Abstract
Trask/CDCP1 is a transmembrane glycoprotein widely expressed in epithelial tissues whose functions are just beginning to be understood, but include a role as an anti-adhesive effector of Src kinases. Early studies looking at RNA transcript levels seemed to suggest overexpression in some cancers, but immunostaining studies are now providing more accurate analyses of its expression. In an immunohistochemical survey of human cancer specimens we find that Trask expression is retained, reduced, or sometimes lost in some tumors compared with their normal epithelial tissue counterparts. A survey of human cancer cell lines also show a similar wide variation in the expression of Trask, including some cell types with the loss of Trask expression, and additional cell types that have lost the physiological detachment-induced phosphorylation of Trask. Three experimental models were established to interrogate the role of Trask in tumor progression including two gain-of-function models with tet-inducible expression of Trask in tumor cells lacking Trask expression, and one loss-of-function model to suppress Trask expression in tumor cells with abundant Trask expression. The induction of Trask expression and phosphorylation in MCF-7 cells and in 3T3v-src cells was associated with a reduction in tumor metastases while the shRNA induced knockdown of Trask in L3.6pl cancer cells was associated with increased tumor metastases. The results from these three models are consistent with a tumor suppressing role for Trask. These data identify Trask as one of several potential candidates for functionally relevant tumor suppressors on the 3p21.3 region of the genome frequently lost in human cancers.
Keywords: Trask, CDCP1, SIMA135, 3p21.3, metastasis
Introduction
Epithelial cell biology and homeostasis is in part regulated by interactions with the surrounding matrix. One of the goals of cancer biology is to understand how cell-matrix interactions change in the course of epithelial tumorigenesis (Hirohashi and Kanai 2003, Pietras and Ostman 2010, Tlsty and Coussens 2006). Changes are known to occur in cell adhesion molecules, in membrane or secreted proteases, in growth factor receptors, and in many other pathways that regulate the interaction of cells with their extracellular environment (Kessenbrock et al 2010, Rathinam and Alahari 2010, Spiegelberg and Hamm 2007, Srinivasan et al 2005).
We have been studying the functions of a transmembrane protein named Trask (also known as CDCP1, SIMA135, gp140). Trask/CDCP1 is a 140kd type I transmembrane glycoprotein with a larger extracellular and smaller intracellular region. Trask is widely expressed in most epithelial tissues as well as certain hematopoeitic stem cells (Conze et al 2003, Spassov et al 2009). Trask is a substrate of Src family kinases and is phosphorylated by Src kinases when anchorage is lost (Brown et al 2004, Spassov et al 2009). When phosphorylated, Trask inhibits cell adhesion through the inhibition of integrin clustering and disruption of focal adhesion complexes (Spassov et al 2011b). In a survey of archival epithelial cancer tissues we found that Trask is phosphorylated in many cancers, in a patchy distribution, and not phosphorylated in many others. The phosphorylation of Trask is seen in some pre-invasive cancers as well as in invasive tumors and in tumor metastases (Wong et al 2009). The functional implications of Trask phosphorylation in tumors is currently unknown.
Some groups have proposed a function for Trask/CDCP1 in tumor promotion, however the data has been mostly descriptive and at least partly conflicting. Some have reported that Trask expression is increased in tumor tissues compared to normal tissues (Miyazawa et al 2010, Perry et al 2007, Scherl-Mostageer et al 2001, Uekita et al 2008). This has led to the suggestion that Trask may function to promote tumor metastasis. One group found increased surface expression of Trask in a more metastatic subline of HEp3 carcinoma cells, but the causal role of Trask as a metastasis promoter was not supported in a comparative analysis of its expression across cancer cell lines with different metastatic attributes (Hooper et al 2003). In another study, Uekita et al found that the shRNA knockdown of Trask reduced invasiveness in an in vivo gastric carcinoma cell model (Uekita et al 2008).
Other evidence does not support a tumor-associated expression pattern for Trask. In the first immuno-histochemical study of Trask expression in human tissues we found that Trask is widely expressed in most epithelial tissues, and quite abundantly in some (Spassov et al 2009). In an immuno-histochemical survey of cancer specimens we found great variability in tumor expression of Trask, but in comparison to many normal tissue sample controls for each disease type, we did not find a general increase in Trask expression associated with tumors (Wong et al 2009). Furthermore, the Trask gene maps to chromosome 3 at 3p21.3, an area associated with high frequency allelic loss in many human cancers, at least conceptually conflicting with a putative role as a tumor promoter (Ji et al 2005). In contrast and in more agreement with a possible tumor suppressive role, some cancers have low expression of Trask due to promoter hypermethylation (Ikeda et al 2006). Attempts to correlate the level of expression of Trask in human tumors with clinical outcome has also produced conflicting data sets as both poorer and better prognoses have been linked with higher Trask expression (Ikeda et al 2009, Mamat et al 2010, Miyazawa et al 2010).
Clearly our understanding of Trask/CDCP1/gp140 function is still evolving. We need better reagents to study its expression, more insight into the cellular functions of Trask for hypothesis generation, and more experimental models to interrogate its functions in cancer biology. In this study we begun by more closely looking at tumor expression of Trask in relation to the normal epithelium, and interrogated Trask function in tumor growth and metastasis using both gain-of-function and loss-of-function experimental models, and the data is most consistent with a tumor suppressive role for Trask.
