Abstract
Spinal tuberculosis is a destructive form of tuberculosis. It accounts for approximately half of all cases of musculoskeletal tuberculosis. Spinal tuberculosis is more common in children and young adults. The incidence of spinal tuberculosis is increasing in developed nations. Genetic susceptibility to spinal tuberculosis has recently been demonstrated. Characteristically, there is destruction of the intervertebral disk space and the adjacent vertebral bodies, collapse of the spinal elements, and anterior wedging leading to kyphosis and gibbus formation. The thoracic region of vertebral column is most frequently affected. Formation of a ‘cold’ abscess around the lesion is another characteristic feature. The incidence of multi-level noncontiguous vertebral tuberculosis occurs more frequently than previously recognized. Common clinical manifestations include constitutional symptoms, back pain, spinal tenderness, paraplegia, and spinal deformities. For the diagnosis of spinal tuberculosis magnetic resonance imaging is more sensitive imaging technique than x-ray and more specific than computed tomography. Magnetic resonance imaging frequently demonstrates involvement of the vertebral bodies on either side of the disk, disk destruction, cold abscess, vertebral collapse, and presence of vertebral column deformities. Neuroimaging-guided needle biopsy from the affected site in the center of the vertebral body is the gold standard technique for early histopathological diagnosis. Antituberculous treatment remains the cornerstone of treatment. Surgery may be required in selected cases, e.g. large abscess formation, severe kyphosis, an evolving neurological deficit, or lack of response to medical treatment. With early diagnosis and early treatment, prognosis is generally good.
Keywords: Tuberculosis, Myelopathy, Spinal cord, Antituberculous treatment, Cold abscess, Mycobacterium tuberculosis
Introduction
Spinal tuberculosis is a frequently encountered extrapulmonary form of the disease. In developed nations, most cases of spinal tuberculosis are seen primarily in immigrants from endemic countries. Because the epidemic of human immunodeficiency virus (HIV) infection caused resurgence in all forms of tuberculosis, increased awareness about spinal tuberculosis is necessary. Despite its common occurrence and the high frequency of long-term morbidity, there are no straightforward guidelines for the diagnosis and treatment of spinal tuberculosis. Early diagnosis and prompt treatment is necessary to prevent permanent neurological disability and to minimize spinal deformity.1,2
Spinal tuberculosis is one of the oldest diseases known to mankind and has been found in Egyptian mummies dating back to 3400 BC.3 The disease is popularly known as Pott's spine. The name traces back its origin from the description of tuberculous infection of the spine by Sir Percival Pott in his monograph in 1779.4 The majority of his patients were infants and young children. The classic destruction of the disk space and the adjacent vertebral bodies, destruction of other spinal elements, severe and progressive kyphosis subsequently became known as Pott's disease. Currently, the term ‘Pott's disease/Pott's spine’ describes tuberculous infection of the spine and the term ‘Pott's paraplegia’ describes paraplegia resulting from tuberculosis of the spine.
This review focuses on the various aspects of spinal tuberculosis. An extensive review of the literature published in English was carried out using the PubMed and Google Scholar databases. The search terms included tuberculosis, skeletal tuberculosis, spinal tuberculosis, Pott's disease, Pott's paraplegia, and central nervous system tuberculosis.
Epidemiology
Tuberculosis is a disease of poverty that affects mostly young adults in their most productive years. The risk of developing tuberculosis is estimated to be 20–37 times greater in people co-infected with HIV than among those without HIV infection. In 2009, approximately 1.2 million new tuberculosis cases were reported among people living with HIV; 90% of these cases were concentrated in the African and South East Asian regions. The highest number of tuberculosis-related deaths were in Africa.5,6 Although multidrug-resistant tuberculosis is not common in spinal disease, there have been a few recent case reports.7
The exact incidence and prevalence of spinal tuberculosis in most parts of the world are not known. In countries with a high burden of pulmonary tuberculosis, the incidence is expected to be proportionately high. Approximately 10% of patients with extrapulmonary tuberculosis have skeletal involvement. The spine is the most common skeletal site affected, followed by the hip and knee. Spinal tuberculosis accounts for almost 50% cases of skeletal tuberculosis.8
Spinal tuberculosis is uncommon in the western world. Most of the patients with spinal tuberculosis in developed countries are immigrants from countries where tuberculosis is endemic. A study evaluated the epidemiology of musculoskeletal tuberculosis in United Kingdom and a review of data of musculoskeletal tuberculosis over a 6-year period was performed. From 1999 to 2004, there were 729 patients with tuberculosis. Approximately 8% (61) cases had musculoskeletal involvement; nearly 50% of these patients had spinal involvement. The majority (74%) of patients was immigrants from the Indian subcontinent.9 In France, in a hospital-based survey, the overall incidence of vertebral osteomyelitis was estimated at 2.4/100 000. In 2002–2003, 1422 and 1425 patients were classified as definite (64%), probable (24%), and possible (12%) vertebral osteomyelitis. The main infectious agents reported were Staphylococcus spp. (38%) and Mycobacterium tuberculosis (31%).10 In endemic countries, spinal tuberculosis is more common in children and younger adults, while the disease affects the adult population in developed Western and Middle East countries.9,11
Spinal tuberculosis in HIV-infected persons
Tuberculosis is the most common HIV-related opportunistic infection worldwide.12 In a Nigerian study, the records of 1320 HIV-infected patients were reviewed. One-hundred and thirty eight (10%) patients were coinfected with tuberculosis. Fifty (36%) coinfected patients had some type of extrapulmonary tuberculosis; 15 had both pulmonary tuberculosis and extrapulmonary tuberculosis. Among the 35 patients with extrapulmonary tuberculosis, 14% patients had spinal tuberculosis.13 A study from South Africa, evaluated 525 medical records of all patients seen for spinal conditions, of which 104 (20%) had spinal tuberculosis. About 90% of patients with spinal tuberculosis were African and 10% from other races. The incidence of spinal tuberculosis was approximately 1 and 3 per 100 000 for Africans and other races, respectively. All the patients had a history of pulmonary tuberculosis. In this study, 28% patients were HIV-positive.14
Multi-level noncontiguous vertebral tuberculosis
Multi-level noncontiguous spinal tuberculosis is an atypical form of spinal tuberculosis that affects two noncontiguous vertebrae without destruction of the adjacent vertebral bodies and intervertebral disks. So far, there have been a few recent case reports with involvement of two or more noncontiguous vertebrae. However, in one study, the incidence of multi-level noncontiguous vertebral tuberculosis was observed as high as 71% and a large proportion of the patients with affected noncontiguous vertebral sites were asymptomatic. In this retrospective analysis, patients were included if spinal infection was identified by whole spine magnetic resonance imaging (MRI) and confirmed as tuberculosis by a combination of histology and microbiology.15 In another study, authors identified 16 cases of noncontiguous spinal tuberculosis from a single surgeon series of 98 patients. Most noncontiguous lesions were evident on plain radiology and noncontiguous tuberculosis was not associated with HIV infection, multidrug-resistant tuberculosis or with chronicity of the disease.16
Pathogenesis and pathology
Predisposing factors for tuberculosis include poverty, overcrowding, illiteracy, malnutrition, alcoholism, drug abuse, diabetes mellitus, immunosuppressive treatment, and HIV infection. These are also predisposing factors for spinal tuberculosis as well.17 In Iran, older age, male gender, chronic peritoneal dialysis, imprisonment, and previous tuberculous infection were identified as risk factors for tuberculous spondylitis.18 Genetic susceptibility to spinal tuberculosis has recently been demonstrated. A group of workers investigated the association between the FokI polymorphism in the vitamin-D receptor gene and spinal tuberculosis in a Chinese population and this gene was found to be associated with susceptibility to spinal tuberculosis.19
Spinal involvement is usually a result of hematogenous spread of M. tuberculosis into the dense vasculature of cancellous bone of the vertebral bodies. The primary infection site is either a pulmonary lesion or an infection of the genitourinary system.20–22 Spread occurs either via the arterial or the venous route. An arterial arcade, in the subchondral region of each vertebra, is derived from anterior and posterior spinal arteries; this arcade form a rich vascular plexus. This vascular plexus facilitates hematogenous spread of the infection in the paradiskal regions. Batson's paravertebral venous plexus in the vertebra is a valve-less system that allows free flow of blood in both directions depending upon the pressure generated by the intra-abdominal and intrathoracic cavities following strenuous activities like coughing. Spread of the infection via the intraosseous venous system may be responsible for central vertebral body lesions. In patients with noncontiguous vertebral tuberculosis, again it is the vertebral venous system that spreads the infection to multiple vertebrae.
