wasps. Wolbachia-infected wasps were closer to Cardinium-infected wasps in male production, and did not differ significantly from any other treatment.
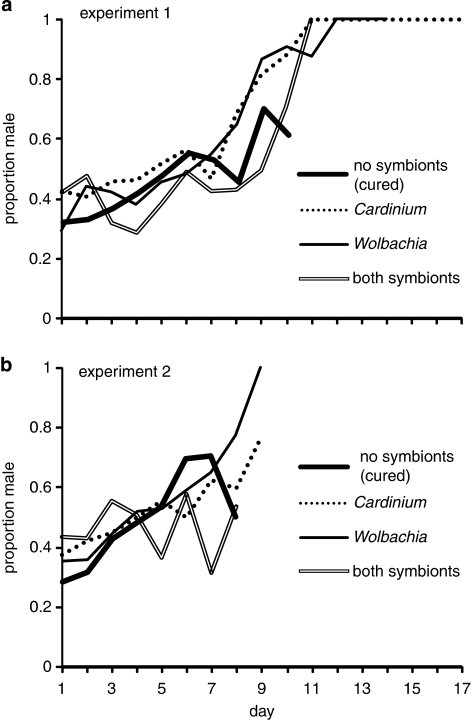
Reproductive allocation toward males increased with parasitoid age in all four cultures (Figure 3). This increase was particularly evident in experiment 1 (Figure 3a), in which wasps lived longer on average, and three out of four treatments produced only males by the end of the experiment. Most of the long-lived females had produced daughters earlier in their lives, but shifted to male production as they aged, with the pattern exhibited by individuals looking similar to the summed sex ratios shown in Figure 3a. We examined the spermatheca from all females that shifted toward male production at the end of their life and found little sign of sperm limitation: of 20 wasps dissected, 17 had no visible depletion of sperm within their spermatheca, 2 were approximately 50% depleted and 1 was approximately 75% depleted. This last individual was one of the most long-lived and productive individuals in experiment 1, producing 153 daughters over her first 11 days, followed by 3 days of male-only production before she died. All of the other females that shifted toward male production did so earlier in their life span than this wasp (5.5±2.7 days) after having produced fewer female offspring (29.0±3.6 females).
Figure 3.
Proportion male offspring produced per day by surviving E. inaron in experiment 1 (a) and experiment 2 (b).
Vertical transmission of symbionts was perfect under our laboratory conditions. All screened wasps (n=555) had the same symbiont status as their mothers.
Discussion
We found that symbionts impose a cost upon E. inaron. Cardinium, in particular, was associated with reduced egg load across treatments and experimental dates. With Wolbachia the egg load cost was less consistent: even though we detected a significant difference relative to cured wasps, inspection of the data shows that egg load reduction in Wolbachia-bearing wasps primarily occurred in experiment 2 (Figure 1). Experiment 2 was conducted under what seemed to be more stressful conditions than experiment 1, likely because of the powdery mildew infection of the host plants at that time, and we speculate that the physiological costs of bearing Wolbachia may be negligible in healthy wasps but have detectable consequences in wasps that have additional sources of stress. Further studies under more controlled conditions would be needed to validate this hypothesis, however. When both symbionts occurred together in the same host, egg load was reduced relative to cured wasps but did not differ from wasps bearing only Cardinium, indicating that there was not an additive cost to bearing two symbionts.
Cardinium also provided a benefit to E. inaron. Wasps that had only Cardinium survived 40% longer, which in turn resulted in 50% more male offspring production than cured wasps. Other studies have found fecundity benefits associated with Cardinium in mites (Weeks and Stouthamer, 2004), but this is the first instance in which adult survivorship has been shown to be positively associated with Cardinium infection. The mechanism by which adult longevity is increased is not clear, but it is possible that increased survivorship in Cardinium-infected wasps may be directly attributable to decreased egg load. In general, reproduction is costly, and is often negatively correlated with life span (Chapman et al., 1998). However, if Cardinium limits daily egg load and consequently daily reproductive investment, egg-limited E. inaron in a host-rich environment may engage in greater host-feeding activities, allocate more resources toward self-maintenance and future reproduction than current reproduction (Heimpel and Collier, 1996), and ultimately increase overall reproduction. This increase is entirely realized as male offspring rather than female, as late life reproduction in this species (across all infection types) tends to be male. Greater male bias in older females is a relatively common phenomenon in haplodiploid hymenopteran species and is generally attributed to sperm limitation (Santolamazza-Carbone et al., 2007; reviewed in Godfray, 1994). Within this experiment E. inaron did not experience sperm limitation per se, because spermatheca dissection confirmed the presence of ample sperm in virtually all mated individuals. However, sperm quality may have diminished with age (Ridley, 1988), or it is possible that female eggs receive greater nutrient provisioning than male eggs, and that older (lower quality) mothers opt to lay less costly male eggs (Giron and Casas, 2003).
