Abstract
Purpose
To determine if the accuracy of the baseline prediction model for the development of primary open-angle glaucoma (POAG) in ocular hypertension patients can be improved by correcting intraocular pressure (IOP) for central corneal thickness (CCT).
Design
Re-analysis of the baseline prediction model for the development of POAG from the Ocular Hypertension Treatment Study (OHTS) substituting IOP adjusted for CCT using 5 different correction formulae for unadjusted IOP.
Participants
1,433 of 1,636 participants randomized to OHTS who had complete baseline data for factors in the prediction model – age, IOP, CCT, vertical cup-to-disc ratio (VCDR) and pattern standard deviation (PSD).
Methods
Re-analysis of the prediction model for the risk of developing POAG using the same baseline variables (age, IOP, CCT, VCDR and PSD) except that IOP was adjusted for CCT using correction formulae. A separate Cox proportional hazards model was run using IOP adjusted for CCT by each of the five formulae published to date. Models were run including and excluding CCT.
Main Outcome Measures
Predictive accuracy of each Cox proportional hazards model was assessed using the c-statistic and calibration chi-square.
Results
C-statistics for prediction models that used IOP adjusted for CCT by various formulas ranged from 0.75 to 0.77, no better than the original prediction model (0.77) that did not adjust IOP for CCT. Calibration chi-square was acceptable for all models. Baseline IOP, whether adjusted for CCT or not, was statistically significant in all models including those with CCT in the same model. CCT was statistically significant in all models including those with IOP adjusted for CCT in the same model.
Conclusion
The calculation of individual risk for developing POAG in ocular hypertensive individuals is simpler and equally accurate using IOP and CCT as measured, rather than applying an adjustment formula to correct IOP for CCT.
Introduction
Central corneal thickness (CCT) is among the strongest, independent predictors for the development of primary open-angle glaucoma (POAG) in the Ocular Hypertension Treatment Study (OHTS)1 and the European Glaucoma Prevention Study (EGPS).2 The risk of developing POAG doubled for every 40 μm decrease in CCT from the overall mean of 573.3 μm in the OHTS and EGPS pooled sample.3 Other independent predictive factors for the development of POAG in the OHTS and EGPS prediction models were baseline age, intraocular pressure (IOP), vertical cup-to-disc ratio (VCDR) and pattern standard deviation (PSD). Other studies have also reported similar associations between CCT and the incidence of POAG4–6 or the prevalence of POAG7–9.
Although CCT and IOP have an independent effect on the risk of developing POAG, in fact, these two factors interact. When Goldmann applanation tonometry (GAT) was introduced in the 1950s, the thickness of the cornea was recognized as a potential confounder to IOP measurement.10 As optical and ultrasonic pachymeters became widely available, researchers realized that CCT varied greatly between individuals. Since then, a number of investigators have developed formulae to “adjust” IOP as measured by GAT for CCT.11–15 These formulae have been based on cannulation studies of eyes during cataract surgery,11, 12, 15 meta-analyses of published datasets14 or engineering models of the applanated cornea.13
Why CCT is such a powerful predictor of POAG is unknown. Some clinicians think that the only reason central corneal thickness is in the prediction model for the development of POAG is to correct IOP for mismeasurement. To address this issue, we correct IOP for CCT using published correction formulae described above. If the influence of CCT on GAT fully explains CCT’s role as a predictive factor, correcting IOP using these formulae should cause CCT to drop out of the predictive model.
Neither the OHTS nor EGPS prediction models for the development of POAG adjusted baseline IOP for corneal thickness by correction formulae. To determine whether doing so might improve the predictive ability of the model, we re-calculated the predictive model for the development of POAG substituting the value of IOP adjusted for CCT for the unadjusted IOP. We compare the predictive accuracy of the original prediction model with unadjusted values of IOP to prediction models with values of IOP adjusted for CCT using correction formulae by Ehlers11, Whitacre12, Orssengo and Pye13, Doughty14, and Kohlhaas.15
Methods
OHTS is an unmasked randomized trial of the safety and efficacy of topical ocular hypotensive medication in preventing or delaying the development of POAG in individuals with ocular hypertension. The design and methods of OHTS have been described previously and can be found at http://ohts.wustl.edu (accessed March 1, 2011) and are briefly summarized here.16, 17 Eligibility criteria included age 40–80 years, a qualifying IOP ≥ 24 mm Hg and ≤ 32 mm Hg in one eye and ≥ 21 mm Hg and ≤ 32 mm Hg in the fellow eye. Both eyes had to meet eye-specific criteria including gonioscopically open angles, normal and reliable visual fields and normal optic discs. Individuals signed an informed consent approved by the Institutional Review Board of each participating clinic.