Spinal tuberculosis is initially apparent in the anterior inferior portion of the vertebral body. Later on it spreads into the central part of the body or disk. Paradiskal, anterior, and central lesions are the common types of vertebral involvement. In the central lesion, the disk is not involved, and collapse of the vertebral body produces vertebra plana. Vertebra plana indicates complete compression of the vertebral body. In younger patients, the disk is primarily involved because it is more vascularized. In old age, the disk is not primarily involved because of its age-related avascularity. In spinal tuberculosis, there is involvement of more than one vertebra because its segmental arteries bifurcate to supply two adjacent vertebrae. Spread of the disease beneath the anterior or posterior longitudinal ligaments involves multiple contiguous vertebrae. A lack of proteolytic enzymes in mycobacterial infections (in comparison with pyogenic infections) has been suggested as the cause of the of the subligamentous spread of infection.1,2,8,17,23–26
In spinal tuberculosis, characteristically, there is destruction of the intervertebral disk space and the adjacent vertebral bodies, collapse of the spinal elements, and anterior wedging leading to the characteristic angulation and gibbus (palpable deformity because of involvement of multiple vertebrae) formation. The upper lumbar and lower thoracic spine are most frequently involved sites. More than one vertebra is typically affected, and the vertebral body is more frequently affected than the posterior arch.27 Distortion of spinal column leads to spinal deformities (Table 1).
Table 1.
Mechanisms of paraplegia/tetraplegia in spinal tuberculosis
| Causes of neurological involvement | |
|---|---|
| Early-onset paraplegia | |
| Mechanical pressure | Mechanical pressure by tuberculous debris, sequestrem of bone or disk, abscess, subluxation and dislocations, concertina collapse, and internal gibbus |
| Tuberculous granuloma | Tuberculoma in extradural, intradural, or intramedullary regions |
| Tuberculous myelitis | Uncommon. May involve spinal cord parenchyma |
| Spinal artery thrombosis | Infective thrombosis of anterior spinal artery |
| Tuberculous arachnoiditis | Meningeal inflammation and fibrosis |
| Late-onset paraplegia | |
| Transection of spinal cord by bony bridge | Transverse ridge of bone produced by severe kyphosis |
| Fibrosis of dura (pachymeningitis) | Formation of tough, fibrous membrane encircling the cord |
Paraplegia is the most devastating complication of spinal tuberculosis. Hodgson, in his classical paper on Pott's paraplegia, classified paraplegia into two groups according to the activity of the tuberculous infection. These two groups were paraplegia of active disease (early-onset paraplegia) and paraplegia of healed disease (late-onset paraplegia).
Early-onset paraplegia develops in the active stage of spinal tuberculosis and requires active treatment. This type of paraplegia has a better prognosis and is frequently seen in adults with Pott's spine. In these patients, paraplegia is caused by formation of debris, pus, and granulation tissue due to destruction of bone and intervertebral disk. Destruction of the anterior vertebral column leads to subluxation and subsequent dislocation of the spine. Concertina collapse (compression fracture without involvement of the intervertebral disk) may occur because of extensive tuberculous destruction. Concertina collapse bulges into the parenchyma of the spinal cord. Intrinsic factors cause meningomyelitis by direct involvement of spinal cord, surrounding meninges and roots or by involvement of blood vessels supplying the spinal cord. In addition, some rarer causes of paraplegia include infective thrombosis of arteries supplying the spinal cord and nonosseous intramedullary or extramedullary tuberculoma of the spinal cord (Table 1). Late-onset paraplegia is a neurological complication that develops after a variable period in a patient with healed tuberculosis. Late-onset paraplegia may develop two to three decades after active infection. It is often associated with marked spinal deformities.28
Clinical features
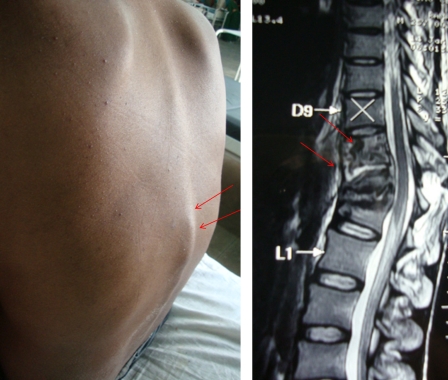
The characteristic clinical features of spinal tuberculosis include local pain, local tenderness, stiffness and spasm of the muscles, a cold abscess, gibbus, and a prominent spinal deformity. The cold abscess slowly develops when tuberculous infection extends to adjacent ligaments and soft tissues. Cold abscess is characterized by lack of pain and other signs of inflammation (Fig. 1).
Figure 1.
‘Gibbus formation’ in the thoraco-lumbar region of a patient with spinal tuberculosis (left). The magnetic resonance shows spinal tuberculosis at T10–T12. Spinal tuberculosis causes the destruction, collapse of vertebrae, and angulation of vertebral column (right).
The progression of spinal tuberculosis is slow and insidious. The total duration of the illness varies from few months to few years, with average disease duration ranging from 4 to 11 months. Usually, patients seek advice only when there is severe pain, marked deformity, or neurological symptoms.29–31
Constitutional symptoms are present in approximately 20–30% of cases of osteoarticular tuberculosis. The classical constitutional features of tuberculosis indicating presence of an active disease are malaise, loss of weight and appetite, night sweats, evening rise in temperature, generalized body aches, and fatigue.
Back pain is the most frequent symptom of spinal tuberculosis. The intensity of pain varies from constant mild dull aching to severe disabling. Pain is typically localized to the site of involvement and is most common in the thoracic region. The pain may be aggravated by spinal motion, coughing, and weight bearing, because of advanced disk disruption and spinal instability, nerve root compression, or pathological fracture. Chronic back pain as the only symptom was observed in 61% of cases of spinal tuberculosis.32,33
Neurologic deficits are common with involvement of thoracic and cervical regions. Left untreated, early neurologic involvement may progress to complete paraplegia or tetraplegia. Paraplegia may occur at any time and during any stage of the vertebral disease. The reported incidence of neurological deficit in spinal tuberculosis varies from 23 to 76%.30 The level of spinal cord involvement determines the extent of neurological manifestations. In cervical spinal tuberculosis, patients manifest with symptoms of cord or root compression. The earliest signs are pain, weakness, and numbness of the upper and lower extremities, eventually progressing to tetraplegia. If the thoracic or lumbar spine is involved, upper extremity function remains normal while lower-extremity symptoms progress over time eventually leading to paraplegia.20 Patients with cauda equina compression due to lumbar and sacral vertebral damage have weakness, numbness, and pain, but have decreased or absent reflexes among the affected muscle groups. This is in contrast to the hyperreflexia seen with spinal cord compression along with bladder involvement (cauda-equina syndrome).
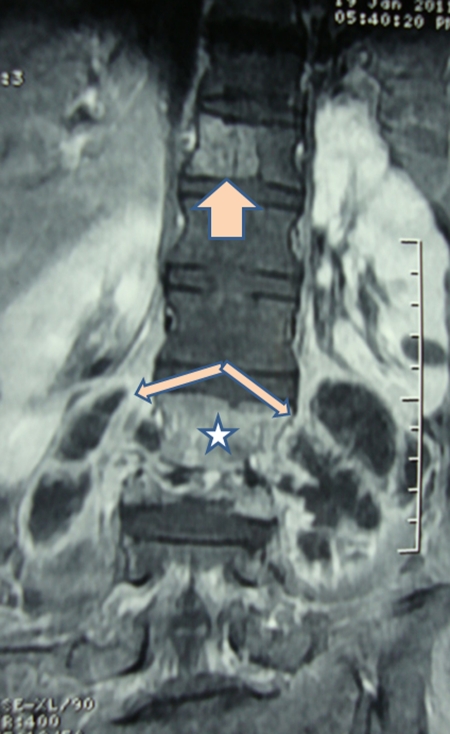
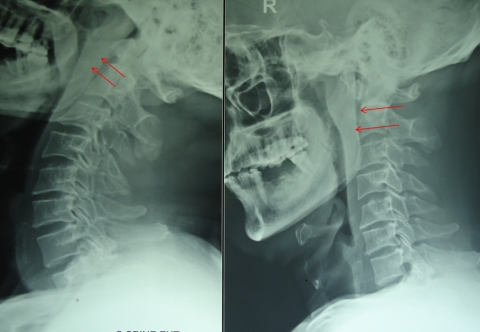
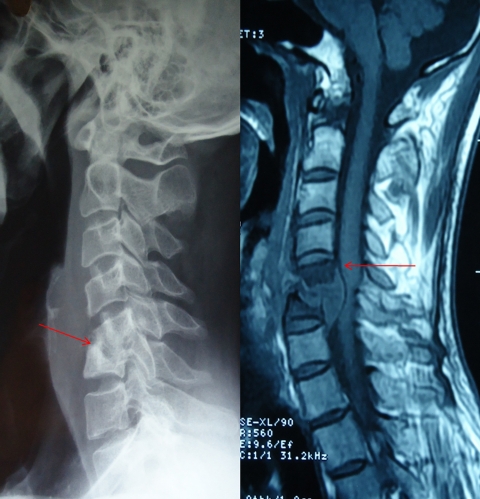
Formation of a cold abscess around the vertebral lesion is another characteristic feature of spinal tuberculosis. Abscess formation is common and can grow to a very large size. The site of cold abscess depends on the region of the vertebral column affected. In the cervical region, the pus accumulates behind prevertebral fascia to form a retropharyngeal abscess (Fig. 2). The abscess may track down to the mediastinum to enter into the trachea, esophagus, or the pleural cavity. Retropharyngeal abscess can produce considerable pressure effects such as dysphagia, respiratory distress, or hoarseness of voice. In the thoracic spine, the cold abscess usually presents as a fusiform or bulbous paravertebral swellings and may produce posterior mediastinal lumps (Fig. 3). The cold abscesses formed at lumbar vertebrae most commonly present as a swelling in the groin and thigh. Abscess can descend down beneath the inguinal ligament to appear on the medial aspect of thigh. Pus collection can follow the blood vessels to form an abscess in gluteal region if it follows femoral or gluteal vessels, respectively.1,17
Figure 2.