Results
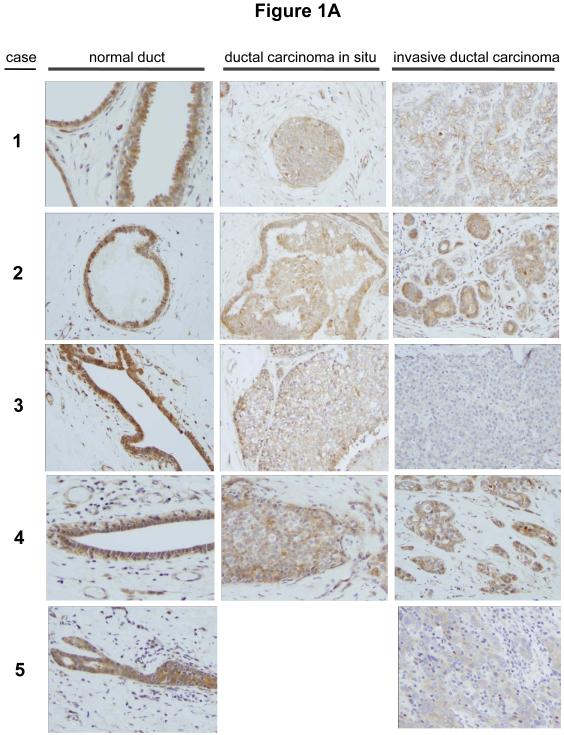
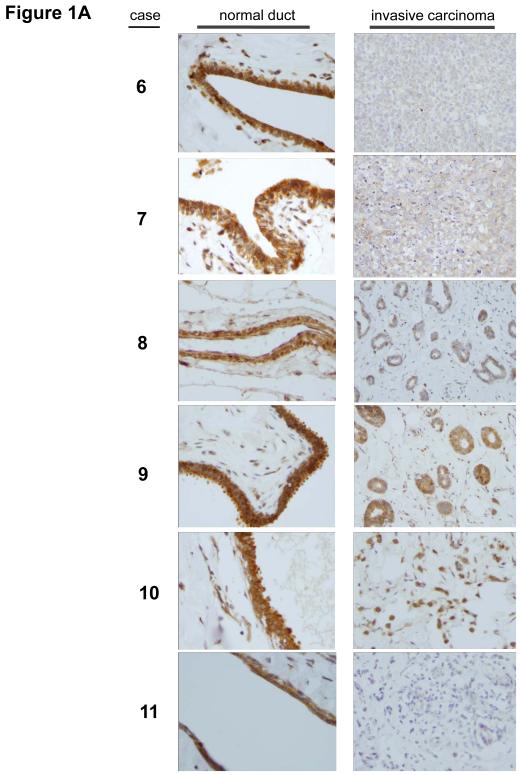
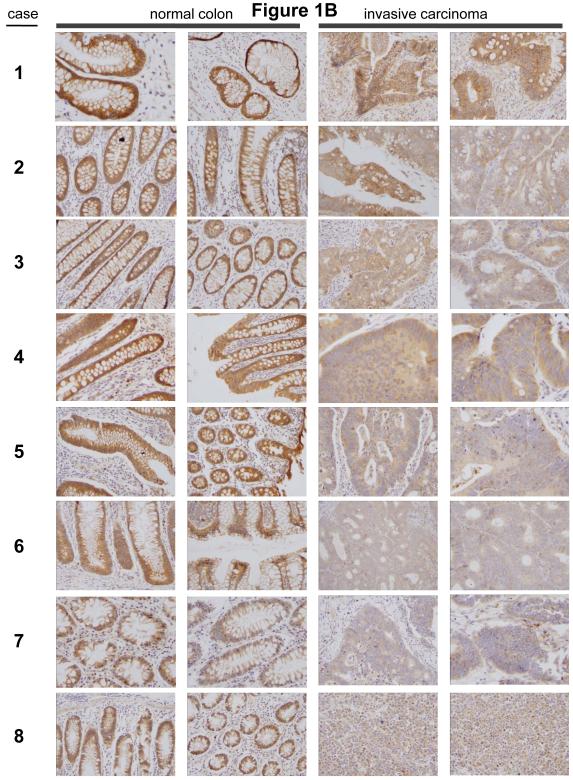
In our previous survey of human epithelial cancers, we graded the expression of Trask in about 90 carcinomas of the breast, colon, and lung as well as more than 30 surgical or biopsy specimens of normal breast, colon, and lung tissues (Wong et al 2009). This analysis, which included pre-invasive tumors as well as primary invasive tumors and tumor metastases did not reveal an increase in Trask expression in cancers, although phosphorylation of Trask was often seen in tumors. In fact some tumors appeared to have a Trask expression score that was lower than normal tissues. The normal tissue samples and the tumor tissue samples were from different patients in these studies. To more accurately and directly determine whether Trask expression is reduced or lost in some tumors we undertook to compare the expression of Trask in a series of breast and colon cancers compared with their adjacent normal epithelial tissue counterparts. We specifically looked for specimens in which both normal epithelium and cancer can be seen on the same slide so as to minimize variations in immunostaining intensity. In a survey of breast cancer tissues there is much variation in the expression of Trask. Some cancers have preserved Trask expression compared to the normal ductal epithelium, some cancers have reduced Trask expression, and some cancer have lost Trask expression (figure 1A). In a survey of colon cancer tissues there is also variation in the expression of Trask with preservation of expression in some cases, and a reduction in expression in many cases (figure 1B and supplementary figure S1). The expression of Trask in tumors is often heterogeneous with a patchy distribution. We do not see an overexpression of Trask in these cancers.
Figure 1. Comparative expression of Trask in normal and malignant human epithelium.
Archived paraffin embedded tissues from surgically resected breast and colon cancers were retrieve and stained with anti-Trask polyclonal antibodies. Images were taken from areas of normal tissue and tumor tissue within the same slide so as to most reliably compare Trask expression. A) 11 cases of breast cancer are shown comparing normal duct with tumor on the same slide. In cases where ductal carcinoma in situ was also present on the same slide, this is shown as well. Cases 1-9 are ductal carcinomas whereas cases 10-11 are lobular carcinomas. B) 8 cases of colon cancer are shown comparing normal epithelium with tumor. For each case, two different areas of normal epithelium and two different areas of tumor are shown from the same slide. Larger images are included in the supplementary materials for closer inspection. All stainings included positive and negative controls (supplementary figure S2).
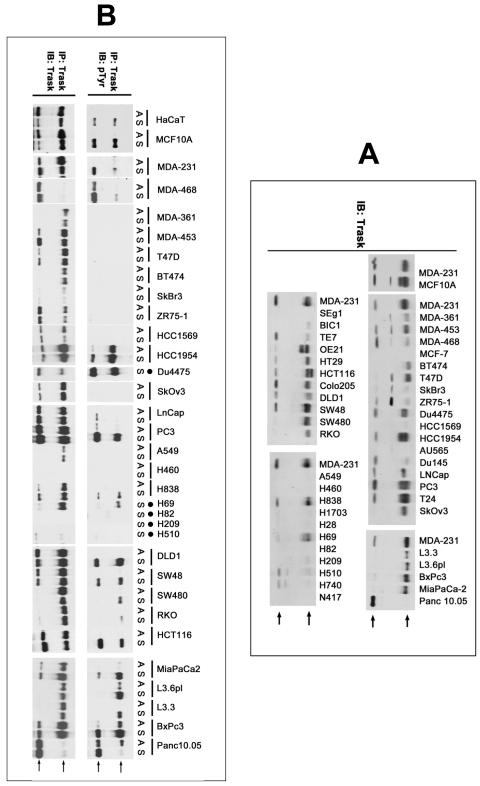
We have also determined the expression of Trask in a panel of epithelial cancer cell lines by western blotting. There is wide variation in the expression of Trask, including some cancer cells with very low or no expression (figure 2A). None of the cancer cell lines show Trask expression that is significantly greater than non-cancer cells such as MCF10A or HaCaT cells. We also considered that some cancers may have impaired Trask function rather than reduced expression. Although we don’t yet fully understand all the functions of Trask, we know that Trask is phosphorylated by Src kinases during anchorage deprivation and p-Trask functions in reciprocity with focal adhesion signaling and functions to inhibit integrin clustering and cell adhesion (Spassov et al 2009, Spassov et al 2011b). This appears to be a general attribute of epithelial cells and can be experimentally induced in cultured cells or in the mouse epidermis, or seen in detached epithelial cells in vivo (Spassov et al 2009, Spassov et al 2011b). Therefore we sought to determine whether the detachment-induced phosphorylation of Trask is preserved in all cancer cells. In our analysis of a panel of epithelial cancer cell lines we identified cancer cell lines that do express Trask, but fail to phosphorylate it when detached (figure 2B, see MDA-361, MDA-453, T47D, BT474, ZR75-1, SkOv3). RT-PCR amplification and sequencing of the Trask mRNA showed no mutations associated with Trask in these cells. These data from our comparative immunohistochemical survey of tissues and from the cell line panel suggest that Trask may contribute to tumor progression through a reduction or loss of function.