Beginning February, 1994, 1,636 individuals were randomized to either observation or treatment with topical ocular hypotensive medication. All topical ocular hypotensive medications available commercially in the United States were available from the OHTS central pharmacy. Medication was selected at the clinician’s discretion.
Follow-up Visits and Tests
Semi-annual visits included an ocular and medical history, refraction, best corrected visual acuity, full threshold Humphrey white-on-white 30-2 visual field test, slit lamp examination, IOP measurement, and direct ophthalmoscopy. In addition, annual visits included dilated fundus examination and stereoscopic optic disc photography.
Intraocular pressure was measured by two certified study personnel, an operator and a recorder, using a calibrated Goldmann applanation tonometer. The operator initially set the dial at 10 mm Hg and then looked through the slit-lamp and adjusted the dial while the recorder read and recorded the results. This procedure was repeated on the same eye. If the two readings differed by 2 mm Hg or less, the average of the two readings served as the visit IOP. If the two readings differed by more than 2 mm Hg, a third reading was performed and the median of the three readings served as the visit IOP.
Central corneal thickness (CCT) was measured at the clinical center by calibrated ultrasonic pachymeters (Pachette 500, DGH Technologies, Exton, PA). We began to collect CCT measurements in early 1999, about 2 years after randomization of the last participant. The protocol for measurement of CCT is described in a previously published article.18
Determination of Primary Open-Angle Glaucoma
Primary open-angle glaucoma was defined as the development of a reproducible visual field abnormality or reproducible, clinically significant optic disc deterioration attributed to POAG by the masked Endpoint Committee. Criteria for reproducible visual field abnormality were three consecutive reliable visual fields judged abnormal (corrected pattern standard deviation of P < 5% or a glaucoma hemifield test outside normal limits by STATPAC 2 criteria) by masked readers at the Visual Field Reading Center, University of California-Davis, Sacramento, California.19
Criteria for reproducible optic disc deterioration were two consecutive sets of optic disc photographs showing generalized or localized thinning of the optic disc neuroretinal rim compared to baseline stereoscopic optic disc photographs as determined by masked certified readers at the Optic Disc Reading Center, Bascom Palmer Eye Institute, Miami, Florida.20
When either Reading Center determined the occurrence of a reproducible endpoint, the masked Endpoint Committee reviewed the participant’s clinical and medical history to determine if the endpoint was due to POAG.
Statistical Analysis
The analysis dataset for this report consists of data collected prospectively in OHTS from the start of randomization February 1994 through June, 2002 when participants were managed according to their randomization assignment. This includes participants with complete data for factors in the prediction model for the development of POAG - baseline age, IOP, CCT, VCDR and PSD3. A total of 1,433 of the 1,636 randomized participants had complete baseline data (717 observation participants and 716 medication participants). Participants completed a median follow-up of 7.0 years. The analysis dataset included 102 incident cases of POAG in the observation group and 41 incident cases of POAG in the medication group. Cox proportional hazards models stratified by randomization group were run with the same predictors as the OHTS/EGPS prediction model - baseline age, IOP, CCT, VCDR and PSD. For eye-specific variables, the average of right and left eyes was used. Separate Cox proportional hazards models stratified by randomization group were rerun with values for baseline IOP corrected for CCT using formulae published by Ehlers11, Whitacre12, Orssengo and Pye13, Doughty14, and Kohlhaas15. Models with baseline IOP adjusted for CCT by various formulae were run with/without CCT in the model to determine if CCT made an independent contribution to the risk of developing POAG. The Pearson correlation coefficient between unadjusted IOP and CCT in this sample is −0.03.1 The Orssengo/Pye formula assumed a mean CCT for this sample of 1,433 participants (580um) and a value for the radius of curvature of the central anterior surface of 7.80.21 The formulae are given in Table 1 (available at http://aaojournal.org).
Table 1.