T1-weighted image of an MRI scan shows a bilateral paravertebral abscess with destruction of lumbar vertebrae as well as the intervertebral disks.
Figure 3.
X-rays of cervical region showing retropharyngeal abscess.
Spinal deformity is a hallmark feature of spinal tuberculosis. Type of spinal deformity depends on the location of the tuberculous vertebral lesion. Kyphosis, the most common spinal deformity, occurs with lesions involving thoracic vertebrae. The severity of the kyphosis depends on the number of vertebrae involved. An increase in kyphotic deformity by 10° or more may be seen in up to 20% of cases, even after the treatment. Atlanto-axial tuberculosis may present as torticollis.8,34
Diagnosis
Diagnosis of spinal tuberculosis depends on presence of characteristic clinical and neuroimaging findings. Etiological confirmation requires the demonstration of acid-fast bacilli on microscopy or culture of material obtained following biopsy the lesion. Polymerase chain reaction is also an effective method for bacteriological diagnosis of tuberculosis. Screening of the whole spine should be done to look for noncontiguous vertebral lesions.
Imaging
Conventional radiographs give a good overview; computed tomography (CT) visualizes the disko-vertebral lesions and paravertebral abscesses, while MRI is useful in determining the spread of the disease to the soft tissues and to determine the extent of spinal cord involvement.35
Plain radiographs
In resource-poor countries, vertebral radiography still remains the cornerstone of spinal imaging. It often provides enough information for diagnosis and treatment of spinal tuberculosis. The plain radiograph described changes consistent with tuberculosis spine in up to 99% of cases.29,36–38 The characteristic radiographic findings include rarefaction of the vertebral end plates, loss of disk height, osseous destruction, new-bone formation and soft-tissue abscess. Often, multiple vertebrae are involved and late fusion or collapse of vertebrae is not uncommon39 (Table 2).
Table 2.
Various types of vertebral involvement in spinal tuberculosis
| Type of involvement | Mechanisms of involvement | Radiological appearances |
|---|---|---|
| Paradiskal | Spread of disease via the arteries | Involves adjacent margins of two consecutive vertebrae. The intervening disk space is reduced |
| Central | Spread of infection along Batson's plexus of veins | Involves central portion of a single vertebra; proximal and distal disk spaces intact |
| Anterior marginal | Abscess extension beneath the anterior longitudinal ligament and the periosteum | Begins as destructive lesion in one of the anterior margins of the body of a vertebra, minimally involving the disk space but sparing the vertebrae on either side |
| Skipped lesions | Spread of infection along Batson's plexus of veins | circumferentially involvement of two noncontiguous vertebral levels without destruction of the adjacent vertebral bodies and intervertebral disks |
| Posterior | Spread via the posterior external venous plexus of vertebral veins or direct spread | Involves posterior arch without involvement of vertebral body |
| Synovial | Hematogenous spread through subsynovial vessels | Involves synovial membrane of atlanto-axial and atlanto-occipital joints |
Tuberculous cold abscesses may also be seen on plain radiographs as soft tissue shadows adjacent to the spine. In cervical spine, increase in prevertebral soft tissue space is a reliable radiological parameter suggesting inflammatory pathology (retropharyngeal abscess).40 Widening of the superior mediastinum in antero-posterior x-ray and increased prevertebral soft tissue shadow with anterior convexity of tracheal shadow in lateral x-rays of the upper dorsal spine are strong indicators of the disease in the underlying vertebrae. Dorsal and lumbar spine abscesses are seen as paravertebral soft tissue shadows. The presence of calcification within the abscess is virtually diagnostic of spinal tuberculosis. Such calcifications are formed because of the lack of proteolytic enzymes in M. tuberculosis.41
Certain vertebral sites where it is difficult to appreciate bony changes on conventional x ray are craniovertebral junction and cervico-dorsal junction.40 The main disadvantage is that radiographs generally remain normal in the early stages of the disease. Approximately one-third of calcium must be lost from a particular area for osteolysis to be appreciated radiographically.42 It is also difficult to assess spinal cord compression, soft tissue involvement, abscesses and extent of disease on plain x-rays. On the contrary, by the time disease is apparent on x-rays, the patient has already reached an advanced stage of illness with, with the majority having vertebral collapse and neurological deficits.
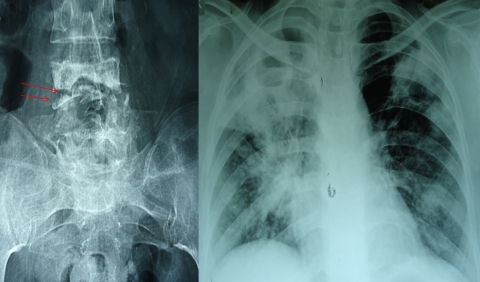
Concomitant pulmonary tuberculosis is frequent in patients with spinal tuberculosis. From 50 and 75% of patients with osteoarticular tuberculosis and up to 67% of patients with spinal tuberculosis have an associated primary lung focus or have a reported history of pulmonary tuberculosis.22,43 In a series of 60 patients with miliary tuberculosis and neurological complications, 3 patients had Pott's paraplegia44 (Fig. 4).
Figure 4.
X-ray of sacral region of spine shows destruction of vertebrae which is suggestive of spinal tuberculosis (left). X-ray chest of same patient which shows presence of extensive pulmonary tuberculosis (right).
Computed tomography
CT demonstrates abnormalities earlier than plain radiography. The pattern of bone destruction may be fragmentary in 47% of the cases; osteolytic in 34%, localized and sclerotic in 10%, and subperiosteal in 30% cases.45 Other findings include soft tissue involvement and paraspinal tissue abscess. CT is of great value in the demonstration of any calcification within the cold abscess or visualizing epidural lesions containing bone fragments. CT is of the greatest value in the delineation of encroachment of the spinal canal by posterior extension of inflammatory tissue, bone or disk material, and in the CT-guided biopsy.38,46
Magnetic resonance imaging
MRI is the neuroimaging of choice for spinal tuberculosis. MRI is more sensitive than x-ray and more specific than CT in the diagnosis of spinal tuberculosis. MRI allows for the rapid determination of the mechanism for neurologic involvement.23,25,47,48
MRI readily demonstrates involvement of the vertebral bodies, disk destruction, cold abscess, vertebral collapse, and spinal deformities. In the early stages, however, only disk degeneration with alteration of bone marrow signal intensity of vertebra is seen, which may not be sufficiently diagnostic of spinal tuberculosis. Abscess formation and collection and expansion of granulation tissue adjacent to the vertebral body is highly suggestive of spinal tuberculosis. MRI is also useful in detecting intramedullary or extramedullary tuberculoma, spinal cord cavitation, spinal cord edema, and possibly unsuspected noncontiguous lesions of the spine.23,25,36,49 The subligamentous spread of a paraspinal mass and the involvement of multiple contiguous bones and intramedullary spinal changes can be very well demonstrated by MRI50 (Table 2) (Figs. 1, 2 and 5).
Figure 5.
X-ray of cervical region which shows spinal tuberculosis of cervical six to seven vertebrae and a retropharyngeal abscess (left). T1-weighted image of an MRI of same patient, which shows destruction of C6–C7 vertebrae.