Regardless of mechanism, the net result for wasps bearing Cardinium alone is an increase in male production and no change in female production; which makes little evolutionary sense from the perspective of a maternally inherited symbiont. We have found no evidence for paternal or horizontal transmission of Cardinium in this system (White et al., 2009; White J, unpublished data), confirming that maternal transmission is the only mode of transmission. For symbionts with strict maternal inheritance, spread and maintenance within a host population is dependent on production of female offspring (Bull, 1983); consequently, the fitness benefit associated with Cardinium infection provides no explanatory power for the presence of Cardinium in E. inaron.
Our study does, however, illustrate interactive effects of symbionts within a shared host. Cardinium alone increased parasitoid survival (and male offspring production), whereas Cardinium in conjunction with Wolbachia did not. A simple comparison of these naturally doubly infected wasps to uninfected individuals would lead to the conclusion that multiple symbiont infection has no realized consequences for the survival or fecundity of E. inaron under our experimental conditions. This lack of phenotypic effect is apparently masking a complex interplay of costs and benefits among symbionts. For example, we speculate that in doubly infected E. inaron the fitness benefit associated with Cardinium may serve to counter the increased metabolic cost of bearing two symbionts, resulting in no net fitness effect for the host. Disentangling such interactive effects among symbionts will become increasingly important as both the frequency of multiple infections (Duron et al., 2008; Burke et al., 2009), and the dynamic nature of symbiont effects over time (Weeks et al., 2007; Oliver et al., 2009) become evident.
Given this dynamism, it seems likely that present-day Cardinium within E. inaron does not have the same functionality as the symbiont that initially invaded the host population. Our study data indicate that neither Cardinium alone nor Cardinium in combination with Wolbachia promotes production of female offspring relative to individuals lacking Cardinium. However, we have found that a Cardinium in a cryptic sibling species of E. inaron causes CI (MS Hunter et al, unpublished data), suggesting that the initial invasion of E. inaron may have been accomplished through CI that has subsequently been lost in this population. This loss of function would be particularly likely if the CI induced by Wolbachia in this species was somehow able to supersede Cardinium-induced CI. Little is known about the mechanisms of CI caused by either symbiont, nor has a host been discovered in which both Cardinium and Wolbachia induce CI, so such potential interactions among the symbionts remain speculative.
Alternatively, it is possible that Cardinium has a function in E. inaron that remains obscure because we have not yet examined it in the right context. For example, a Wolbachia that seemed to lack a strong phenotype (Hoffmann et al., 1996; Harcombe and Hoffmann, 2004) has recently been found to protect its host from viral infection (Hedges et al., 2008; Teixeira et al., 2008). A defensive phenotype is a prime example of a context-dependent benefit unlikely to be discovered in simple laboratory assay. As our understanding of the ecological context of symbionts in hosts become more sophisticated, the role of additional ‘asymptomatic' symbionts may yet be characterized.
Acknowledgments
We thank A Kozuch, J Bergen, N Doidge, N Dowdy, J Garcia, H Kim, T Montgomery and C Ross for technical assistance, and three anonymous reviewers for comments on previous versions of this article. This research was supported by the Center for Insect Science through NIH Training Grant no. 2 K12 Gm00708-06 and an NSF DEB grant (DEB-0542961) to MSH and SJP. SJP is a member of the Canadian Institute for Advanced Research's Integrated Microbial Biodiversity Program. This is publication 10-08-080 of the Kentucky Agricultural Experiment Station.
The authors declare no conflict of interest.
References
- Baldo L, Hotopp J, Jolley K, Bordenstein SR, Biber S, Choudhury R, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ.1983Evolution of Sex Determining Mechanisms Benjamin/Cummings Publishing: Menlo Park; 316pp. [Google Scholar]
- Burke GR, Normark BB, Favret C, Moran NA. Evolution and diversity of facultative symbionts from the aphid subfamily Lachninae. Appl Environ Microbiol. 2009;75:5328–5335. doi: 10.1128/AEM.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari E, Watson GS. On the evolutionary importance of cytoplasmic sterility in mosquitos. Evolution. 1959;13:568–570. [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly Ceratitis capitata. Proc R Soc London Ser B Biol Sci. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan DJ, McLauchlan A, Wilson GDF, Livingston SP, Edgecombe GD, Macaranas J. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust J Zool. 1998;46:419–437. [Google Scholar]
- Douglas AE. Mycetocyte symbiosis in insects. Biol Rev Camb Philos Soc. 1989;64:409–434. doi: 10.1111/j.1469-185x.1989.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstadter J, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstadter J, Hammerstein P, Hurst GDD. The evolution of endosymbiont density in doubly infected host species. J Evol Biol. 2007;20:685–695. doi: 10.1111/j.1420-9101.2006.01257.x. [DOI] [PubMed] [Google Scholar]
- Giron D, Casas J. Mothers reduce egg provisioning with age. Ecol Lett. 2003;6:273–277. [Google Scholar]