Formulae to adjust intraocular pressure measured by tonometry for central corneal thickness.
| Formula Name (adjusted for IOP) | Formula | |
|---|---|---|
| Ehlers11 |
|
|
| Whitacre12 |
|
|
| Orssengo/Pye13 | , where | |
| i. |
|
|
| ii. |
|
|
| iii. |
|
|
| iv. |
|
|
| v. |
|
|
| Doughty14 |
|
|
| Kohlhaas15 |
|
IOP = intraocular pressure; CCT = central corneal thickness, π = 3.14159265
To compare the predictive accuracy of the prediction models, we calculated the c-statistic and calibration chi-square. The c-statistic ranges from 0.5 (chance) to 1.0 (perfect agreement) and indicates degree of agreement between the ordering of the estimated and observed probability of a dichotomous endpoint.22 When CCT is included in the model, all formulae except the one by Orssengo/Pye13 will have nearly identical c-statistics by definition because the formulae are linear adjustments to IOP which do not affect the relative ranking of unadjusted IOP to the risk of developing POAG. The calibration chi-square indicates whether the model over/under estimates the actual number of POAG events for each model by decile of risk. A calibration chi-square of 20.00 and below indicates acceptable agreement between the predicted and observed event rates.22 The c-statistic and calibration chi-square were calculated using the Design library from the Comprehensive R Archive Network (R Development Core Team, v2.3-0, http://cran.r-project.org/web/packages/Design/index.html). Accessed March 1, 2011.
Results
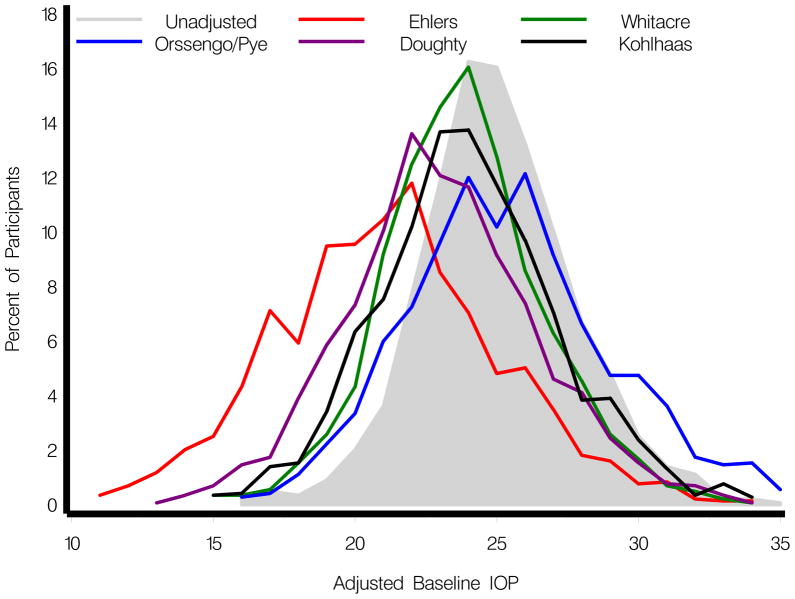
Figure 1 shows the distribution of the baseline IOP adjusted using the formulae from Ehlers11, Whitacre12, Orssengo and Pye13, Doughty14, and Kohlhaas15. The correlations between adjusted IOP’s calculated from these formulae were high (range from 0.87 to 0.99). Table 2 (available at http://aaojournal.org) reports baseline age, VCDR, PSD, CCT and IOP (unadjusted and adjusted for CCT) for participants who did and did not develop POAG. Table 3 reports the multivariate hazard ratio for the risk of developing POAG for baseline IOP, as adjusted for CCT by the aforementioned formulae. In all models, the hazard ratio for adjusted IOP was statistically significant with and without CCT in the model. There was little difference in the size of the hazard ratio in the models among the various IOP correction formulae. The hazard ratio with the lowest value for CCT adjusted IOP was 1.10 (95% CI, 1.04, 1.15) for the Orssengo/Pye formula in a model that included CCT. The hazard ratio with the highest value for CCT adjusted IOP was 1.17 (95% CI, 1.12, 1.23) for the Doughty formula and the Kohlhaas formula in a model that did not include CCT (Table 3).
Figure 1.
Distribution of baseline intraocular pressure (IOP) adjusted using the formulae from Ehlers11, Whitacre12, Orssengo and Pye13, Doughty14, and Kohlhaas15
Table 2.