Figure 2. Expression of Trask in human epithelial cancer cell lines.
A) Total cell lysates from the indicated cancer cell lines were immunoblotted using anti-Trask monoclonal antibodies. MDA-231 cell lysates appear repeatedly on each of the separate blots to provide a reference basis for comparison among the blots. Arrows point to the two Trask bands. The intermediate band appearing in the lysates of some cells is a non-specific band and does not appear if the lysate is purified by anti-Trask immunoprecipitation. B) The indicated cell lines were harvested in the adherent (A) or suspended (S) states and lysates immunoprecipitated with anti-Trask antibodies and immunoblotted as indicated. The cells that lack an A lane are cells that grow naturally in suspension and do not have an adherent state in culture.
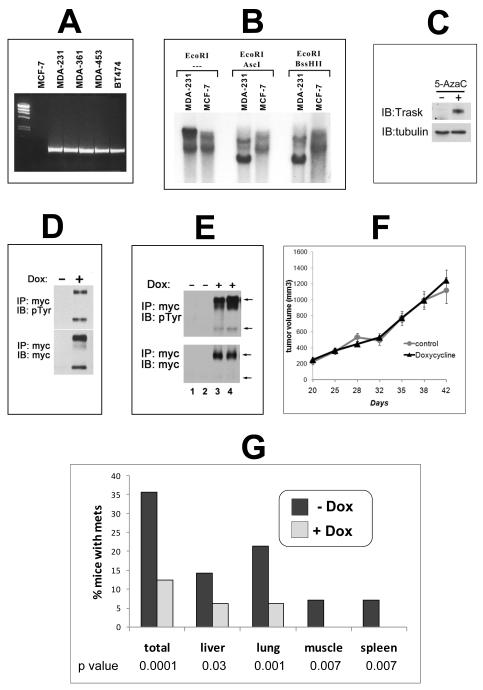
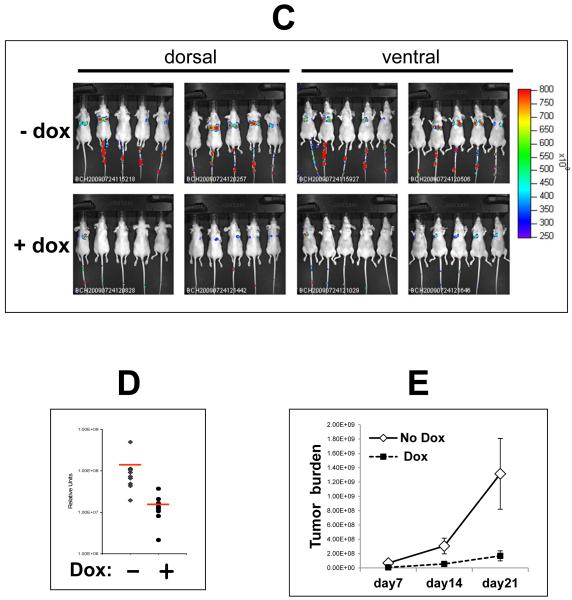
In order to experimentally interrogate the role of Trask in tumor growth and progression we re-expressed Trask in a tumor cell line that lacks its expression. MCF-7 cells have no expression of Trask protein (Figure 1A) and no expression of Trask mRNA (figure 3A). This is likely due to methylation silencing of the Trask promoter region as determined by southern blot analysis of the Trask promoter region using methylation-sensitive restriction enzymes (figure 3B). The promoter region of Trask is dense with many CpG repeats and its methylation silencing in cancer cells has been previously shown (Ikeda et al 2006). Treatment of MCF-7 cells with 5-azacytidine induces the re-expression of Trask, confirming that its silencing in these cells is mediated through genome methylation (figure 3C). MCF-7 cells were engineered to express the luciferase gene to aid with in vivo imaging, and also engineered to express Trask in a tet-inducible fashion. When Trask is induced to express in MCF-7/Luc/TR/Trask cells, it is constitutively phosphorylated, similar to its overexpression in other cancer cell lines (figure 3D) (Spassov et al 2011b). This may be due to the high activity of Src kinases in these tumor cells and/or the saturation of dephosphorylation mechanisms. When grown as orthotopically implanted tumors in mice, Trask expression can be induced in the MCF-7/Luc/TR/Trask tumors in vivo by administration of doxycycline to the mice (figure 3E). The expression of Trask in MCF-7/Luc/TR/Trask tumors in vivo has no significant effect on tumor growth (figure 3F). To determine whether tumor metastasis is affected by Trask expression mice were sacrificed at 7 weeks of tumor growth post-implantation, and the development of tumor metastases was assessed by necropsy analysis assisted by ex-vivo bioluminescence imaging. Metastases were identified at necropsy in a variety of organs, including lungs, liver, bone, muscle, lymph nodes and spleen (supplementary figure S3). Quantifying the number of mice with or without mets shows a reduction due to Trask expression such that 35.7% of the control mice had detectable metastases whereas only 12.5% of doxycycline-treated mice had detectable metastases (figure 3G). The difference was evident across all organs. Quantifying the number of metastases per mouse also shows a significant decrease in metastatic burden due to Trask expression with the doxycycline-fed mice having 4 times less metastatic disease (p=0.03; supplementary figure S4).
Figure 3. The induction of Trask expression in MCF-7 breast cancer cells.
A) Total cellular RNA from the indicated cells were used to detected Trask RNA expression by RT-PCR. B) Genomic DNA from MCF-7 and control MDA-231 cells were digested with EcoRI as well as the methylation-sensitive AscI and BssHII restriction enzymes and subjected to southern blot analysis using a radiolabelled Trask genomic probe corresponding to the promoter region of Trask. C) MCF-7 cells were cultured in media containing 10μM 5-azacytidine or control for one week and total cellular lysates immunoblotted with anti-Trask or anti-α-tubulin antibodies. D) MCF-7/Luc/TR/Trask cells were induced to express Trask by doxycycline treatment for 24 hours and cell lysates were immunoblotted as indicated to show the expression and phosphorylation of Trask. E) MCF-7/Luc/TR/Trask cells growing as orthotopic tumors in mice were harvested and cell lysates immunoblotted as indicated. The lanes correspond to 4 different mice in the control (1,2) or doxycycline-treated (3,4) arms. F) Tumor volumes are shown for mice bearing MCF-7/Luc/TR/Trask orthotopic tumors in the control or doxycycline treated arms. The data shown is the average with SEM for n=24 in each arm. G) The development of metastases was assessed by necropsy analysis including ex-vivo bioluminescence imaging in 31 randomized mice. The number of mice with metastases was determined within each arm and shown for the control (n=15) and doxycycline-treated (n=16) arms. The significance for each comparison was determined by the chi square test with the indicated p values.
