Summary
The development of super-resolution microscopy techniques using molecular localization, such as Photoactivated Localization Microscopy (PALM), Fluorescence Photoactivated Localization Microscopy (F-PALM), Stochastic Optical Reconstruction Microscopy (STORM), PALM with Independent Running Acquisition (PALMIRA) and many others, has heightened interest in molecules that will be grouped here into a category referred to as “optical highlighter” fluorescent proteins. This review will survey many of the advances in fluorescent protein for optically highlighting molecule subsets within a population of fluorescently labeled molecules.
Keywords: photoactivation, photoconversion, photoswitching, fluorescence microscopy
Introduction
The term “optical highlighter” describes molecules that are initially non-fluorescent or can be made non-fluorescent at the activated fluorescence wavelength and increase in fluorescence with contrast over a darker background after photoactivation, photoconversion, or photoswitching. In addition to super-resolution techniques (Patterson, 2009), these offer an alternative to photobleaching approaches in the study of protein kinetics, gene expression, organelle dynamics, and even cellular dynamics within living specimen. Numerous advances and discoveries have been made in developing optical highlighter fluorescent proteins, of which only a subset is discussed here. These molecules have been classified in several manners. Here, a selection of fluorescent proteins is generally grouped into three subclasses: photoactivatable (off state to on state), photoconvertible (converts from one wavelength to another), or photoswitchable (switches on and off repeatedly). Readers should note that synthetic optical highlighter probe development has progressed much over recent several years. And although these have been developed or modified for many of the same uses, discussion is limited here to their fluorescent protein counterparts.
Fluorescent proteins: Green photoactivation
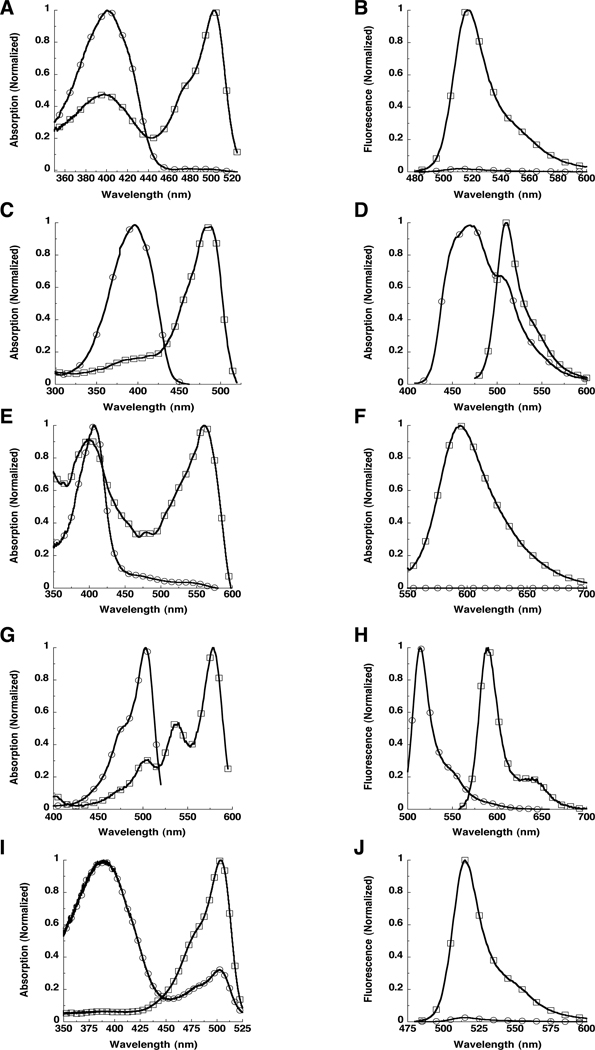
The wild type GFP (wtGFP) from Aequorea victoria was the first GFP used as highlighter. The chromophore population is a mixture of neutral phenols (Y66 is protonated) and anionic phenolates (Y66 is deprotonated) which produce absorption peaks at ~397 nm and ~475 nm, respectively. The technique relied on the photoconversion taking place after irradiation with ~400 nm light (Yokoe & Meyer, 1996), which produces an absorption increase at the minor peak and gives a subsequent increase in the fluorescence intensity when excited at this wavelength. This approach was improved using mutants at the T203 position (Patterson & Lippincott-Schwartz, 2002). A T203I mutant produces a variant that reduces the minor absorbance peak while maintaining the major peak (Heim et al., 1994, Ehrig et al., 1995). Several substitutions at this position, including T203I, were found to undergo photoconversion (Patterson & Lippincott-Schwartz, 2002). The T203H variant (named PA-GFP) decreased 488 nm absorption (Fig 1A) and produced >60 fold fluorescence (Fig. 1B) increases after ~400 nm irradiation (Patterson & Lippincott-Schwartz, 2002). (Table 1)
Figure 1. Example spectra for several optical highlighter fluorescent proteins.