Baseline predictors (mean, standard deviation) and adjusted intraocular pressure by primary open-angle glaucoma status.
| Not POAG N=1290 |
POAG N=143 |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age at baseline (years) | 55.2 | 9.5 | 58.8 | 9.0 |
| Vertical cup-to-disc ratio at baseline | 0.4 | 0.2 | 0.5 | 0.2 |
| Pattern standard deviation at baseline (dB) | 1.9 | 0.2 | 2.0 | 0.2 |
| Central corneal thickness (μm) | 574.9 | 37.8 | 551.4 | 37.8 |
| Unadjusted baseline IOP (mm Hg) | 24.8 | 2.7 | 25.7 | 3.0 |
| Ehlers11 adjusted baseline IOP (mm Hg) | 20.9 | 3.8 | 23.5 | 4.1 |
| Whitacre12 adjusted baseline IOP (mm Hg) | 23.7 | 2.8 | 25.1 | 3.1 |
| Orssengo/Pye13 adjusted baseline IOP (mm Hg) | 25.3 | 3.7 | 27.8 | 4.3 |
| Doughty14 adjusted baseline IOP (mm Hg) | 22.8 | 3.3 | 24.9 | 3.6 |
| Kohlhaas15 adjusted baseline IOP (mm Hg) | 23.8 | 3.1 | 25.7 | 3.5 |
SD = standard deviation; POAG = primary open-angle glaucoma; IOP = intraocular pressure.
Table 3.
Comparison of prediction models for the development of primary open-angle glaucoma when the model uses unadjusted intraocular pressure (IOP) vs. IOP adjusted by formula for central corneal thickness.
| Baseline IOP (mm Hg) HR & 95% CI | CCT (40 μm decrease) HR & 95% CI | C statistic | Calibration Chi Square | |
|---|---|---|---|---|
| OHTS prediction model†: | ||||
| Baseline age, unadjusted IOP, CCT, PSD & VCDR | ||||
| Model with CCT (as above) | 1.11 (1.05,1.17) | 1.84 (1.53,2.21) | 0.774 | 7.86 |
| Model without CCT | 1.12 (1.06,1.19) | n/a | 0.747 | 3.57 |
|
| ||||
| Ehlers11 adjusted IOP | ||||
| Model with CCT | 1.11 (1.05,1.17) | 1.38 (1.07,1.77) | 0.774 | 7.86 |
| Model without CCT | 1.16 (1.12,1.21) | n/a | 0.770 | 4.60 |
|
| ||||
| Whitacre12 adjusted IOP | ||||
| Model with CCT | 1.11 (1.05,1.17) | 1.69 (1.40,2.05) | 0.774 | 7.86 |
| Model without CCT | 1.16 (1.10,1.22) | n/a | 0.754 | 2.59 |
|
| ||||
| Orssengo/Pye13 adjusted IOP | ||||
| Model with CCT | 1.10 (1.04,1.15) | 1.42 (1.12,1.80) | 0.775 | 5.32 |
| Model without CCT | 1.15 (1.11,1.19) | n/a | 0.770 | 3.83 |
|
| ||||
| Doughty14 adjusted IOP | ||||
| Model with CCT | 1.11 (1.05,1.17) | 1.50 (1.20,1.87) | 0.774 | 7.86 |
| Model without CCT | 1.17 (1.12,1.23) | n/a | 0.766 | 2.14 |
|
| ||||
| Kohlhaas15 adjusted IOP | ||||
| Model with CCT | 1.11 (1.05,1.17) | 1.55 (1.25,1.91) | 0.774 | 7.86 |
| Model without CCT | 1.17 (1.12,1.23) | n/a | 0.763 | 2.43 |
POAG = primary open-angle glaucoma; CCT = central corneal thickness; IOP = intraocular pressure; PSD = pattern standard deviation; VCDR = vertical cup-to-disc ratio; OHTS = ocular hypertension treatment study; HR = hazard ratio; CI = confidence interval; n/a = not applicable.
All models are run with and without CCT in the model.
Hazard ratios and 95% confidence intervals may differ slightly from previous reports because of slight differences in dataset freeze date. This report includes data from February, 1994 through June 1, 2002.
CCT was a statistically significant independent predictor for the development of POAG in all prediction models that included baseline age, VCDR, PSD, CCT and IOP (unadjusted and adjusted for CCT). In models that included both adjusted IOP and CCT, the hazard ratio for CCT ranged from 1.38 (95% CI, 1.07, 1.77) to 1.69 (95% CI, 1.40, 2.05) depending on the correction formula (Table 3). The Pearson correlation coefficient between CCT and CCT adjusted IOP ranged from −0.53 (Kohlhaas) to −0.71 (Ehlers).