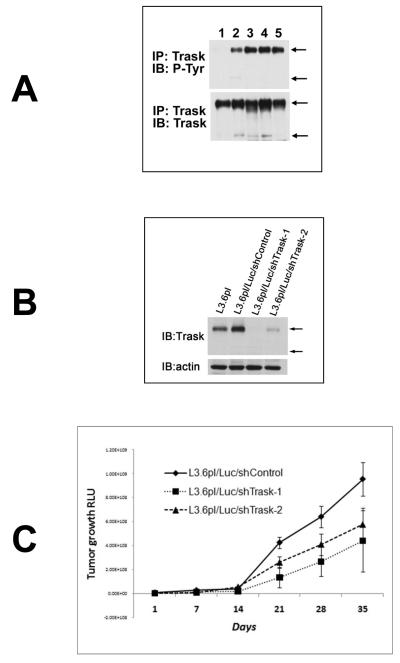
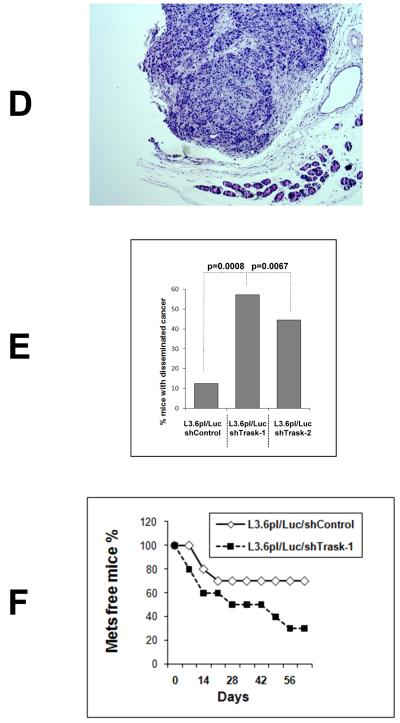
To further interrogate the role of Trask in tumor growth and progression we conducted a loss-of-function experiment in vivo. L3.6pl pancreatic cancer cells have expression of Trask and proper phosphorylation of Trask when anchorage deprived or when grown as orthotopically implanted tumors in the mouse pancreas (figure 4A). L3.6pl cells were engineered to express the firefly luciferase gene as well as a control non-silencing shRNA (L3.6pl/Luc/shControl) or either of two Trask shRNA sequences (L3.6pl/Luc/shTrask-1 and -2) leading to near-complete knockdown of Trask protein expression (figure 4B). The engineered L3.6pl/Luc/shTrask cells and controls were grown as orthotopically implanted tumors in the mouse pancreas and tumor growth rate and the development of tumor metastases was monitored by bioluminescence imaging. The L3.6pl/Luc/shTrask tumors showed slightly slower tumor growth rate compared with the L3.6pl/Luc/shControl tumors (Figure 4C). Mice were sacrificed at six weeks and the development of tumor metastases was assessed on necropsy analysis including ex-vivo bioluminescence imaging. At necropsy, metastases were frequently seen through peritoneal dissemination, as well as in the lungs and liver (figure 4D and supplementary figure S5). Trask knockdown tumors had an increased chance of metastasizing to the lung or liver, although not statistically significant (Table 1). But peritoneal dissemination was significantly increased in the Trask knockdown tumors. Peritoneal metastases was quantitatively assessed by bioluminescence imaging of the body cavity after removal of the primary tumor by pancreatectomy. Mice bearing tumors with Trask knockdown had significantly increased peritoneal metastatic disease compared with control mice (figure 4E). To further interrogate the metastatic potential of this tumor model, L3.6pl/Luc/shControl and L3.6pl/Luc/shTrask-1 tumor cells were introduced into the systemic circulation of mice by tail-vein injection and the development of systemic metastasis was assessed by weekly in vivo bioluminescence imaging. The development of tumor metastases was significantly accelerated in the Trask knockdown tumors compared with controls (figure 4F, p=0.02 by chi square test).
Figure 4. The knockdown of Trask expression in L3.6 pancreatic cancer cells.
A) L3.6pl pancreatic cancer cell lysates growing under different conditions are assayed as indicated. Lanes correspond to growth in vitro in the adherent (1) or suspended (2) states, growth in vivo as an orthotopic tumor in the pancreas (3,4) or a liver metastasis (5). B) L3.6pl cells expressing luciferase were engineered to express either of two Trask shRNA constructs or a control shRNA were assayed as indicated to confirm the knockdown of Trask. C) The indicated cell types were grown as orthotopically implanted tumors in mice. The pancreatic tumor burden was quantified weekly by bioluminescence imaging and the mean for each arm (n=10) is shown with SEM. D) The development of peritoneal metastasis was confirmed by histologic sections of formalin-fixed paraffin-embedded peritoneal tissue from necropsy. Shown here is a microscopic image of a representative H&E stained peritoneal metastasis. E) The development of peritoneal tumor dissemination was assessed on necropsy analysis with the help of bioluminescence imaging and the fraction of mice within each arm that had disseminated cancer is shown. F) The indicated cell types were introduced into the systemic circulation of mice by tail-vein injection and mice were imaged regularly to detect the onset of systemic metastases. The data is shown for each arm (n=10) as metastasis-free survival.
Table 1.
Metastases in L3.6pl-Luc-shRNA tumors
| Arm | N | %mice with liver mets |
%mice with lung mets |
% mice with intraperitoneal dissemination |
|---|---|---|---|---|
| L3.6pl-Luc-shControl | 8 | 25 | 25 | 12 |
| L3.6pl-Luc-shTrask-1 | 7 | 57 | 29 | 57 |
| L3.6pl-Luc-shTrask-2 | 9 | 55 | 33 | 44 |
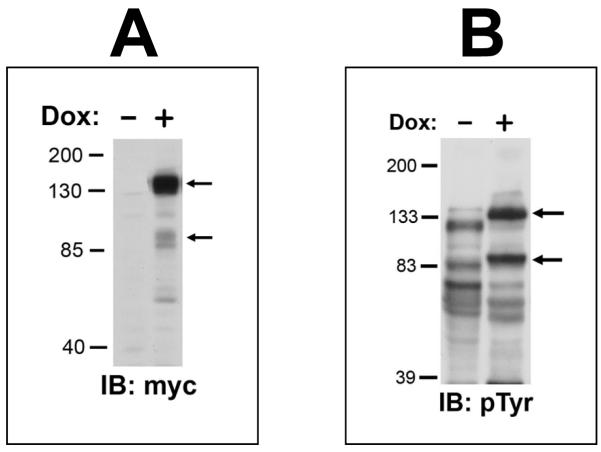
Since the data suggests that the functions of Trask may be tumor suppressing, we sought additional evidence in a third experimental model of in vivo tumor metastasis. v-src transformed fibroblasts are highly metastatic in a tail-vein injection model and have low expression of Trask. We engineered 3T3v-src cells to express firefly luciferase as well as myc-tagged Trask in a doxycycline-inducible fashion (figure 5A). The overexpression of Trask in these cells leads to abundant tyrosine phosphorylation of Trask similar to other cancer cells with active Src kinases (figure 5B). 3T3v-src/Luc/TR/Trask cells were introduced into the systemic circulation of mice by tail-vein injection in two arms consisting of mice treated with doxycycline or control. Doxycycline treatment was initiated the day prior to injection. Mice were monitored for the development of metastases weekly by in vivo bioluminescence imaging. Control mice develop metastases with high frequency and short latency, and the induction of tumor Trask expression is associated with a significant reduction in tumor metastastic burden (Figure 5C). Both the rate of development and the burden of tumor metastasis is significantly reduced by the induction of Trask expression (Figure 5D,E).