A. Absorption spectra of PAGFP before (open circles) and after (open squares) photoactivation. B. Emission spectra of PAGFP before (open circles) and after (open squares) photoactivation. C. Absorption spectra of PSCFP before (open circles) and after (open squares) photoactivation. D. Emission spectra of PSCFP before (open circles) and after (open squares) photoactivation. E. Absorption spectra of PAmCherry1 before (open circles) and after (open squares) photoactivation. F. Emission spectra of PAmCherry1 before (open circles) and after (open squares) photoactivation. G. Absorption spectra of mKiKGR before (open circles) and after (open squares) photoconversion. H. Emission spectra of mKiKGR before (open circles) and after (open squares) photoconversion. I. Absorption spectra of Dronpa in the OFF state (open circles) and after photoswitching to the ON state (open squares). J. Emission spectra of Dronpa in the OFF state (open circles) and after photoswitching to the ON state (open squares). Note that the Dronpa protein sample was not entirely switched to the dark state (open circles) in this example.
Table 1.
Selected fluorescent protein optical highlighters
| Protein | Wavelengths (nm)a λem |
λem | Class | Fold contrast (post/pre)b |
Extinction coefficient (M−1 cm−1) |
Quantum yield |
Reference |
|---|---|---|---|---|---|---|---|
| PA-GFP | 400 (Pre) 504 (Post) |
515 517 |
Photoactivatable | 70 | 20,700 17,400 |
0.13 0.79 |
(Patterson & Lippincott-Schwartz, 2002) |
| PS-CFP | 402 (Pre) 490 (Post) |
468 511 |
Photoactivatable | 300 green increase 1,500 green to cyan ratio |
34,000 27,000 |
0.16 0.19 |
(Chudakov et al., 2004) |
| PS-CFP2 | 400 (Pre) 490 (Post) |
470 511 |
Photoactivatable | 43,000 47,000 |
0.20 0.23 |
||
| PAmRFP1-1 | 578 (Post) | 605 | Photoactivatable | 70 | 10,000 | 0.08 | (Verkhusha & Sorkin, 2005) |
| PAmCherry1 | 564 (Post) | 595 | Photoactivatable | 4,000 | 18,000 | 0.46 | (Subach et al., 2009) |
| PATagRFP | 562 (Post) | 595 | Photoactivatable | 66,000 | 0.38 | (Subach et al., 2010) | |
| Kaede | 508 (Pre) 572 (Post) |
518 582 |
Photoconvertible | 2,000 red to green ratio |
98,800 60,400 |
0.80 0.33 |
(Ando et al., 2002) |
| Kikume Green- Red (KikGR) |
507 (Pre) 583 (Post) |
517 593 |
Photoconvertible | 2,000 red to green ratio |
28,200 32,600 |
0.70 0.65 |
(Tsutsui et al., 2005) |
| mKikGR | 505 (Pre) 580 (Post) |
515 591 |
Photoconvertible | 49,000 28,000 |
0.69 0.63 |
(Habuchi et al., 2008) | |
| EosFP | 506 (Pre) 571 (Post) |
516 581 |
Photoconvertible | 72,000 41,000 |
0.70 0.55 |
(Wiedenmann et al., 2004) | |
| mEosFP | 505 (Pre) 569 (Post) |
516 581 |
Photoconvertible | 67,200 37,000 |
0.64 0.62 |
(Wiedenmann et al., 2004) | |
| tdEosFP | 506 (Pre) 571 (Post) |
516 581 |
Photoconvertible | 200 | 84,000 (×2) 33,000 (×2) |
0.66 0.6 |
(Wiedenmann et al., 2004) |
| mEos2 | 506 (Pre) 573 (Post) |
519 584 |
Photoconvertible | 56,000 46,000 |
0.84 0.66 |
(McKinney et al., 2009) | |
| Dendra | 486 (Pre) 558 (Post) |
505 575 |
Photoconvertible | 4,500 red to green ratio |
21,000 20,000 |
0.72 0.70 |
(Gurskaya et al., 2006) |
| Dendra2 | 490 (Pre) 553 (Post) |
507 573 |
Photoconvertible | 300 | 45,000 35,000 |
0.50 0.55 |
|
| Dronpa | 503 (ON) | 518 | Photoswitchable | 95,000 | 0.85 | (Ando et al., 2004) | |
| Dronpa-2 | 486 (ON) | 487 | Photoswitchable | 56,000 | 0.28 | (Ando et al., 2007) | |
| Dronpa-3 | 487 (ON) | 514 | Photoswitchable | 58,000 | 0.33 | (Ando et al., 2007) | |
| rsFastlime | 496 (ON) | 518 | Photoswitchable | 67 | 39,100 | 0.77 | (Stiel et al., 2007) |
| Padron | 503 (ON) | 522 | Photoswitchable | 143 | 43,000 | 0.64 | (Andresen et al., 2008) |
| bsDronpa | 460 (ON) | 504 | Photoswitchable | 17 | 45,000 | 0.50 | (Andresen et al., 2008) |
| rsCherry | 572 (ON) | 610 | Photoswitchable | 6.7 | 80,000 | 0.02 | (Stiel et al., 2008) |
| rsCherryRev | 572 (ON) | 608 | Photoswitchable | 20 | 84,000 | 0.005 | (Stiel et al., 2008) |
| IrisFP | 488 (Pre) 551 (Post) |
516 580 |
Photoconvertible / Photoswitchable |
52,200 35,400 |
0.43 0.47 |
(Adam et al., 2008) |
(Pre) and (Post) indicate before and after irradiation to turn on fluorescence, respectively. (ON) indicates the fluorescent state of the protein.