C-statistics for predictive accuracy of models using baseline IOP corrected for CCT by formula ranged from 0.754 to 0.775 overall, no better than the original prediction model for the development of glaucoma (0.774) that used unadjusted IOP (Table 3). There was virtually no difference in the c-statistic among models using various correction formulae for CCT. The maximum difference between the highest and lowest c-statistic among all models in Table 3 was 0.027.
Calibration chi-squares of all prediction models in Table 3 were under 20.00 indicating acceptable fit. The calibration chi-square for the original prediction model with unadjusted IOP was 7.86 and ranged from 2.43 to 7.86 for models using CCT adjusted IOP. Models that included both CCT adjusted IOP and CCT performed slightly worse (higher calibration chi-square of 5.32 to 7.86) due partly to the high correlation between CCT adjusted IOP and CCT.
Discussion
The predictive accuracy of the OHTS/EGPS prediction model for the development of POAG was not improved by correcting IOP for CCT using formulae published by Ehlers11, Whitacre12, Orssengo and Pye13, Doughty14, and Kohlhaas.15 C-statistics for these prediction models using corrected IOP ranged from 0.75 to 0.77, no better than the original OHTS prediction model (0.77) that did not adjust IOP for CCT. Calibration chi squares did not differ meaningfully among these models (2.14 to 7.86, less than 20 is acceptable) and was no better than the original prediction model (7.86). This finding should not be a surprise. Tonometry is influenced by the material properties of the cornea, of which CCT is but one component. Current correction formulae for IOP use only CCT11, 12, 14, 15, (or CCT + corneal curvature)13 to ‘adjust’ IOP estimates. One engineering model of applanation suggests that Young’s modulus, a measure of material ‘stiffness’ known to vary widely between individuals, has a stronger impact on GAT error than does CCT.23 Available formulae do not appear to adequately correct the measurement of IOP for the biomechanical properties of the cornea on GAT.
There are several reasons that might explain why CCT is a strong predictor for the development of POAG. CCT can be measured with high reliability in one sitting18. Because CCT is relatively stable over the lifetime of an adult, a single measurement of CCT is adequate in most patients. By comparison, IOP reflects transient factors that may or may not be relevant to the risk of developing POAG. The test-retest agreement between multiple IOP’s at a given OHTS visit is very high, but the test-retest agreement between 6 month visits is low to moderate.24 OHTS may not have captured information that is important to ascertaining the relationship of IOP to the risk of developing POAG. In OHTS, IOP was measured 2–3 times per visit during normal office hours. No diurnal measurements were taken. Our study highlights the fact that all tonometry techniques provide only an estimate of ‘true’ IOP, a physiologic parameter that can vary greatly within the individual. Goldmann tonometry is widely considered a reference standard in the conduct of clinical and regulatory trials but even when IOP is adjusted for CCT using specialized formulae, the adjusted IOP suffers from the inherent variability of IOP and inaccuracy of IOP measurement.
In the OHTS, IOP measured by GAT was used to determine participant eligibility, to guide treatment decisions and finally to construct the predictive model for the development of POAG. Had the OHTS been carried out with a perfectly accurate, cornea-independent tonometer (something that does not exist), it is entirely possible that IOP might have been a more powerful predictor for the development of POAG and CCT a less powerful predictor. However, it is worth noting that in the Early Manifest Glaucoma Trial (EMGT), IOP was not used to determine eligibility or treatment decisions and thus the influence of CCT on GAT measurements had no opportunity to affect the incidence rate of glaucoma progression. In the EMGT, CCT was found to be an independent predictive factor for progression of POAG.25 In the population-based, longitudinal Barbados Eye Studies (BES), CCT measured at 9 years from baseline was an independent risk factor for incident glaucoma.6 In the population-based Los Angeles Latino Eye Study (LALES), the prevalence of glaucoma was higher among individuals with thin CCTs than individuals with normal or thick CCTs across all levels of IOP.9 The LALES investigators explored whether adjusting each IOP individually for CCT using the Doughty & Zaman algorithm14 changed this relationship and found almost no change in the association between thin CCT and higher prevalence of glaucoma. LALES investigators concluded “… there is an independent risk related to CCT itself.”9 In combination with the present study, the findings of the EMGT, BES and LALES suggest that the influence of CCT on glaucoma risk is caused by more than just tonometry artifact.