Figure 5. The induction of Trask overexpression in v-src transformed cells.
A) V-Src transformed fibroblasts expressing luciferase were engineered to overexpress myc-tagged Trask in a tet-inducible fashion. The doxycycline-induced expression of Trask was confirmed in this anti-myc immunoblot. Arrows indicate the two forms of Trask. B) The doxycycline-induced expression of phosphorylated Trask was confirmed in this anti-phosphotyrosine immunoblot. C) 3T3v-src/Luc/TR/Trask cells were injected into the tail vein of mice being fed water with or without doxycyline (n=10) and the development of lung metastases was monitored weekly using bioluminescence imaging. D) The total luminescence over the lung fields was captured for each mouse and the quantitative results shown here according to the experimental arm. The red lines indicate the average of the values for each arm. The 8-fold difference in the average values is highly significant (2-sided t-test, p=0.04) E) Quantitative analysis of the total luminescence signal over time from the control and doxycycline-treated mice at 7, 14 and 21 days after inoculation. Error bars represent ± SEM.
These experiments, in three in vivo models, show that the functions of Trask are negatively associated with tumor progression, a function consistent with a tumor suppressing function. We recently described that Trask, when phosphorylated, functions to inhibit integrin clustering and outside-in integrin signaling (Spassov et al 2011b). Since integrin signaling is important in tumor progression and metastasis, we looked to determine whether the induction of Trask overxpression in our experimental models is also associated with the suppression of integrin outside-in signaling. In both the MCF-7/Luc/TR/Trask cells and in the 3T3v-src/Luc/TR/Trask cells, the induction of Trask expression and phosphorylation by doxycycline leads to the dephosphorylation of FAK, direct evidence of the inhibition of integrin outside-in signaling (figure 6).
Figure 6. The inhibition of integrin signaling by Trask overexpression.
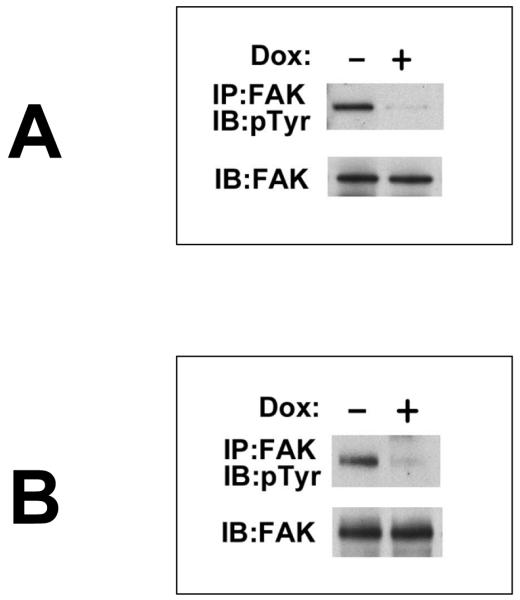
A) MCF-7/Luc/TR/Trask cells were treated with 2ug/ml doxycycline overnight and the phosphorylation state of FAK was determined by anti-FAK immunoprecipitation and immunoblotting as indicated. B) 3T3v-src/Luc/TR/Trask cells were induced with doxycycline and assayed exactly as in part A.
Discussion
Trask/CDCP1 is a transmembrane protein with little homology to known human proteins and its functions are difficult to predict by informatic methodologies which depend on structural homologies. Trask has only been studied in the past few years and the body of data that has emerged thus far has been conflicting with regards to a role in human cancer. It localizes to chromosome 3 on 3p21.3, an area associated with high frequency allelic loss in human cancers, suggesting that its functions may be tumor suppressive. This area of the genome has been studied extensively and a high resolution map developed that identifies the areas of genomic loss in cancers (Wistuba et al 2000) (Ji et al 2005). The Trask/CDCP1 gene is flanked by the markers D3S1029 and D3S1478 (from the NCBI genome map) referenced in the study by Wistuba et al, identifying it as a gene with loss of heterozygosity in a large fraction of lung cancers, but not normal or pre-neoplastic bronchial epithelium (Wistuba et al 2000). Further consistent with a potential tumor suppressing role is the evidence showing that its expression is reduced or silenced by promoter methylation in some cancer cell lines (Ikeda et al 2006). In this study we find that the expression of Trask relative to the normal epithelium is reduced or lost in some cancers of the breast or colon. This is also evident in panels of cancer cell lines. Furthermore, a functional analysis in the panel of cancer cell lines reveals that some aspects of Trask function appear to be lost in some cancers. These data led us to interrogate the role of tumor Trask expression in experimental models.