The posterior spinal element, specifically the pedicle involvement, is generally not a characteristic feature of spinal tuberculosis. In a study, pedicle involvement was noted in unusually high (65%) number of patients. In this study the highest involvement was at the thoracic level. The mean vertebral body, disk collapse, prevertebral abscess, and kyphosis were more severe in the pedicle-involved group.51 There is no pathognomonic finding on MRI that reliably distinguishes tuberculosis from other spinal infections or from a possible neoplasm.47,48
Bone scan
There are no pathognomonic scintigraphic features of spinal tuberculosis. Infection usually causes a hot spot, but avascular bone fragments may produce a cold spot. Bone scan is, however, helpful in differentiating from metastatic lesions, which usually show uptake of radioactive substance at multiple sites. The technetium 99 m bone scan was negative in 35% of an series of patients with Pott's spine.52,53 In another study, the pattern of uptake similar to metastatic disease was seen in 63% of the patients of Pott's spine.39 Gallium scintigraphic scans were also negative in most of the patients with active tuberculosis of the spine.53
Cytological and microbiological confirmation
Etiological confirmation can be made either by demonstration of acid-fast bacilli on pathological specimen or histological evidence of a tubercle or the mere presence of epithelioid cells on the biopsy material.54
Neuroimaging guided-needle biopsy from the affected site is the gold standard technique for the early histopathological diagnosis of spinal tuberculosis.38 CT-guided needle biopsy usually yields sufficient material either from the spine itself or from an adjacent abscess. Open biopsy of the spine is usually performed when either closed techniques have proved insufficient or other procedures, such as decompression and possibly arthrodesis, are planned. In an Indian study, fine needle aspiration biopsy done under CT guidance was successful in diagnosing spinal tuberculosis in 34 out of 38 patients.55 Surgery may be required in up to 10% of cases to establish the etiological diagnosis.33 Material obtained from biopsy should be submitted for cytologic, histologic, and bacteriologic studies. Smear positivity for acid-fast bacilli may be seen in up to 52% of cases and culture positivity in about 83% of cases.56 However, as with respiratory tuberculosis, culture is not the gold standard for diagnosing spinal tuberculosis because mycobacterial bacilli are not readily detected from extrapulmonary sites.57
Histologic studies confirm the diagnosis of spinal tuberculosis in approximately 60% of patients. The most common cytological findings observed are epithelioid cell granulomas (90%), granular necrotic background (83%), and lymphocytic infiltration (76%). Scattered multinucleated and Langhans' giant cells may be seen in up to 56% of cases.58–61 False-negative results of biopsy are common and, therefore, diagnosis of spinal tuberculosis must be made on ground of clinical manifestations and radiology when bacteriology proves negative.62
Polymerase chain reaction and other immunological tests
Conventional microbiological methods like Ziehl–Neelsen staining for acid-fast bacilli and culture of M. tuberculosis on Lowenstein Jensen media have low sensitivity and specificity.53,63 In addition, culturing M. tuberculosis is time consuming, taking 6–8 weeks for the growth to appear.64 So, mostly, the diagnosis of tuberculosis depends on histological evidence. Polymerase chain reaction has shown very promising results for the early and rapid diagnosis of the disease. This technique is able to detect as few as 10–50 tubercle bacilli in various clinical samples.65 This test offers better accuracy than a smear and can be performed at greater speed than cultures.66 Various studies have reported sensitivity of polymerase chain reaction ranging from 61 to 90% and a specificity of 80–90%.67–71
The QuantiFERON-TB Gold assay detects cell-mediated inflammatory responses in vitro to tuberculosis infection by measuring interferon-gamma harvested in plasma from whole blood incubated with the M. tuberculosis-specific antigens. In a study, 70 consecutive patients with vertebral collapse underwent a battery of investigations including the QuantiFERON assay. The patients were classified as having tuberculosis on the basis of positive smear or culture, a biopsy consistent with tuberculosis, or a therapeutic response to antituberculosis chemotherapy. Tuberculosis was diagnosed in 51 patients, and 19 had vertebral collapse that was attributable to other causes. In this study, sensitivity was estimated as 84% and specificity was 95%.72
Other tests
Erythrocyte sedimentation rate (ESR) is generally raised many folds in the majority of patients with spinal tuberculosis. ESR declines to normal or near normal when the active tuberculous lesion is controlled. In pyogenic infection, leucocytosis parallels with raised ESR, while in patients with spinal tuberculosis, there is markedly elevated ESR with a normal white blood cell count.73
Differential diagnosis
Spinal tuberculosis should be considered in the differential diagnosis of chronic back pain (with or without constitutional, neurological, or musculoskeletal manifestations) and in young persons. The spinal tuberculosis should also be considered in immigrant patients of chronic back pain coming from endemic countries.33 Several spinal diseases need be differentiated from spinal tuberculosis. Common differential diagnosis includes pyogenic spondylitis, brucellar spondylitis, sarcoidosis, metastasis, multiple myeloma, and lymphoma (Table 3).
Table 3.
Diagnosis of spinal tuberculosis: summary points
| 1. X-ray, CT, or MRI of the spine should be performed in all patients |
| 2. Spinal MRI determines extent and nature of the bony destructions as well as soft tissue involvement (including spinal cord) |
| 3. Screening of whole spine should be done to look for skipped lesions |
| 4. All patients should have a chest x-ray to detect coexisting pulmonary tuberculosis |
| 5. Advantages and disadvantages of both biopsy and needle aspiration should be discussed with the patient, with the aim of obtaining adequate material for diagnosis |
| 6. Material obtained from the site of disease by needle biopsy or open surgery should be submitted for microbiology, histology, and culture |
| 7. Appropriate treatment regimen should be started without waiting for culture results |
| 8. Clinicians should consider spinal tuberculosis even if histology and rapid diagnostic tests are negative, but clinical suspicion is strong |
| 9. The appropriate drug regimen should be continued even if subsequent culture results are negative |
The characteristic imaging characteristics of vertebral tuberculosis include extensive paraspinal soft tissue shadows, thoracic region involvement, well-defined paraspinal abnormal signal, subligamentous spread, and presence of spinal deformities.74 In brucellar spondylitis, the lumbar vertebrae are the most frequently affected followed by thoracic and cervical segments of the vertebral column. The differentiating imaging characteristics of brucellar spondylitis include disk space involvement, minimal paraspinal soft tissue shadow, and absence of gibbus deformity. A pyogenic infection of vertebrae is found more often in the lumbar and cervical regions. Pyogenic spondylitis does not involve the vertebrae, posterior arch and spinous process, and often there is no gibbus deformity. Intervertebral disk destruction is more frequent in pyogenic spondylitis.74–76 Sarcoidosis can produce multifocal lesions of vertebrae and disks, along with paraspinal masses that appear identical to tuberculosis77,78 (Table 4). Osteoporotic vertebral involvement is more common in thoracic regions. Osteoporotic vertebral lesions do not involve the pedicle or have contour abnormalities.
Table 4.
Differential diagnosis of vertebral involvement because of pyogenic, tuberculous, brucellar or metastatic diseases
| Pyogenic | Tuberculous | Brucellar | Metastatic | |
|---|---|---|---|---|
| Duration of illness (in months) | 2-3 | 3-6 | 2-6 | <2 |
| Usual age of presentation | Any age | Children and young adults | Middle-aged | Middle-aged and elderly |
| Anatomical location | Lumbar | Lumbo-thoracic | Lumbar | Thoracic |
| Vertebral and other structures involved | Vertebral bodies and the intervening disk, minimal soft tissue involvement | Vertebral bodies and the intervening disk, extensive soft tissue involvement (cold abscess) | Vertebral bodies and the intervening disk, minimal paraspinal soft tissue involvement, sacroiliitis | Posterior wall of the vertebral body (60%), pedicles and lamina (50%) |
| Common predisposing factors | Systemic illnesses like diabetes mellitus | Exposure to tuberculous infection | Ingestion of unpasteurized milk | Presence of systemic malignancy |
| Common clinical features | Fever and marked back pain, myelopathy | Fever, malaise and weight loss, backache, myelopathy | Fever, malaise, weight loss, backache | Bone pain at night, backache, back pain followed by radicular pain, myelopathy |
| Laboratory features | ||||
| Leukocytosis | Present | Absent | Present | Absent |
| Raised ESR | Raised | Raised | Raised | Not raised |
| C-reactive protein | Raised | May be raised | May be raised | Not raised |
| Neuroimaging (salient features) | Destruction of vertebral bodies and disc spaces, marked enhancement of the lesion, epidural abscess | Destruction of vertebral bodies and disc spaces, rim enhancement of the soft-tissue masses | Intact vertebral architecture despite diffuse vertebral osteomyelitis | Low signal intensity on T1-weighted images, hypersignal on T2-weighted images and heterogeneous enhancement |
In patients with metastatic spinal cord involvement, the intervertebral disk height is usually preserved, but this may be affected in lymphoma and multiple myeloma. The vertebral end-plates are also distinct and usually regular. The posterior vertebral segments are more extensively affected early on. It is difficult to differentiate spinal tuberculosis from a metastatic disease on MRI if there is prominent central body tuberculosis and epidural tuberculous granuloma without osseous involvement.30 In elderly patients with vertebral damage, metastatic disease of the spine should always be considered (Table 4).