We conclude that available formulae to correct IOP measurements for CCT do not improve accuracy of the original prediction model for the development of POAG. Nor did we find that any formula outperformed the other formulae as judged by the c-statistic and calibration chi-square. We caution that the accuracy of the original OHTS prediction model is estimated from data averaged over a large sample of ocular hypertensive individuals and that its accuracy in predicting outcome for a single ocular hypertensive individual cannot be reliably estimated.
The 5-year risk of developing POAG for an individual with ocular hypertension can be simply calculated from age, IOP, CCT, VCDR and PSD using the risk calculator available at http://ohts.wustl.edu/risk (accessed March 1, 2011) which can be downloaded free of charge.3 The results of our analyses suggest that the influence of corneal thickness as a prognostic factor for the development of POAG is not entirely through its effect on IOP measurement but that CCT is a biomarker for structural or physical factors involved in the pathogenesis of POAG.
Supplementary Material
Acknowledgments
Financial Support: Supported by grants from the National Eye Institute, and the National Center on Minority Health and Health Disparities, National Institute of Health, Bethesda. MD (EY09341, EY09307, EY091369 and Core Grant EY02687); Merck Research Laboratories, White House Station New Jersey; Pfizer Inc., New York, New York and an unrestricted grant from Research to Prevent Blindness, New York, New York.
Footnotes
Meeting Presentations: The Association for Research in Vision and Ophthalmology (ARVO) May 2010.
On-line only material: This article contains online-only material. The following should appear online-only: Table 1 and Table 2.
Financial Conflict of Interest: James D. Brandt. Please see his Financial Disclosure Form.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 2.European Glaucoma Prevention Study (EGPS) Group. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 3.Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–13. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 5.Zeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. Eur J Ophthalmol. 2005;15:196–201. doi: 10.1177/112067210501500203. [DOI] [PubMed] [Google Scholar]
- 6.Leske MC, Wu SY, Hennis A, et al. BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Wolfs RC, Klaver CC, Vingerling JR, et al. Distribution of central corneal thickness and its association with intraocular pressure: the Rotterdam Study. Am J Ophthalmol. 1997;123:767–72. doi: 10.1016/s0002-9394(14)71125-0. [DOI] [PubMed] [Google Scholar]
- 8.Nemesure B, Wu SY, Hennis A, Leske MC Barbados Eye Study Group. Corneal thickness and intraocular pressure in the Barbados Eye Studies. Arch Ophthalmol. 2003;121:240–4. doi: 10.1001/archopht.121.2.240. [DOI] [PubMed] [Google Scholar]
- 9.Francis BA, Varma R, Chopra V, et al. Los Angeles Latino Eye Study Group. Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;146:741–6. doi: 10.1016/j.ajo.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldmann H, Schmidt T. Applanation tonometry [in German] Opthalmologica. 1957;134:221–42. doi: 10.1159/000303213. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–6. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 13.Orssengo GJ, Pye DC. Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull Math Biol. 1999;61:551–72. doi: 10.1006/bulm.1999.0102. [DOI] [PubMed] [Google Scholar]
- 14.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 15.Kohlhaas M, Boehm AG, Spoerl E, et al. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 2006;124:471–6. doi: 10.1001/archopht.124.4.471. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. . The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 17.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 18.Brandt JD, Beiser JA, Kass MA, Gordon MO Ocular Hypertension Treatment Study (OHTS) Group Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108:1779–88. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 19.Johnson CA, Keltner JL, Cello KE, et al. Ocular Hypertension Study Group. Baseline visual field characteristics in the Ocular Hypertension Treatment Study. Ophthalmology. 2002;109:432–7. doi: 10.1016/s0161-6420(01)00948-4. [DOI] [PubMed] [Google Scholar]
- 20.Feuer WJ, Parrish RK, II, Schiffman JC, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: reproducibility of cup/disk ratio measurements over time at an optic disc reading center. Am J Ophthalmol. 2002;133:19–28. doi: 10.1016/s0002-9394(01)01338-1. [DOI] [PubMed] [Google Scholar]
- 21.Emsley HH. Optics of Vision. 5. Vol. 1. London: Butterworths; 1973. Visual Optics; p. 346. AQ: must provide specific inclusive pagination for material being cited. [Google Scholar]
- 22.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–55. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Bhorade AM, Gordon MO, Wilson B, et al. Ocular Hypertension Treatment Study Group. Variability of intraocular pressure measurements in observation participants in the Ocular Hypertension Treatment Study. Ophthalmology. 2009;116:717–24. doi: 10.1016/j.ophtha.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leske MC, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.