To interrogate the functional role of Trask in experimental models of cancer progression, we used both gain-of-function models as well as loss of function models. These results across the three models that we studied are all consistent with a tumor suppressing function, in particular a function that suppresses tumor metastasis rather than tumor growth. The mechanisms by which Trask may suppress tumor progression is unknown. We have shown that Trask, when phosphorylated, functions to inhibit integrin signaling, disrupt focal adhesions, and oppose cell adhesion (Spassov et al 2011b). Clearly integrin signaling and cell adhesion are functions that tumor cells require for processes of migration and metastases and this function of Trask may be tumor suppressive (Brakebusch et al 2002, Felding-Habermann 2003, Hood and Cheresh 2002, Ramsay et al 2007). We also show (Figure 2B) that some cancer cell lines that do express Trask, fail to phosphorylate it upon anchorage deprivation. This could also be consistent with the hypothesis that the phosphorylation of Trask and the consequent inactivation of integrin signaling is the tumor suppressive aspect of its functions. But in a survey of human cancers we found that Trask is phosphorylated in many cancers and not phosphorylated in others (Wong et al 2009). There are several hypotheses that can be proposed to reconcile the tumor survey data with the experimental data. The most likely explanation is that the phosphorylation of Trask in epithelial tumors is a physiological phenomenon due to the loss of normal adhesion to underlying basement membrane. This physiological phosphorylation may function to suppress the premature adhesion of unanchored epithelial cells until the appropriate basement membrane context is established. Such a function would be suppressive for tumor progression and consistent with a selective pressure to lose Trask or its phosphorylation because of its integrin inhibitory functions. In our doxycycline-inducible tumor models, we see an inhibition of FAK signaling that is consistent with this. The role of integrin-mediated FAK signaling in tumor progression and metastasis is now well established. In experimental models, the activation of FAK signaling enhances tumor metastases, and the suppression of FAK signaling by genetic ablation, dominant negatives, knockdown, or pharmacologic inhibitors inhibits tumor metastases (Abdel-Ghany et al 2002, Chen et al 2010, Hanada et al 2005, Lahlou et al 2007, Provenzano et al 2008, Shibue and Weinberg 2009, Sun et al 2010, Tang et al 2008, Trimmer et al 2010, van Nimwegen et al 2005, Walsh et al 2010). Considering the abundance of evidence regarding the role of FAK in promoting tumor metastases, it is easily conceivable that a physiological inhibitor of integrin-FAK signaling, such as phosphorylated Trask, could have tumor suppressing functions and subject to negative selection pressure during tumor evolution. The phosphorylation of Trask seen in many tumors may be also be non-physiologic and a direct consequence of elevated Src kinase activity, prematurely activating this Src-driven anti-adhesive pathway.
It is also possible that some of the tumor suppressive functions of Trask are embodied within its extracellular domain (ECD). Much less is currently known about the functions of the Trask ECD. We previously showed that Trask interacts with the tumor-associated protease MT-SP1 and its ECD is cleaved by MT-SP1 (Bhatt et al 2005). But the functional significance of this interaction is not yet known. The anti-adhesive functions of Trask are mediated entirely through tyrosine phosphorylation of its intracellular domain (Spassov et al 2011a), therefore a tumor suppressing function carried within its ECD would likely invoke a different mechanism of action. It is however possible that the tumor suppressing functions of Trask are a composite of intracellular and extracellular domain functions. It is also possible that the functions of Trask are more complex than the designation of a tumor suppressor or promoter and it may in fact have roles as both in different contexts.
A number of previous studies have reported an overexpression of Trask/CDCP1 in tumors, leading some to propose that Trask may function as a tumor promoter. But looking at some of these data in hindsight, and with the benefit of our current knowledge of Trask expression and the many newer reagents that have since been developed, these data sets can now be seen in a new perspective and their conclusions reassessed. Two groups have reported that Trask/CDCP1 RNA expression is elevated in cancer tissues and cancer cell lines compared with normal tissue controls including colon cancers (Perry et al 2007, Scherl-Mostageer et al 2001). Trask/CDCP1 protein expression was not evaluated in these studies and may not parallel its RNA expression. More importantly, we know now that Trask/CDCP1 is widely expressed in epithelial cells but not in the mesenchymal compartment (Spassov et al 2009). The use of normal tissue controls in studies of RNA expression is complicated by the fact that the normal tissue samples contain only a thin epithelial layer with much underlying stromal mesenchymal and sometimes muscle components, while tumor tissues are often dense with the epithelial tumor cells. Therefore the increased Trask/CDCP1 RNA expression in tumor tissues observed in these studies likely reflects the much higher epithelial content in the tumor tissue samples and the purely epithelial nature of cancer cell lines, when compared with samples obtained from normal tissues, which have only a thin layer of epithelium. There are very few immuno-histochemical studies comparing tumor Trask/CDCP1 expression to normal epithelial tissue counterparts. In one study of five cases of prostate cancers, Siva et al found that Trask/CDCP1 expression was most abundant in the normal prostate and less intense and occasionally lost in prostate cancer (Siva et al 2008). In our previous survey of 90 cancers of the breast, colon, and lung that included 30 normal tissue controls, we found that Trask/CDCP1 was expressed in all the normal tissues and there was no evidence of overexpression in tumor tissues. In one contradictory immuno-histochemical study, Miyazawa et al reported the absence of Trask/CDCP1 expression in normal pancreatic ducts but widespread expression in pancreatic cancers (Miyazawa et al 2010). This may have been due to antibody specificity since we find abundant Trask/CDCP1 expression in the normal pancreatic ducts (data not shown) in concordance with the pancreatic ductal expression reported by Siva et al (Siva et al 2008). Our current study now examines Trask/CDCP1 expression by immunohistochemistry with a direct comparative analysis of tumor cells and their normal epithelial counterparts side-by-side and this analysis shows no evidence of Trask/CDCP1 overexpression, rather evidence that its expression is in fact reduced in some cancers and sometimes lost. The western blot evidence in panels of epithelial cell lines also is consistent with a reduction or absence of Trask/CDCP1 expression or its functions in some cancer cell types.
Several clinical correlative studies have looked for associations between tumor Trask/CDCP1 expression and prognosis and the results have been mixed with both positive and negative correlations observed (Awakura et al 2008, Ikeda et al 2009, Mamat et al 2010, Miyazawa et al 2010). Correlative studies do not by themselves reveal a functional role for Trask/CDCP1 since its expression levels may correlate with other functional parameters including other genes or distinct molecular subtypes of the specific cancer. Some of the discordance may also be related to difference in antibody reagents. The function of Trask/CDCP1 was interrogated by siRNA knockdown in one study of gastric cancer cells with the finding that the knockdown tumor cells had a reduction in tumor invasion and dissemination (Uekita et al 2008). These results appear contradictory to ours, and the discordance is difficult to explain. One possibility is that the functions of Trask/CDCP1 are cell type or tissue type dependent. It is also possible that the functions of Trask are more complex than and include tumor suppressive and promoting functions under different contexts.
Materials and Methods
Cell Culture and reagents
Cell lines were obtained from the American Type Culture Collection. To force cells into suspension, they were washed in PBS and exposed to a 2mM solution of EDTA in Hank’s buffer, and when fully detached, were washed and cultured in growth media in ULC plates (Corning) for 2 hours. ULC plates are not permissive to cell adhesion.
Anti-phosphotyrosine antibodies (PY99) were from SantaCruz Biotechnology, Inc (SantaCruz, CA). Generation of polyclonal and monoclonal anti-Trask antibodies were previously described (Wong et al 2009).