Treatment
In patients with spinal tuberculosis, antituberculous treatment should be started as early as possible. Antituberculous treatment often needs to be instituted empirically, much before an etiological diagnosis is established. In resource-poor countries, etiological diagnoses may not be established at all. In patients with established complications of spinal tuberculosis, surgery may also be required. Sequelae like kyphosis require surgical intervention.79,80
Almost all antituberculous drugs penetrate well into tuberculous vertebral lesions. The distribution of antituberculosis drugs such as rifampin, isoniazid, and pyrazinamide was evaluated in affected vertebral tissues of spinal tuberculosis. In patients without vertebral sclerotic wall around the tuberculous foci, the isoniazid concentrations in foci were of bactericidal levels. Levels of rifampin and pyrazinamide in foci corresponded to the minimal inhibitory concentrations of each drug, respectively. The sclerotic bone of affected vertebra played a role in blocking the antituberculosis drug's penetration. In another study, three drugs resulted in an effective bactericidal concentration level in osseous tissues around the foci of spinal tuberculosis except for the 4 mm of osseous tissue surrounding the sclerotic wall. The results suggested that osseous tissues within 4 mm surrounding the sclerotic wall should be removed during the surgery.81,82
Antituberculous treatment
Various studies have shown that the majority (82–95%) patients of spinal tuberculosis respond very well to medical treatment. The treatment response is apparent in form of pain relief, decrease in neurological deficit, and even correction of spinal deformity.58–60,79,80 Patients with potentially dangerous craniovertebral junction tuberculosis also respond satisfactorily to medical treatment.83 Patients with medically resistant spinal tuberculosis need careful reassessment of the differential diagnosis before surgery is planned surgery.59,84
Therapeutic regimen
The total duration of treatment and numbers of drugs needed for adequate treatment have always been subject to controversy.84 World Health Organization (WHO) recommends a category-based treatment for tuberculosis. Spinal tuberculosis falls under category-1 of the WHO treatment category. The category-1 antituberculosis treatment regimen is divided into two phases: an intensive (initial) phase and a continuation phase. In the 2-month intensive phase, antituberculous therapy includes a combination of four first-line drugs: isoniazid, rifampicin, streptomycin, and pyrazinamide. In the continuation phase, two drugs (isoniazid and rifampicin) are given for 4 months. Because of the serious risk of disability and mortality and because of difficulties of assessing treatment response, WHO recommends 9 months of treatment for tuberculosis of bones or joints.85 The American Thoracic Society recommends 6 months of chemotherapy for spinal tuberculosis in adults and 12 months in children.86 The British Thoracic Society recommends 6 months of daily treatment with rifampicin and isoniazid, supplemented in the initial 2 months with pyrazinamide and either ethambutol or streptomycin (the 6-month four-drug regimen), irrespective of age.87 Although 6 months of treatment is considered sufficient, many experts still prefer a durations of 12–24 months or until radiological or pathological evidence of regression of disease occurs.30,33,88,89 To avoid poor compliance, directly observed treatment and short-course regimens may be administered.90 There is no definite role for corticosteroids in spinal tuberculosis except in cases of spinal arachnoiditis or nonosseous spinal tuberculosis.57,91
Supportive measures
General supportive measures, together with prolonged recumbency and rest, formed the basis of treatment for a patient with tuberculosis of the spine before the era of antituberculous chemotherapy. Sanatorium care was formerly the avenue of treatment for patients with pulmonary and bone tuberculosis. Today, the majority of patients with bone tuberculosis are treated with ambulatory care without prolonged recumbency and rest. Although cast or brace immobilization was a classic form of treatment, it was found to be inefficient and has generally been abandoned.10
Surgery
There is controversy about the precise role of surgery in the management of spinal tuberculosis. This difference of opinion goes back to 1960 when Hodgson and Stock advocated surgical treatment, and Konstam and colleagues advocated conservative treatment.92–94 However, many experts feel that neither all cases of tuberculosis of spine should be treated conservatively, nor do all cases require surgery. Approximately 40% of the cases of tuberculosis of the spine with paraplegia show recovery with antituberculous treatment, rest, and/or traction. Tuli, in 1975, proposed a ‘middle-path regimen’ for treatment of spinal tuberculosis. It advocates conservative treatment with multi-drug chemotherapy and surgery reserved for specific indications.95
The Medical Research Council of the United Kingdom, on the basis of results of some hallmark studies, had shown that antituberculous treatment alone can be effective, with a resolution of neurological sequelae and prevention of substantial progression of kyphosis. A Cochrane Database Review assessing the role of routine surgery in addition to chemotherapy in spinal tuberculosis also concluded that evidence was insufficient for the routine use of surgery. There were no statistically significant differences for any of the outcome measures: kyphosis angle, neurological deficit (none went on to develop this), bony fusion, absence of spinal tuberculosis, death from any cause, activity level regained, change of allocated treatment, or bone loss.58 The guidelines published by the Royal College of Physicians noted that there was no additional advantage of routinely carrying out anterior spinal fusion over standard chemotherapy.57
A randomized trial performed primarily among ambulatory patients by the Medical Research Council Working Party on Tuberculosis of the Spine demonstrated no additional benefit of surgical debridement or radical operation (resection of the spinal focus and bone grafting) in combination with chemotherapy compared with chemotherapy alone. Myelopathy with or without functional impairment most often responds to chemotherapy.96 In two Medical Research Council studies conducted in Korea, 24 of 30 patients in one study and 74 of 85 patients in an earlier study had complete resolution of myelopathy or complete functional recovery when treated medically.97,98
In some circumstances, however, surgery appears to be beneficial and may be indicated.97,98 Potential benefits of surgery were less kyphosis, immediate relief of compressed neural tissue, quicker relief of pain, higher percentage of bony fusion, quicker bony fusion, less relapse, earlier return to previous activities, and less bone loss. It may also prevent late neurological problems due to kyphosis of the spine if fusion has not occurred.32,99 One expert suggested that indications for surgery were pan-vertebral lesions, refractory disease, severe kyphosis, an evolving neurological deficit, and clinical deterioration or lack of clinical improvement.100,101
Two types of surgical procedures are performed. One is debridement of the infected material. In this form of surgery no attempt is made to stabilize the spine. The other procedure is debridement with stabilization of the spine (spinal reconstruction). This is a more extensive procedure and the reconstructions are performed with bone grafts. Stabilization may also be done using artificial materials like steel, carbon fiber, or titanium58 (Table 5).
Table 5.
Indications of surgery in spinal tuberculosis
| Indications for surgery in patients without neurological complications | Indications for surgery in patients with neurological complications |
|---|---|
| Progressive bone destruction in spite of ATT | New or worsening neural complications or lack of improvement with conservative treatment |
| Failure to respond to conservative therapy | Paraplegia of rapid onset or severe paraplegia |
| Evacuation of paravertebral abscess when it has increased in size despite medical treatment | Late-onset paraplegia |
| Uncertainty of diagnosis, for biopsy | Neural arch disease |
| Mechanical reasons: spinal instability caused by destruction or collapse, destruction of two or more vertebrae, kyphosis | Painful paraplegia in elderly patients |
| Prevention of severe kyphosis in young children with extensive dorsal lesions | Spinal tumor syndrome (epidural spinal tuberculoma without osseous involvement) |
| Large paraspinal abscess |
ATT, antituberculous treatment.
Treatment of spinal tuberculosis in HIV infection
Treatment of tuberculosis in patients with HIV infection follows the same principles as treatment of uninfected patients. Spinal tuberculosis in HIV-infected patients can also be satisfactorily treated with good clinical outcome irrespective of HIV status and treatment with antiretroviral therapy.102,103 However, there are several important differences between patients with and without HIV infection. These differences include the potential for drug interactions, especially between the rifamycins and antiretroviral agents, paradoxical reactions that may be interpreted as clinical worsening, and the potential for the development of acquired resistance to rifamycins when treated with highly intermittent therapy. Major orthopedic surgery in HIV-positive patients has potential for increased risk for sepsis.104
Management of complications
Measures that are helpful in minimizing the increase in kyphosis include recumbency in the early active stage of the disease and prolonged protection of the spine with suitable braces in later stages. Most experts believe that kyphosis greater than 30° is likely to generate back pain and may deteriorate further, and hence, requires surgical correction.105–107 Aspiration or surgical drainage was carried out for some patients with large cold abscesses because it was thought to improve the patient's general condition, and prevent rapid progression of the abscess along the spine. This has been shown to be ineffective, however, and surgical drainage of a cold abscess alone is no longer recommended.23 Abscesses usually resolve with medical therapy as antituberculosis drugs penetrate very well.108,109
Prognosis
Prognosis is generally good in patients without neurological deficit and deformity. Various studies show that 82–95% cases respond to medical treatment alone in the form of pain relief, improving neurological deficit, and correction of spinal deformity.23,108 In a recently published study among patients with neurologic deficit, significant recovery occurred in 92%, with 74% improving from nonambulatory to ambulatory status. This study included 82 patients; 52% of patients presented in a nonambulatory state, 21% had mild neurologic deficits, and 27% had intact neurological function.110 In a study from an endemic country, the majority (79 patients, 61%) of patients had severe motor and sensory impairment. Imaging revealed multiple vertebral involvements in 90 patients (80%). All patients were managed using antituberculous treatment; however, 33 patients required operative treatment as well. Marked clinical improvement was seen in 91 patients (70%) within 6 months of treatment.111
In Korea, a retrospective study examined the treatment outcome in patients with spinal tuberculosis. A total of 116 patients with spinal tuberculosis were analyzed. Forty-seven patients (35%) had severe symptoms. Radical surgery was carried out in 84 (62%) patients. Twenty patients were treated with short-term chemotherapy, while 96 underwent long-term antituberculous treatment. At the end of chemotherapy, 94 patients had achieved a favorable status and 22 an unfavorable one. Age and radical surgery were significantly related to a favorable outcome by logistic analysis.112 Patients with craniovertebral junction tuberculosis can be managed conservatively regardless of the extent of bony destruction and the majority have satisfactory outcome.113 In a retrospective study of 71 patients, 11 patients underwent early surgery. Five (8%) patients required delayed surgery for reducible atlantoaxial dissociation. The remaining 82% patients were effectively managed conservatively.114
Conclusion
The prognosis for spinal tuberculosis is improved by early diagnosis and rapid intervention. A high degree of clinical suspicion is required if patients present with chronic back pain, even in the absence of neurological symptoms and signs. Medical treatment is generally effective. Surgical intervention is necessary in advanced cases with marked bony involvement, abscess formation, or paraplegia. Spinal tuberculosis affects young people, so efforts should be made for its effective prevention. Controlling the spread of tuberculosis is only way available to prevent spinal tuberculosis.