Reported here if indicated in the original reference.
Fluorescent proteins: Cyan-to-green photoactivation
Photoswitchable cyan fluorescent protein (PS-CFP) changes both excitation and emission spectra in a shift from a cyan to a green fluorescent protein after irradiation (Chudakov et al., 2004) and could perhaps be considered photoconvertible, but is thought to have a structural alteration similar to wtGFP and PAGFP when photoactivated (Lukyanov et al., 2005). PS-CFP initially displays excitation at 402 nm and emission at 468 nm (Chudakov et al., 2004). (Fig 1C) After activation with ~400 nm light, new excitation and emission peaks are observed at 490 nm and 511 nm, respectively (Fig. 1D) producing ~1500-fold increase in the green-to-cyan fluorescence ratio. An improved version, PS-CFP2, that is brighter and develops fluorescence more efficiently at 37°C is also available through the commercial source, Evrogen.
Fluorescent proteins: Red photoactivation
The monomeric version of DsRed, mRFP1 (Campbell et al., 2002), was made into a series of photoactivatable fluorescent proteins, PAmRFP1-1, PAmRFP1-2, and PAmRFP1-3 (Verkhusha & Sorkin, 2005). The brightest, PAmRFP1-1, has a quantum yield of only 0.08, but produces an ~70-fold increase in red fluorescence upon ultraviolet light irradiation (Verkhusha & Sorkin, 2005). This was expanded by development of mCherry (a derivative of mRFP1) into a photoactivatable marker. PAmCherry1 (Subach et al., 2009) also has little fluorescence before photoactivation but was found to display a fluorescence increase of ~4000 fold and be ~10 times brighter than PAmRFP1-1 after ~400 nm irradiation. (Fig. 1E and 1F) The brightness of the TagRFP (Merzlyak et al., 2007) made it an appealing target for developing a photoactivatable fluorescent protein. Indeed, PATagRFP (Subach et al., 2010) is ~3 times brighter than PAmCherry1 with an extinction coefficient and a quantum yield of 66,000 M−1 cm−1 and 0.38, respectively. Both PAmCherry1 (Subach et al., 2009) and PATagRFP (Subach et al., 2010) have been used successfully in diffraction-limited photolabeling and super-resolution PALM experiments.
Fluorescent proteins: Green-to-red photoconversion
Kaede was one of the first green-to-red photoconvertible proteins utilized in mammalian cell biology (Ando et al., 2002). Before photoconversion with ~400 nm light, it has a major absorbance peak at 508 nm and emission at 518 nm. (Table 1) After photoconversion, it has a new red-shifted absorbance peak at 572 nm, which upon excitation fluoresces with a new emission peak at 582 nm. This shift in both the excitation and emission peaks results in a >2000 fold increase in the red-to-green fluorescence ratio. Kaede forms tetramers (Ando et al., 2002), which is a limitation for some experiments, but its large contrast over background after photoconversion makes it appealing as a marker for cells in developing organisms.
Another green-to-red photocovertible fluorescent protein (Tsutsui et al., 2005) was developed from KikG (Favia favus) which did not originally have highlighting properties. Using structural characterizations of Kaede, KikG was engineered into the KikGR (Fig. 1G and 1H), which produced >2000-fold increase in fluorescence contrast during ratio imaging of the red and green components. (Table 1) The purified KikGR protein lacked the brightness of Kaede, but displayed several fold more fluorescence than Kaede when expressed in cells. It was also found to be a tetramer (Tsutsui et al., 2005), but has since been modified into the monomeric version, mKikGR (Habuchi et al., 2008), which expands the use of this highlighter in cell biology applications.