Immunohistochemical studies
Tissue blocks were obtained under an IRB approved protocol for studies of archival tissue samples in the tissue banks of our center. Deparaffinized sections were rehydrated and antigen retrieval was performed by 15 minutes incubation in warm trypsin followed by microwave in 10mM citrate buffer for total of 10 minutes in 1 minute intervals. Slides were then washed and blocked with 3% H2O2 followed by blocking in goat serum and primary incubation at 4C overnight. Secondary staining was performed using biotinylated goat anti-rabbit antibodies (Vector Labs, Burlingame, CA) and colorized using Vectastain ABC Kit (Vector Labs) and 3,3′-diaminobenzidine (DAB)-H2O2 substrate (Sigma, St, Louis, MO). Slides were then counterstained with hematoxylin, dehydrated through graded alcohols and xylene and mounted. Slides were studied and imaged under brightfield microscopy. All staining procedures included positive and negative controls. The positive control was MDA-MB-468 cells and the negative control was MCF-7 cells which do and don’t express Trask. Immunohistochemically stained tissue sections were viewed and imaged using an Olympus BX41 brightfield microscope fitted with a DP70 digital camera. Images were acquired using the Olympus DP Controller software and gamma adjusted for optimal representation.
Generation of doxycycline inducible cell lines
V-src transformed NIH3T3 cells were stably transfected with pcDNA6/TR followed by pcDNA4-TO-MycHis-Trask to generate cells with dox-inducible expression of Trask. Several clones were expanded and the doxycycline-inducible expression of Trask was confirmed by myc immunoblotting. These were then stably transfected with a pcDNA3.1-CMV-luciferase reporter construct. MCF-7 cells were stably transfected with pcDNA6/TR to levels sufficient for suppression of expression of a GFP reporter construct in the absence of doxycycline. These cells were then stably transfected with pcDNA4-TO-MycHis-Trask to generate cells with dox-inducible expression of Trask and confirmed by anti-myc immunoblotting.
Generation of Trask knockdown cells
shRNA sequences were cloned into pSico-RGFP and pSico-RNeo vectors. pSico-RGFP expresses GFP as a selectable marker and pSico-RNeo contains neomycin resistant cassette. To create the shTrask-1 construct, the oligo: 5′-TGAATGTTGCTTTCTCGTGGCAGTTCAAGAGACTGCCACGAGAAAGCAACATTTTTTTTGGATCC-3′ was annealed to 5′-TCGAGGATCCAAAAAAAATGTTGCTTTCTCGTGGCAGTCTCTTGAACTGCCACGAGAAAGCAACATTCA-3′ and cloned into HPA-I and XhoI sites of the pSicoR vector. To create the shTrask-2 construct, the oligo: 5′-TGATAGATGAGCGGTTTGCAATGCTGATTCAAGAGATCAGCATTGCAAACCGCTCATCTATTTTTTTTGGCGCGCC-3′ was annealed to 5′-TCGAGGCGCGCCAAAAAAAATAGATGAGCGGTTTGCAATGCTGATCTCTTGAATCAGCATTGCAAACCGCTCATCTATCA-3′ and cloned into HPA-I and XhoI sites of the vector. For generation of the non-silencing construct the oligo 5′- TGTCTCGCTTGGGCGAGAGTAAGTTCAAGAGACTTACTCTCGCCCAAGCGAGATTTTTTTGGCGCGCC-3′ and 5′-TCGAGGCGCGCCAAAAAAATCTCGCTTGGGCGAGAGTAAGTCTCTTGAACTTACTCTCGCCCAAGCGAGACA-3′ were similarly annealed and cloned. The pSico-shRNA constructs were transfected into 293T cells along with the appropriate packaging vectors to generate lentiviral particles for infection. L3.6pl cells expressing firefly luciferase were infected with the pSico-RNeo-shRNA lentiviral particles and selected in G418 (300 ug/ml).
Animal studies
All xenograft studies were conducted under the guidelines of the UCSF Institutional Animal Care and Use Committee under an IACUC approved protocol. 3T3vsrcTR/Trask/Luc cells were introduced into the systemic circulation of 20 nude mice by tail-vein injection at an inoculum of 1,000,000 cells per mouse in two arms. In the experimental arm, both cells and mice were treated with doxycycline beginning the day before injection and mice continued treatment with doxycycline in drinking water for the duration of the experiment. In the control arm, there was no exposure to doxycycline and cells and mice were only exposed to the vehicle. 7-21 days after inoculation, tumor burden was assayed in all mice by injection with luciferin and in vivo bioluminescence imaging. Both dorsal and ventral views were imaged and shown.
MCF7TR/Trask/Luc cells were implanted orthotopically into the mammary fat pad of nude mice (2,000,000 cells per mouse). Once implanted, the recipient mice were continuously fed regular water or water containing doxycycline (2mg/ml). Primary tumor growth was monitored biweekly after implantation. After 7 weeks, mice were injected with luciferin, immediately sacrificed, the primary tumor rapidly resected, and the development of metastases was assessed with the assistance of bioluminescence imaging of the body cavity and organs ex vivo. Quantifying the total number of micro-metastasis foci is impossible, therefore we quantified the number of organs with metastatic disease.
L3.6pl cells were engineered to express the firefly luciferase reporter gene and Trask or control shRNA. The tumor cells were initially grown as a subcutaneously implanted tumor in nude mice and tumor tissue was used for the pancreatic implantation. The pancreas implantion was done under sterile conditions, using sterilized surgical instruments. A 1.0-1.5 cm left abdominal flank incision was made and the spleen and adherent pancreas tissue exteriorized. Sterilely dissected tumor tissue derived from a subcutaneously implanted xenograft sample was cut into small fragments and a 2-4 mm3 chunk of tumor was implanted into a small pocket made using microscissors, and the pocket closed using 8-0 nylon suture. The spleen was returned to its original position, the muscle layer adapted with 6-0 nylon suture, and skin closed with 9mm wound clamps. After implantation, the mice were checked for tumor formation twice a week by palpation and by weekly bioluminescent imaging.
L3.6pl/Luc/shControl and L3.6pl/Luc/shTrask cells were introduced by tail-vein injection at an inoculum of 1,000,000 cells per mouse. Subsequent imaging was performed weekly. In all cases mice were imaged with in vivo imaging system (IVIS, Caliper Life Sciences). The bioluminescence was quantified using Living images software (Caliper Life Sciences).
Supplementary Material
Acknowledgements
This work was funded by the National Institutes of Health CA113952 (MMM). DS is funded by a Susan G. Komen for the Cure Postdoctoral Fellowship. CHW is funded by a California Breast Cancer Research Program Postdoctoral Fellowship. We wish to thank Michael McManus and the UCSF Sandler Lentiviral RNAi core facility. We acknowledge the use of core facilities of the UCSF Helen Diller Family Comprehensive Cancer Center including the Preclinical Therapeutics Core, the immunohistochemistry core, and the mouse pathology core.
