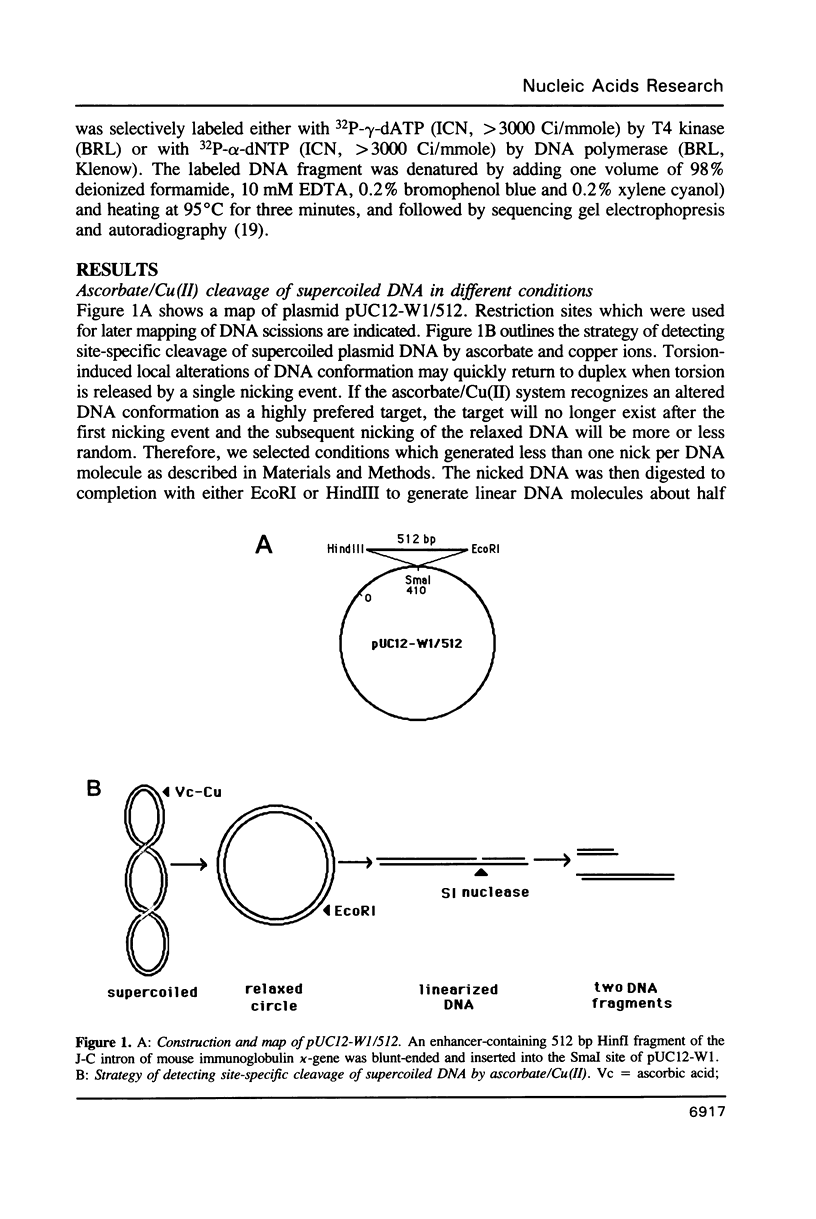
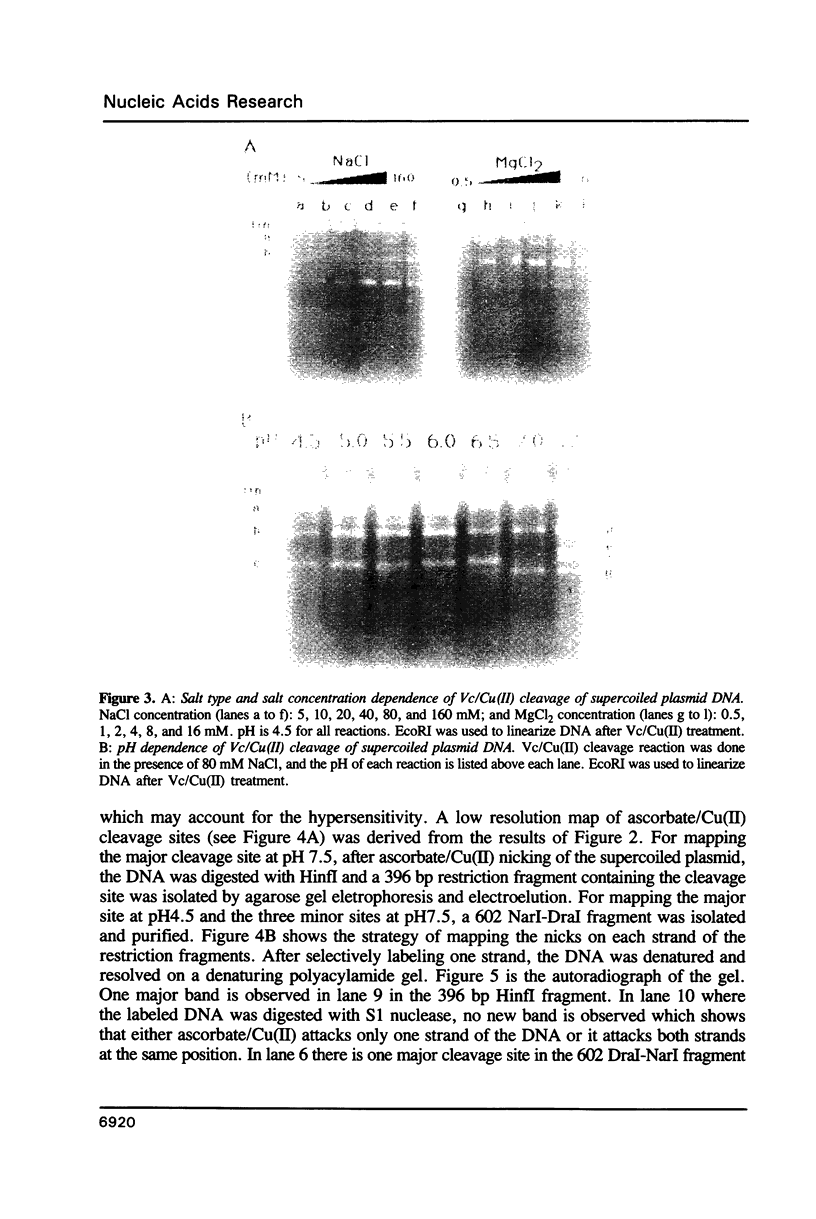
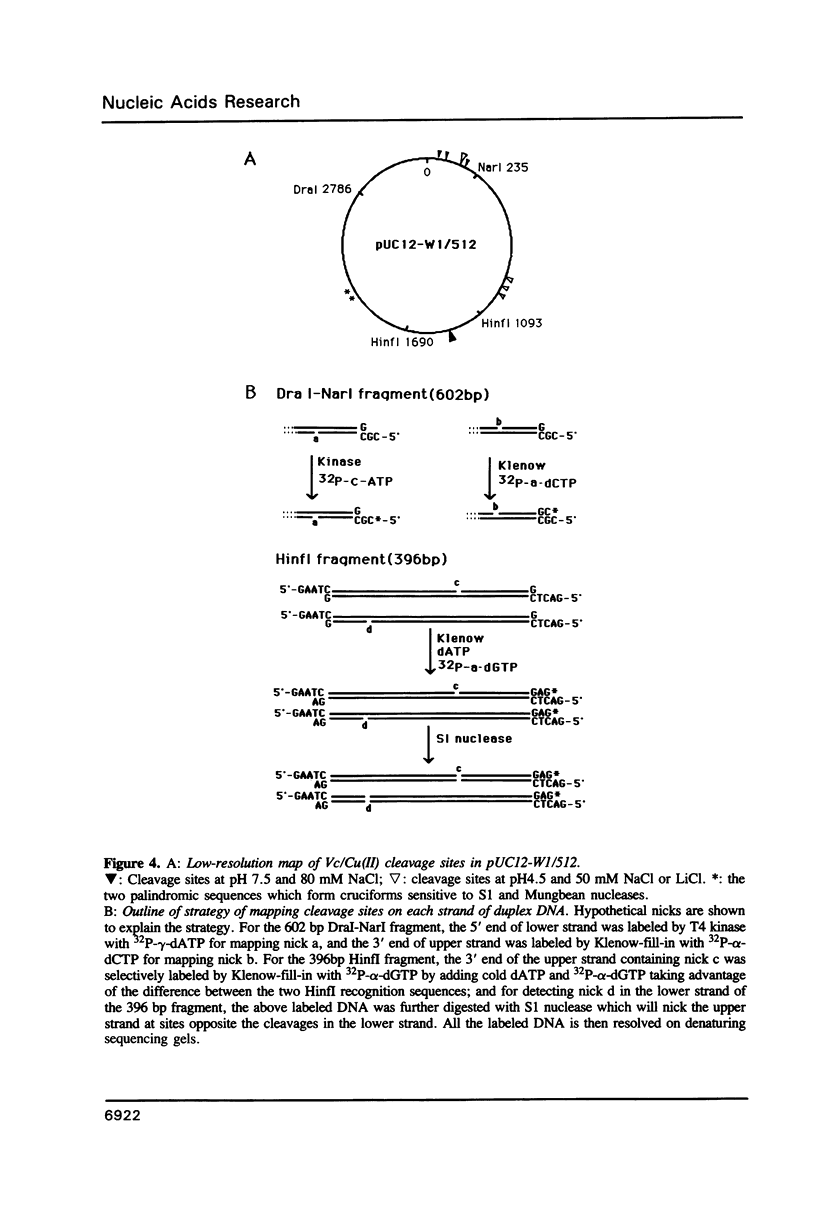
Abstract
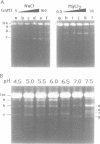
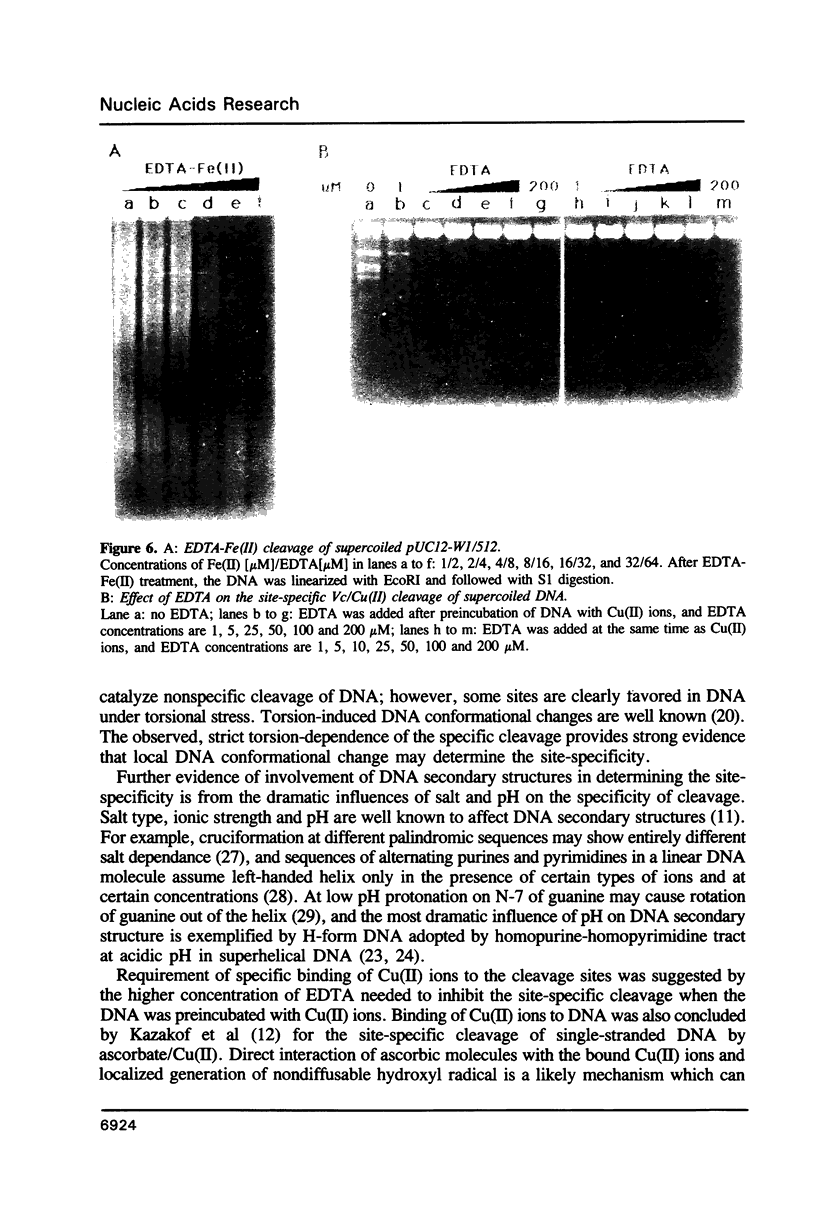
We have investigated ascorbate/Cu(II) cleavage of double-stranded DNA in the presence and absence of DNA negative torsion. We found that ascorbate/Cu(II) cleavage shows a site-specificity that is dependent on negative torsion and is influenced by the nature of the salt, ionic strength, and pH. This provides strong evidence for involvement of local DNA conformation in ascorbate/Cu(II) specific cleavage sites, that differs from the previous reports on cleavage of linear double-stranded DNA and secondary structures assumed by single-stranded DNA. The data indicate specific binding of Cu(II) ions to sites in the negatively supercoiled DNA. Fining mapping of the cleavage sites does not reveal any known DNA conformation, nor does it indicate any sequence identity among the sites cleaved. However, identification of a major site of cleavage of supercoiled DNA at physiological ionic strength, pH and temperature, along with fact that ascorbate and Cu(II) are normal cell constituents, suggests the torsion-dependent, site-specific interactions could have biological significance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bram S., Froussard P., Guichard M., Jasmin C., Augery Y., Sinoussi-Barre F., Wray W. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980 Apr 17;284(5757):629–631. doi: 10.1038/284629a0. [DOI] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Chiou S. H., Chang W. C., Jou Y. S., Chung H. M., Lo T. B. Specific cleavages of DNA by ascorbate in the presence of copper ion or copper chelates. J Biochem. 1985 Dec;98(6):1723–1726. doi: 10.1093/oxfordjournals.jbchem.a135445. [DOI] [PubMed] [Google Scholar]
- Chiou S. H. DNA- and protein-scission activities of ascorbate in the presence of copper ion and a copper-peptide complex. J Biochem. 1983 Oct;94(4):1259–1267. doi: 10.1093/oxfordjournals.jbchem.a134471. [DOI] [PubMed] [Google Scholar]
- Courtois Y., Fromageot P., Guschlbauer W. Protonated polynucleotide structures. 3. An optical rotatory dispersion study of the protonation of DNA. Eur J Biochem. 1968 Dec 5;6(4):493–501. doi: 10.1111/j.1432-1033.1968.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Ellison M. J., Kelleher R. J., 3rd, Wang A. H., Habener J. F., Rich A. Sequence-dependent energetics of the B-Z transition in supercoiled DNA containing nonalternating purine-pyrimidine sequences. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8320–8324. doi: 10.1073/pnas.82.24.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J., Stafford J., Queen C. An immunoglobulin promoter displays cell-type specificity independently of the enhancer. 1985 May 30-Jun 5Nature. 315(6018):423–425. doi: 10.1038/315423a0. [DOI] [PubMed] [Google Scholar]