References
- 1.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br 2010;92(7):905–13 [DOI] [PubMed] [Google Scholar]
- 2.Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res 2007;460(July):39–49 [DOI] [PubMed] [Google Scholar]
- 3.Taylor GM, Murphy E, Hopkins R, Rutland P, Chistov Y. First report of Mycobacterium bovis DNA in human remains from the Iron Age. Microbiology 2007;153(4):1243–9 [DOI] [PubMed] [Google Scholar]
- 4.Dobson J. Percivall Pott. Ann R Coll Surg Eng 1972;50(1):54–65 [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. HIV/TB Facts 2011 [assessed on 2011 Jun 11]. http://www.who.int/hiv/topics/tb/hiv_tb_factsheet_june_2011.pdf .
- 6.World Health Organization. 2010/2011 tuberculosis global facts. Facts sheet no 104, 2010 Nov [accessed on 2011 Apr 16]. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/
- 7.Pawar UM, Kundnani V, Agashe V, Nene A, Nene A. Multidrug-resistant tuberculosis of the spine – is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine (Phila Pa 1976) 2009;34(22):E806–10 [DOI] [PubMed] [Google Scholar]
- 8.Gautam MP, Karki P, Rijal S, Singh R. Pott's spine and Pott's paraplegia. J Nep Med Assoc 2005;44(159):106–15 [PubMed] [Google Scholar]
- 9.Talbot JC, Bismil Q, Saralaya D, Newton DA, Frizzel RM, Shaw DL. Musculoskeletal tuberculosis in Bradford – a 6-year review. Ann R Coll Surg Engl 2007;89(4):405–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect 2008;136(5):653–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currier BC, Eismont FJ. Infection of the spine. In: Herkowitz HN, Garfin SR, Balderston RA, et al. (eds.) Rothman-Simeone The Spine. 4th ed Philadelphia, PA: W.B. Saunders; 1999. p. 1207–58 [Google Scholar]
- 12.Sterling TR, Pham PA, Chaisson RE. HIV infection-related tuberculosis: clinical manifestations and treatment. Clin Infect Dis 2010;50Suppl 3:S223–30 [DOI] [PubMed] [Google Scholar]
- 13.Iliyasu Z, Babashani M. Prevalence and predictors of tuberculosis coinfection among HIV-seropositive patients attending the Aminu Kano Teaching Hospital, northern Nigeria. J Epidemiol 2009;19(2):81–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godlwana L, Gounden P, Ngubo P, Nsibande T, Nyawo K, Puckree T. Incidence and profile of spinal tuberculosis in patients at the only public hospital admitting such patients in KwaZulu-Natal. Spinal Cord 2008;46(5):372–4 [DOI] [PubMed] [Google Scholar]
- 15.Kaila R, Malhi AM, Mahmood B, Saifuddin A. The incidence of multiple level noncontiguous vertebral tuberculosis detected using whole spine MRI. J Spinal Disord Tech 2007;20(1):78–81 [DOI] [PubMed] [Google Scholar]
- 16.Polley P, Dunn R. Noncontiguous spinal tuberculosis: incidence and management. Eur Spine J 2009;18(8):1096–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLain RF, Isada C. Spinal tuberculosis deserves a place on the radar screen. Cleve Clin J Med 2004;71(7):543–9 [DOI] [PubMed] [Google Scholar]
- 18.Alavi SM, Sharifi M. Tuberculous spondylitis: risk factors and clinical/paraclinical aspects in the south west of Iran. J Infect Public Health 2010;3(4):196–200 [DOI] [PubMed] [Google Scholar]
- 19.Zhang HQ, Deng A, Guo CF, Wang YX, Chen LQ, Wang YF, et al. Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch Med Res 2010;41(1):46–9 [DOI] [PubMed] [Google Scholar]
- 20.Tuli SM. Tuberculosis of the shoulder. Tuberculosis of the skeletal system. 1st ed New Delhi: JayPee Brothers Medical Publisher (P) Ltd; 1993 [Google Scholar]
- 21.Boachie-Adjei O, Squillante RG. Tuberculosis of the spine. Orthop Clin North Am 1996;27(1):95–103 [PubMed] [Google Scholar]
- 22.Schirmer P, Renault CA, Holodniy M. Is spinal tuberculosis contagious? Int J Infect Dis 2010;14(8):e659–66 [DOI] [PubMed] [Google Scholar]
- 23.Shanley DJ. Tuberculosis of the spine: imaging features. Am J Roentgenol 1995;164(3):659–64 [DOI] [PubMed] [Google Scholar]
- 24.Moghtaderi A, Alavi-Naini R, Rahimi-Movagar V. Tuberculous myelopathy: current aspects of neurological sequels in the southeast of Iran. Acta Neurol Scand 2006;113(4):267–72 [DOI] [PubMed] [Google Scholar]
- 25.Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. Am J Roentgenol 2002;179(4):979–83 [DOI] [PubMed] [Google Scholar]
- 26.Quiñones-Hinojosa A, Jun P, Jacobs R, Rosenberg WS, Weinstein PR. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus 2004;17(6):E1. [DOI] [PubMed] [Google Scholar]
- 27.Moon MS. Tuberculosis of the spine. Controversies and a new challenge. Spine (Phila Pa 1976) 1997;22(15):1791–7 [DOI] [PubMed] [Google Scholar]
- 28.Hodgson AR, Yau A. Pott's paraplegia: A classification based upon the living pathology. Paraplegia 1967;5(1):1–16 [DOI] [PubMed] [Google Scholar]
- 29.Pertuiset E, Beaudreuil J, Lioté F, Horusitzky A, Kemiche F, Richette P, et al. Spinal tuberculosis in adults. A study of 103 cases in a developed country, 1980–1994. Medicine (Baltimore) 1999;78(5):309–20 [DOI] [PubMed] [Google Scholar]
- 30.Kotil K, Alan MS, Bilge T. Medical management of Pott disease in the thoracic and lumbar spine: a prospective clinical study. J Neurosurg Spine 2007;6(3):222–8 [DOI] [PubMed] [Google Scholar]
- 31.Tuli SM. Judicious management of tuberculosis of bone joints and spines. Indian J Orthop 1985;19(2):147–66 [Google Scholar]
- 32.Hsu LC, Cheng CL, Leong JC. Pott's paraplegia of late onset: the cause of compression and results after anterior decompression. J Bone Joint Surg Br 1988;70(4):534–8 [DOI] [PubMed] [Google Scholar]
- 33.Cormican L, Hammal R, Messenger J, Milburn HJ. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J 2006;82(963):46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owolabi LF, Nagoda MM, Samaila AA, Aliyu I. Spinal tuberculosis in adults: a study of 87 cases in Northwestern Nigeria. Neurology Asia 2010;15(3):239–44 [Google Scholar]
- 35.Lindhal S, Nymann RS, Brismar J. Imaging of tuberculosis. IV. Spinal manifestations in 63 patients. Acta Radiol 1996;37(4):506–11 [DOI] [PubMed] [Google Scholar]
- 36.Polley P, Dunn R. Noncontiguous spinal tuberculosis: incidence and management. Eur Spine J 2009;18(8):1096–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azzam NI, Tammawy M. Tuberculous spondylitis in adults: diagnosis and treatment. Br J Neurosurg 1988;2(1):85–91 [DOI] [PubMed] [Google Scholar]
- 38.Jain R, Sawhney S, Berry M. Computed tomography of tuberculosis: patterns of bone destruction. Clin Radiol 1993;47(3):196–9 [DOI] [PubMed] [Google Scholar]
- 39.Watts HG, Lifeso RM. Tuberculosis of bones and joints. J Bone Joint Surg Am 1996;78(2):288–98 [DOI] [PubMed] [Google Scholar]
- 40.Jain AK, Arora A, Kumar S, Sethi A, Avtar R. Measurement of prevertebral soft tissue space (PVSTS) in cervical spine in an Indian population. Indian J Orthop 1994;28(1):27–31 [Google Scholar]
- 41.Dass B, Puet TA, Watanakunakorn C. Tuberculosis of the spine (Pott's disease) presenting as ‘compression fractures’. Spinal Cord 2002;40(11):604–8 [DOI] [PubMed] [Google Scholar]
- 42.Hodgson AR, Wong W, Yau A. X-ray appearance of tuberculosis of the spine. Springfield, IL: Charles C Thomas; 1969 [Google Scholar]
- 43.Dharmalingam M. Tuberculosis of the spine – the Sabah experience. Epidemiology, treatment and results. Tuberculosis (Edinb) 2004;84(1–2):24–8 [DOI] [PubMed] [Google Scholar]
- 44.Garg RK, Sharma R, Kar AM, Kushwaha RA, Singh MK, Shukla R, et al. Neurological complications of miliary tuberculosis. Clin Neurol Neurosurg 2010;112(3):188–92 [DOI] [PubMed] [Google Scholar]
- 45.Perronne C, Saba J, Behloul Z. Pyogenic and tuberculosis spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis 1994;19(4):746–50 [DOI] [PubMed] [Google Scholar]
- 46.Ridley N, Shaikh MI, Remedios D, Mitchell R. Radiology of skeletal tuberculosis. Orthopedics 1998;21(11):1213–20 [DOI] [PubMed] [Google Scholar]