Footnotes
Disclosure of Potential Conflicts of Interest The authors have no conflicts to disclose
References
- Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. Focal adhesion kinase activated by beta(4) integrin ligation to mCLCA1 mediates early metastatic growth. J Biol Chem. 2002;277:34391–34400. doi: 10.1074/jbc.M205307200. [DOI] [PubMed] [Google Scholar]
- Awakura Y, Nakamura E, Takahashi T, Kotani H, Mikami Y, Kadowaki T, et al. Microarray-based identification of CUB-domain containing protein 1 as a potential prognostic marker in conventional renal cell carcinoma. J Cancer Res Clin Oncol. 2008;134:1363–1369. doi: 10.1007/s00432-008-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Yang TM, Zaitsevskaia T, Xia Y, Dunn CA, Sigle RO, et al. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J Biol Chem. 2004;279:14772–14783. doi: 10.1074/jbc.M309678200. [DOI] [PubMed] [Google Scholar]
- Chen JS, Huang XH, Wang Q, Chen XL, Fu XH, Tan HX, et al. FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin Exp Metastasis. 2010;27:71–82. doi: 10.1007/s10585-010-9306-3. [DOI] [PubMed] [Google Scholar]
- Conze T, Lammers R, Kuci S, Scherl-Mostageer M, Schweifer N, Kanz L, et al. CDCP1 is a novel marker for hematopoietic stem cells. Ann N Y Acad Sci. 2003;996:222–226. doi: 10.1111/j.1749-6632.2003.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- Hanada M, Tanaka K, Matsumoto Y, Nakatani F, Sakimura R, Matsunobu T, et al. Focal adhesion kinase is activated in invading fibrosarcoma cells and regulates metastasis. Clin Exp Metastasis. 2005;22:485–494. doi: 10.1007/s10585-005-3733-6. [DOI] [PubMed] [Google Scholar]
- Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hooper JD, Zijlstra A, Aimes RT, Liang H, Claassen GF, Tarin D, et al. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22:1783–1794. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- Ikeda J, Oda T, Inoue M, Uekita T, Sakai R, Okumura M, et al. Expression of CUB domain containing protein (CDCP1) is correlated with prognosis and survival of patients with adenocarcinoma of lung. Cancer Sci. 2009;100:429–433. doi: 10.1111/j.1349-7006.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda JI, Morii E, Kimura H, Tomita Y, Takakuwa T, Hasegawa JI, et al. Epigenetic regulation of the expression of the novel stem cell marker CDCP1 in cancer cells. J Pathol. 2006;210:75–84. doi: 10.1002/path.2026. [DOI] [PubMed] [Google Scholar]
- Ji L, Minna JD, Roth JA. 3p21.3 tumor suppressor cluster: prospects for translational applications. Future Oncol. 2005;1:79–92. doi: 10.1517/14796694.1.1.79. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamat S, Ikeda J, Enomoto T, Ueda Y, Rahadiani N, Tian T, et al. Prognostic significance of CUB domain containing protein expression in endometrioid adenocarcinoma. Oncol Rep. 2010;23:1221–1227. doi: 10.3892/or_00000753. [DOI] [PubMed] [Google Scholar]
- Miyazawa Y, Uekita T, Hiraoka N, Fujii S, Kosuge T, Kanai Y, et al. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010;70:5136–5146. doi: 10.1158/0008-5472.CAN-10-0220. [DOI] [PubMed] [Google Scholar]
- Perry SE, Robinson P, Melcher A, Quirke P, Buhring HJ, Cook GP, et al. Expression of the CUB domain containing protein 1 (CDCP1) gene in colorectal tumour cells. FEBS Lett. 2007;581:1137–1142. doi: 10.1016/j.febslet.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol. 2008;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26:567–578. doi: 10.1007/s10555-007-9078-7. [DOI] [PubMed] [Google Scholar]
- Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene. 2001;20:4402–4408. doi: 10.1038/sj.onc.1204566. [DOI] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva AC, Wild MA, Kirkland RE, Nolan MJ, Lin B, Maruyama T, et al. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008;68:3759–3766. doi: 10.1158/0008-5472.CAN-07-1657. [DOI] [PubMed] [Google Scholar]
- Spassov DS, Baehner FL, Wong CH, McDonough S, Moasser MM. The transmembrane src substrate Trask is an epithelial protein that signals during anchorage deprivation. Am J Pathol. 2009;174:1756–1765. doi: 10.2353/ajpath.2009.080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassov DS, Ahuja D, Wong CH, Moasser MM. The Structural Features of Trask That Mediate Its Anti-Adhesive Functions. PLoS One. 2011a;6:e19154. doi: 10.1371/journal.pone.0019154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassov DS, Wong CH, Sergina N, Ahuja D, Fried M, Sheppard D, et al. Phosphorylation of Trask by Src kinases inhibits integrin clustering and functions in exclusion with focal adhesion signaling. Mol Cell Biol. 2011b;31:766–782. doi: 10.1128/MCB.00841-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg BD, Hamm HE. Roles of G-protein-coupled receptor signaling in cancer biology and gene transcription. Curr Opin Genet Dev. 2007;17:40–44. doi: 10.1016/j.gde.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Srinivasan DM, Kapoor M, Kojima F, Crofford LJ. Growth factor receptors: implications in tumor biology. Curr Opin Investig Drugs. 2005;6:1246–1249. [PubMed] [Google Scholar]
- Sun H, Pisle S, Gardner ER, Figg WD. Bioluminescent imaging study: FAK inhibitor, PF-562,271, preclinical study in PC3M-luc-C6 local implant and metastasis xenograft models. Cancer Biol Ther. 2010;10:38–43. doi: 10.4161/cbt.10.1.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci. 2008;65:2933–2942. doi: 10.1007/s00018-008-8315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Trimmer C, Whitaker-Menezes D, Bonuccelli G, Milliman JN, Daumer KM, Aplin AE, et al. CAV1 inhibits metastatic potential in melanomas through suppression of the integrin/Src/FAK signaling pathway. Cancer Res. 2010;70:7489–7499. doi: 10.1158/0008-5472.CAN-10-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekita T, Tanaka M, Takigahira M, Miyazawa Y, Nakanishi Y, Kanai Y, et al. CUB-domain-containing protein 1 regulates peritoneal dissemination of gastric scirrhous carcinoma. Am J Pathol. 2008;172:1729–1739. doi: 10.2353/ajpath.2008.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen MJ, Verkoeijen S, van Buren L, Burg D, van de Water B. Requirement for focal adhesion kinase in the early phase of mammary adenocarcinoma lung metastasis formation. Cancer Res. 2005;65:4698–4706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. 2010;9:778–790. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba II, Behrens C, Virmani AK, Mele G, Milchgrub S, Girard L, et al. High resolution chromosome 3p allelotyping of human lung cancer and preneoplastic/preinvasive bronchial epithelium reveals multiple, discontinuous sites of 3p allele loss and three regions of frequent breakpoints. Cancer Res. 2000;60:1949–1960. [PubMed] [Google Scholar]
- Wong CH, Baehner FL, Spassov DS, Ahuja D, Wang D, Hann B, et al. Phosphorylation of the SRC epithelial substrate Trask is tightly regulated in normal epithelia but widespread in many human epithelial cancers. Clin Cancer Res. 2009;15:2311–2322. doi: 10.1158/1078-0432.CCR-08-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.