- Frieden E. The biochemistry of copper. Sci Am. 1968 May;218(5):103–114. [PubMed] [Google Scholar]
- Kazakov S. A., Astashkina T. G., Mamaev S. V., Vlassov V. V. Site-specific cleavage of single-stranded DNAs at unique sites by a copper-dependent redox reaction. Nature. 1988 Sep 8;335(6186):186–188. doi: 10.1038/335186a0. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Poly(dG)-poly(dC) sequences, under torsional stress, induce an altered DNA conformation upon neighboring DNA sequences. Cell. 1985 Nov;43(1):199–206. doi: 10.1016/0092-8674(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Kuwabara M., Yoon C., Goyne T., Thederahn T., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion: reaction with CGCGAATTCGCG and its complexes with netropsin and EcoRI. Biochemistry. 1986 Nov 18;25(23):7401–7408. doi: 10.1021/bi00371a023. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Johnson D. A., Morgan A. R. Complexes formed by (pyrimidine)n . (purine)n DNAs on lowering the pH are three-stranded. Nucleic Acids Res. 1979 Jul 11;6(9):3073–3091. doi: 10.1093/nar/6.9.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishanskaya A. I., Mosevitsky M. I. Study of calf thymus deoxyribonucleoproteins by means of gel electrophoresis. Effect of ionic composition on the mode of chromatin fragmentation. Biochem Biophys Res Commun. 1975 Feb 17;62(4):822–829. doi: 10.1016/0006-291x(75)90396-4. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D. Structures of homopurine-homopyrimidine tract in superhelical DNA. J Biomol Struct Dyn. 1986 Feb;3(4):667–669. doi: 10.1080/07391102.1986.10508454. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Cone R. L., Elgert T. M. The mechanism of DNA strand breakage by vitamin C and superoxide and the protective roles of catalase and superoxide dismutase. Nucleic Acids Res. 1976 May;3(5):1139–1149. doi: 10.1093/nar/3.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa M., Stevanato R., Viglino P., Rigo A. Superoxide ion as active intermediate in the autoxidation of ascorbate by molecular oxygen. Effect of superoxide dismutase. J Biol Chem. 1983 Jun 10;258(11):6695–6697. [PubMed] [Google Scholar]
- Shamberger R. J. Genetic toxicology of ascorbic acid. Mutat Res. 1984 Mar;133(2):135–159. doi: 10.1016/0165-1110(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Sissoëff I., Grisvard J., Guillé E. Studies on metal ions-DNA interactions: specific behaviour of reiterative DNA sequences. Prog Biophys Mol Biol. 1976;31(2):165–199. doi: 10.1016/0079-6107(78)90008-1. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Lilley D. M. A dominant influence of flanking sequences on a local structural transition in DNA. Cell. 1986 Dec 5;47(5):817–827. doi: 10.1016/0092-8674(86)90524-6. [DOI] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Hydroxyl radical "footprinting": high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5469–5473. doi: 10.1073/pnas.83.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Iron(II) EDTA used to measure the helical twist along any DNA molecule. Science. 1985 Nov 8;230(4726):679–681. doi: 10.1126/science.2996145. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sauerbier W. Mapping structurally perturbed sites in DNA by replication arrest and run-off replication. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1523–1530. doi: 10.1016/0006-291x(87)90822-9. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sauerbier W. pUC12-W1: a plasmid vector for the study of cloned DNA conformation. Gene. 1987;60(2-3):303–306. doi: 10.1016/0378-1119(87)90239-3. [DOI] [PubMed] [Google Scholar]
- Weitberg A. B. Antioxidants inhibit the effect of vitamin C on oxygen radical-induced sister-chromatid exchanges. Mutat Res. 1987 May;191(1):53–56. doi: 10.1016/0165-7992(87)90170-9. [DOI] [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Wohlrab F., McLean M. J., Wells R. D. The segment inversion site of herpes simplex virus type 1 adopts a novel DNA structure. J Biol Chem. 1987 May 5;262(13):6407–6416. [PubMed] [Google Scholar]
- Wong K., Morgan A. R., Paranchych W. Controlled cleavage of phage R17 RNA within the virion by treatment with ascorbate and copper (II). Can J Biochem. 1974 Nov;52(11):950–958. doi: 10.1139/o74-133. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Fritzsche H., Triebel H. DNA-copper (II) complex and the DNA conformation. Biopolymers. 1971;10(3):441–463. doi: 10.1002/bip.360100303. [DOI] [PubMed] [Google Scholar]