- Godfray HCJ.1994Parasitoids Princeton University Press: Princeton; 473pp. [Google Scholar]
- Groot TVM, Janssen A, Pallini A, Breeuwer JAJ. Adaptation in the asexual false spider mite Brevipalpus phoenicis: evidence for frozen niche variation. Exp Appl Acarol. 2005;36:165–176. doi: 10.1007/s10493-005-3360-6. [DOI] [PubMed] [Google Scholar]
- Haine ER. Symbiont-mediated protection. Proc R Soc B Biol Sci. 2008;275:353–361. doi: 10.1098/rspb.2007.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe W, Hoffmann AA. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J Invertebr Pathol. 2004;87:45–50. doi: 10.1016/j.jip.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- Heimpel GE, Collier TR. The evolution of host-feeding behaviour in insect parasitoids. Biol Rev Camb Philos Soc. 1996;71:373–400. [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- Hunter MS, Perlman SJ, Kelly SE. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc R Soc London Ser B Biol Sciences. 2003;270:2185–2190. doi: 10.1098/rspb.2003.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GDD, Jiggins FM. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Infect Dis. 2000;6:329–336. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzari S, Polaszek A, Belshaw R, Quicke DLJ. Morphometric and molecular analysis of the Encarsia inaron species-group (Hymenoptera: Aphelinidae), parasitoids of whiteflies (Hemiptera : Aleyrodidae) Bull Entomol Res. 2002;92:165–175. doi: 10.1079/BER2001144. [DOI] [PubMed] [Google Scholar]
- Moran NA. Symbiosis. Curr Biol. 2006;16:R866–R871. doi: 10.1016/j.cub.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, Vavre F. Virulence, multiple infections and regulation of symbiotic population in the Wolbachia–Asobara tabida symbiosis. Genetics. 2004;168:181–189. doi: 10.1534/genetics.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita S, Nomura M, Kageyama D. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol Ecol. 2007;61:235–245. doi: 10.1111/j.1574-6941.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Hoffmann AA, Werren JH.1997Influential Passengers: Inherited Microorganisms and Arthropod Reproduction Oxford University Press: Oxford; 232pp. [Google Scholar]
- Oliver KM, Campos J, Moran NA, Hunter MS. Population dynamics of defensive symbionts in aphids. Proc R Soc B Biol Sci. 2008;275:293–299. doi: 10.1098/rspb.2007.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Degnan PH, Hunter MS, Moran NA. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science. 2009;325:992–994. doi: 10.1126/science.1174463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Moran NA, Hunter MS. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc R Soc B Biol Sci. 2006;273:1273–1280. doi: 10.1098/rspb.2005.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SJ, Kelly SE, Zchori-Fein E, Hunter MS. Cytoplasmic incompatibility and multiple symbiont infection in the ash whitefly parasitoid, Encarsia inaron. Biol Control. 2006;39:474–480. [Google Scholar]
- Ridley M. Mating frequency and fecundity in insects. Biol Rev Camb Philos Soc. 1988;63:509–549. [Google Scholar]
- Ros VID, Breeuwer JAJ. The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. Heredity. 2009;102:413–422. doi: 10.1038/hdy.2009.4. [DOI] [PubMed] [Google Scholar]
- Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol. 2003;12:1061–1075. doi: 10.1046/j.1365-294x.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- Russell JA, Moran NA. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc R Soc B Biol Sci. 2006;273:603–610. doi: 10.1098/rspb.2005.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolamazza-Carbone S, Nieto MP, Rivera AC. Maternal size and age affect offspring sex ratio in the solitary egg parasitoid Anaphes nitens. Entomol Exp Appl. 2007;125:23–32. [Google Scholar]
- Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:2753–2763. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48:1500–1513. doi: 10.1111/j.1558-5646.1994.tb02192.x. [DOI] [PubMed] [Google Scholar]
- Vautrin E, Charles S, Genieys S, Vavre F. Evolution and invasion dynamics of multiple infections with Wolbachia investigated using matrix-based models. J Theor Biol. 2007;245:197–209. doi: 10.1016/j.jtbi.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Weeks AR, Stouthamer R. Increased fecundity associated with infection by a Cytophaga-like intracellular bacterium in the predatory mite, Metaseiulus occidentalis. Proc R Soc London Ser B Biol Sci. 2004;271:S193–S195. doi: 10.1098/rsbl.2003.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007;5:997–1005. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc London Ser B Biol Sci. 1995;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- White JA, Kelly SE, Perlman SJ, Hunter MS. Cytoplasmic incompatibility in the parasitic wasp Encarsiainaron: disentangling the roles of Cardinium and Wolbachia symbionts. Heredity. 2009;102:483–489. doi: 10.1038/hdy.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein E, Brown JK. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera : Aleyrodidae) Ann Entomol Soc Am. 2002;95:711–718. [Google Scholar]
- Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, et al. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc Natl Acad Sci USA. 2001;98:12555–12560. doi: 10.1073/pnas.221467498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein E, Perlman SJ, Kelly SE, Katzir N, Hunter MS. Characterization of a ‘Bacteroidetes' symbiont in Encarsia wasps (Hymenoptera : Aphelinidae): proposal of ‘Candidatus Cardinium hertigii. Int J Syst Evol Microbiol. 2004;54:961–968. doi: 10.1099/ijs.0.02957-0. [DOI] [PubMed] [Google Scholar]