- 47.Bell GR, Stearns KL, Bonutti PM, Boumphrey FR. MRI diagnosis of tuberculous vertebral osteomyelitis. Spine (Phila Pa 1976) 1990;15(6):462–5 [DOI] [PubMed] [Google Scholar]
- 48.Griffith JF, Kumta SM, Leung PC, Cheng JC, Chow LT, Metreweli C. Imaging of musculoskeletal tuberculosis: a new look at an old disease. Clin Orthop Relat Res 2002;398(May):32–9 [DOI] [PubMed] [Google Scholar]
- 49.Jung N-Y, Jee W-H, Ha K-Y, Park C-K, Byun J-Y. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. Am J Roentgenol 2004;182(6):1405–10 [DOI] [PubMed] [Google Scholar]
- 50.Oguz E, Sehirlioglu A, Altinmakas M, Ozturk C, Komurcu M, Solakoglu C, et al. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop 2008;32(1):127–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yusof MI, Hassan E, Rahmat N, Yunus R. Spinal tuberculosis: the association between pedicle involvement and anterior column damage and kyphotic deformity. Spine (Phila Pa 1976) 2009;34(7):713–7 [DOI] [PubMed] [Google Scholar]
- 52.Pandit HG, Sonsale PD, Shikare SS, Bhojraj SY. Bone scintigraphy in tuberculous spondylodiscitis. Eur Spine J 1999;8(3):205–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer N, Rosenstein ED. Rheumatologic manifestations of tuberculosis. Bull Rheum Dis 1997;46(3):5–8 [PubMed] [Google Scholar]
- 54.Skaf GS, Kanafani ZA, Araj GF, Kanj SS. Non-pyogenic infections of the spine. Int J Antimicrob Agents 2010;36(2):99–105 [DOI] [PubMed] [Google Scholar]
- 55.Mondal A. Cytological diagnosis of vertebral tuberculosis with fine needle aspiration biopsy. J Bone Joint Surg Am 1994;76(2):181–4 [DOI] [PubMed] [Google Scholar]
- 56.Francis IM, Das DK, Luthra UK, Sheikh Z, Sheikh M, Bashir M. Value of radiologically guided fine needle aspiration cytology (FNAC) in the diagnosis of spinal tuberculosis: a study of 29 cases. Cytopathology 1999;10(6):390–401 [DOI] [PubMed] [Google Scholar]
- 57.National Collaborating Centre for Chronic Conditions. TB (partial update) clinical guideline DRAFT (November 2010). Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London: Royal College of Physicians, 2006 [assessed on 2011 Apr 16]. (http://guidance.nice.org.uk/nicemedia/live/12193/51951/51951.pdf .
- 58.Jutte PC, Van Loenhout-Rooyackers JH. Routine surgery in addition to chemotherapy for treating spinal tuberculosis. Cochrane Database Syst Rev 2006;(1):CD004532. DOI: 10.1002/14651858.CD004532 [DOI] [PubMed] [Google Scholar]
- 59.Tuli SM. Treatment of neurological complications in tuberculosis of spine. J Bone Joint Surg Am 1969;51(4):680–92 [PubMed] [Google Scholar]
- 60.Lifeso RM, Weaver P, Harder EH. Tuberculous spondylitis in adults. J Bone Joint Surg Am 1985;67(9):1405–13 [PubMed] [Google Scholar]
- 61.Handa U, Garg S, Mohan H, Garg SK. Role of fine-needle aspiration cytology in tuberculosis of bone. Diagn Cytopathol 2010;38(1):1–4 [DOI] [PubMed] [Google Scholar]
- 62.Wang D. Diagnosis of tuberculous vertebral osteomyelitis (TVO) in a developed country and literature review. Spinal Cord 2005;43(9):531–42 [DOI] [PubMed] [Google Scholar]
- 63.Van der Spoel van Dijk A, MCleod A, Botha PL, Shipley JA, Kapnoudhis MA, Beukes CA. The diagnosis of skeletal tuberculosis by polymerase chain reaction. Cent Afr J Med 2000;46(6):144–9 [DOI] [PubMed] [Google Scholar]
- 64.Cheng VC, Yam WC, Hung IF, Woo PC, Lau SK, Tang BS, et al. Clinical evaluation of the polymerase chain reaction for the rapid diagnosis of tuberculosis. J Clin Pathol 2004;57(3):281–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh UB, Seth P. PCR diagnosis of tuberculosis-experience in India. Indian J Pediatr 2002;69Suppl 1:S20–4 [PubMed] [Google Scholar]
- 66.Eisenach KD, Sifford MD, Cave MD, Bates JH, Crawford JT. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis 1991;144(5):1160–3 [DOI] [PubMed] [Google Scholar]
- 67.Singh KK, Muralidhar M, Kumar A, Chattopadhyaya TK, Kapila K, Singh MK, et al. Comparison of in house polymerase chain reaction with conventional techniques for the detection of Mycobacterium tuberculosis DNA in granulomatous lymphadenopathy. J Clin Pathol 2000;53(5):355–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colmenero JD, Morata P, Ruiz-Mesa JD, Bautista D, Bermúdez P, Bravo MJ, et al. Multiplex real-time polymerase chain reaction: a practical approach for rapid diagnosis of tuberculous and brucellar vertebral osteomyelitis. Spine (Phila Pa 1976) 2010;35(24):E1392–6 [DOI] [PubMed] [Google Scholar]
- 69.Tiwari V, Jain A, Verma RK. Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary and extrapulmonary tuberculosis. Indian J Med Res 2003;118(December):224–8 [PubMed] [Google Scholar]
- 70.Pandey V, Chawla K, Acharya K, Rao S, Rao S. The role of polymerase chain reaction in the management of osteoarticular tuberculosis. Int Orthop 2009;33(3):801–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berk RH, Yazici M, Atabey N, Ozdamar OS, Pabuccuoglu U, Alici E. Detection of Mycobacterium tuberculosis in formaldehyde solution-fixed, paraffin-embedded tissue by polymerase chain reaction in Pott's disease. Spine (Phila Pa 1976) 1996;21(17):1991–5 [DOI] [PubMed] [Google Scholar]
- 72.Kumar R, Das RK, Mahapatra AK. Role of interferon gamma release assay in the diagnosis of Pott disease. J Neurosurg Spine 2010;12(5):462–6 [DOI] [PubMed] [Google Scholar]
- 73.Weisz RD, Errico J. Spinal infection. Diagnosis and treatment. Bull Hosp Jt Dis 2000;59(1):40–6 [PubMed] [Google Scholar]
- 74.Yilmaz MH, Mete B, Kantarci F, Ozaras R, Ozer H, Mert A, et al. Tuberculous, brucellar and pyogenic spondylitis: comparison of magnetic resonance imaging findings and assessment of its value. South Med J 2007;100(6):613–4 [DOI] [PubMed] [Google Scholar]
- 75.Chang MC, Wu HT, Lee CH, Liu CL, Chen TH. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine (Phila Pa 1976) 2006;31(7):782–8 [DOI] [PubMed] [Google Scholar]
- 76.Turunc T, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect 2007;55(2):158–63 [DOI] [PubMed] [Google Scholar]
- 77.Kourbeti IS, Tsiodras S, Boumpas DT. Spinal infections: evolving concepts. Curr Opin Rheumatol 2008;20(4):471–9 [DOI] [PubMed] [Google Scholar]
- 78.Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics 2007;27(5):1255–73 [DOI] [PubMed] [Google Scholar]
- 79.Medical Research Council Working Party on Tuberculosis of the Spine A 15-year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong. Thirteenth Report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 1998;80(3):456–62 [DOI] [PubMed] [Google Scholar]
- 80.Carey ME. Infections of the spine and spinal cord. In: Youmans JR. (ed.) Neurological Surgery. 4th ed Philadelphia, PA: WB Saunders; 1996. p. 3270–304 [Google Scholar]
- 81.Ge Z, Wang Z, Wei M. Measurement of the concentration of three antituberculosis drugs in the focus of spinal tuberculosis. Eur Spine J 2008;17(11):1482–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu P, Zhu Q, Jiang J. Distribution of three anti-tuberculous drugs and their metabolites in different parts of pathological vertebrae with spinal tuberculosis. Spine (Phila Pa 1976) DOI: 10.1097/BRS.0b013e31820beae3 [2011 Feb 9] [Epub ahead of print] [DOI] [PubMed]
- 83.Arora S, Sabat D, Maini L, Sural S, Kumar V, Gautam VK, et al. The results of nonoperative treatment of craniovertebral junction tuberculosis: a review of twenty-six cases. J Bone Joint Surg Am 2011;93(6):540–7 [DOI] [PubMed] [Google Scholar]
- 84.Hazra A, Laha B. Chemotherapy of osteoarticular tuberculosis. Indian J Pharmacol 2005;37(1):5–12 [Google Scholar]
- 85.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. (WHO/HTM/TB/2009.420) World Health Organization; 2010 [assessed on 2011 Apr 16] (http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf .
- 86.Bass JB, Jr, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, Ruben F, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med 1994;149(5):1359–74 [DOI] [PubMed] [Google Scholar]
- 87.Joint Tuberculosis Committee of the British Thoracic Society Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax 1998;53(7):536–48 [PMC free article] [PubMed] [Google Scholar]
- 88.van Loenhout-Rooyackers JH, Verbeek AL, Jutte PC. Chemotherapeutic treatment for spinal tuberculosis. Int J Tuberc Lung Dis 2002;6(3):259–65 [PubMed] [Google Scholar]
- 89.Donald PR. The chemotherapy of osteo-articular tuberculosis with recommendations for treatment of children. J Infect 2011;62(6):411–39 [DOI] [PubMed] [Google Scholar]
- 90.Chan ED, Iseman MD. Current medical treatment for tuberculosis. BMJ 2002;325(7375):1282–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hristea A, Constantinescu RV, Exergian F, Arama V, Besleaga M, Tanasescu R. Paraplegia due to non-osseous spinal tuberculosis: report of three cases and review of the literature. Int J Infect Dis 2008;12(4):425–9 [DOI] [PubMed] [Google Scholar]
- 92.Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott's disease of the spine. Br J Surg 1960;48(September):172–8 [DOI] [PubMed] [Google Scholar]
- 93.Konstam PG, Konstam ST. Spinal tuberculosis in Southern Nigeria with special reference to ambulant treatment of thoracolumbar disease. J Bone Joint Surg Br 1958;40-B(1):26–32 [DOI] [PubMed] [Google Scholar]
- 94.Konstam PG, Blesovsky A. The ambulant treatment of spinal tuberculosis. Br J Surg 1962;50(July):26–38 [DOI] [PubMed] [Google Scholar]
- 95.Tuli SM. Results of treatment of spinal tuberculosis by ‘middle path’ regime. J Bone Joint Surg Br 1975;57(1):13–23 [PubMed] [Google Scholar]
- 96.Fourteenth report of the Medical Research Council Working Party on Tuberculosis of the Spine Five-year assessment of controlled trials of short-course chemotherapy regimens of 6, 9 or 18 months' duration for spinal tuberculosis in patients ambulatory from the start or undergoing radical surgery. Int Orthop 1999;23(2):73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Twelfth report of the Medical Research Council Working Party on Tuberculosis of the Spine Controlled trial of short-course regimens of chemotherapy in the ambulatory treatment of spinal tuberculosis: results at three years of a study in Korea. J Bone Joint Surg Br 1993;75(2):240–8 [DOI] [PubMed] [Google Scholar]
- 98.Pattison PRM. Pott's paraplegia: an account of the treatment of 89 consecutive patients. Paraplegia 1986;24(2):77–91 [DOI] [PubMed] [Google Scholar]
- 99.Leong JC. Tuberculosis of the spine. J Bone Joint Surg Br 1993;75(2):173–5 [DOI] [PubMed] [Google Scholar]
- 100.Jutte PC, Castelein RM. Complications of pedicle screws in lumbar and lumbosacral fusions in 105 consecutive primary operations. Eur Spine J 2002;11(6):594–8 [DOI] [PubMed] [Google Scholar]
- 101.Sell P. Expert's comment concerning grand rounds case entitled ‘Posterior listhesis of a lumbar vertebra in spinal tuberculosis’ (by Matthew A. Kirkman and Krishnamurthy Sridhar). Eur Spine J 2011;20(1):6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leibert E, Schluger NW, Bonk S, Rom WN. Spinal tuberculosis in patients with human immunodeficiency virus infection: clinical presentation, therapy and outcome. Tuber Lung Dis 1996;77(4):329–34 [DOI] [PubMed] [Google Scholar]
- 103.Govender S, Annamalai K, Kumar KP, Govender UG. Spinal tuberculosis in HIV positive and negative patients: immunological response and clinical outcome. Int Orthop 2000;24(3):163–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jellis JE. Orthopaedic surgery and HIV disease in Africa. Int Orthop 1996;20(4):253–6 [DOI] [PubMed] [Google Scholar]
- 105.Kaplan CJ. Pott's disease in South African Bantu children; an analysis of results and comparison with Lancashire figures. Br J Tuberc Dis Chest 1952;46(4):209–13 [DOI] [PubMed] [Google Scholar]
- 106.Rajeswari R, Balasubramanian R, Venkatesan P, Sivasubramanian S, Soundarapandian S, Shanmugasundaram TK, et al. Short-course chemotherapy in the treatment of Pott's paraplegia: report on five year follow-up. Int J Tuberc Lung Dis 1997;1(2):152–8 [PubMed] [Google Scholar]
- 107.Wimmer C, Ogon M, Sterzinger W, Landauer F, Stöckl B. Conservative treatment of tuberculous spondylitis: a long-term follow-up study. J Spinal Disord 1997;10(5):417–9 [PubMed] [Google Scholar]
- 108.Bakhsh A. Medical management of spinal tuberculosis: an experience from Pakistan. Spine (Phila Pa 1976) 2010;35(16):E787–91 [DOI] [PubMed] [Google Scholar]
- 109.Prasad R. Management of multi-drug resistant tuberculosis: practitioners view. Indian J Tuberc 2007;54(1):3–11 [PubMed] [Google Scholar]
- 110.Dunn R, Zondagh I, Candy S. Spinal tuberculosis: magnetic resonance imaging and neurological impairment. Spine (Phila Pa 1976) 2011;36(6):469–73 [DOI] [PubMed] [Google Scholar]
- 111.Mwachaka PM, Ranketi SS, Nchafatso OG, Kasyoka BM, Kiboi JG. Spinal tuberculosis among human immunodeficiency virus-negative patients in a Kenyan tertiary hospital: a 5-year synopsis. Spine J 2011;11(4):265–9 [DOI] [PubMed] [Google Scholar]
- 112.Park DW, Sohn JW, Kim EH, Cho DI, Lee JH, Kim KT, et al. Outcome and management of spinal tuberculosis according to the severity of disease: a retrospective study of 37 adult patients at Korean teaching hospitals. Spine (Phila Pa 1976) 2007;32(4):E130–5 [DOI] [PubMed] [Google Scholar]
- 113.Chadha M, Agarwal A, Singh AP. Craniovertebral tuberculosis: a retrospective review of 13 cases managed conservatively. Spine (Phila Pa 1976) 2007;32(15):1629–34 [DOI] [PubMed] [Google Scholar]
- 114.Teegala R, Kumar P, Kale SS, Sharma BS. Craniovertebral junction tuberculosis: a new comprehensive therapeutic strategy. Neurosurgery 2008;63(5):946–55 [DOI] [PubMed] [Google Scholar]