EosFP also exhibits a green-to-red fluorescence photoconversion upon ultraviolet or near ultraviolet light irradiation (Wiedenmann et al., 2004). It has a green excitation maximum at 506 nm with emission at 516 nm until irradiation at 405 nm, which produces an excitation peak located at 571 nm with emission at 581 nm. (Table 1) Initially determined to be a tetramer, EosFP was engineered into two dimeric forms, d1EosFP and d2EosFP, and into a monomeric mEosFP. The emission maxima of these mutants remain constant while excitation maxima and brightness change slightly (Table 1). The mEosFP inefficiently forms a fluorescent molecule when expressed at 37°C (Wiedenmann et al., 2004), so an alternative for using the better performing dimeric forms and avoiding the problems associated with unintended intermolecular dimerization of tagged proteins has been to link two of them together into a tandem dimer, tdEosFP. Since having twice the size can also result in problems with use, the monomeric version was further developed into an improved protein, mEos2 (McKinney et al., 2009). The mEos2 is one of the brightest optical highlighter fluorescent proteins, has good photostability, and provides localization precisions in PALM imaging on the order of ~10 nm.
Dendra gives a 4500-fold photoconversion from its green-to-red fluorescent forms (Gurskaya et al., 2006). (Table 1) The wild type dendGFP (Labas et al., 2002) was engineered into the monomeric Dendra with its photoconversion properties. An improved version, Dendra2, represents an improvement in folding efficiency at 37°C. Differing from most of the proteins discussed here, Dendra and Dendra2 offer the options of being photoconverted with ~400 nm light or with a potentially less phototoxic wavelength (~488 nm).
Fluorescent proteins: Photoswitchable
One of the first reported proteins which could be efficiently switched on and off in bulk measurements was Dronpa. It initially displays green fluorescence with an absorption maximum at 503 nm (Fig. 1I) and emission maximum of 518 nm (Fig. 1J) (Ando et al., 2004). With intense irradiation at 490 nm, the absorbance at 503nm as well as the green fluorescence emission is decreased. However, after irradiation at 400 nm, the 503 nm absorbance as well as the green fluorescence emission is rapidly restored. (Table 1) Remarkably, Dronpa can undergo the on-off cycling >100 times with a loss of only 25% of the original fluorescence. The reversible nature of the fluorescence allows the same photoactivation experiment to be repeated multiple times within the same region of interest (Ando et al., 2004). This molecule has also undergone numerous alterations to produce several variants. Dronpa-2 and Dronpa-3 (Ando et al., 2007) were found to photoswitch off much more efficiently than the original. The rsFastlime mutant was found to photoswitch in both directions more efficiently (Stiel et al., 2007). The bsDronpa variant (Andresen et al., 2008) has a broad absorption spectrum which is blue-shifted compared with Dronpa and the other variants. And Padron (Andresen et al., 2008) shows photoswitching under opposite irradiation protocols by being turned on by 488 nm and off by 405 nm irradiation (Andresen et al., 2008). In addition to protein tracking, Dronpa and some of its derivatives, rsFastlime and Padron, have also shown their utility in super-resolution molecular localization experiments (Andresen et al., 2008).
Other photoswitchable fluorescent proteins include two versions of mCherry, rsCherry and rsCherryRev (Stiel et al., 2008). (Table 1) These molecules are switched back and forth between on and off states using red and green/yellow light. The rsCherry behaves similarly to Padron in that the more red-shifted irradiation (561 nm) turns it on while the more blue-shifted irradiation (470 nm) turns it off. IrisFP, a derivative of EosFP discussed earlier, can be irreversibly photoconverted just as EosFP, but has also joined the photoswitchable category by having the capability to photoswitch between off and on states while in the unconverted green form as well as photoswitch between on and off red states after photoconversion (Adam et al., 2008).
Fluorescent proteins: Conventional proteins behaving unconventionally
Included in this section are the fluorescent proteins that are normally just expressed and imaged without any irradiation step to increase their fluorescence. The conventional fluorescent proteins, EGFP (Haupts et al., 1998), EYFP (Dickson et al., 1997, Schwille et al., 2000, Biteen et al., 2008), Citrine (Heikal et al., 2000), and PhiYFP (Folling et al., 2008), have all been found to sample dark or other fluorescent states during single molecule imaging and Fluorescence Correlation Spectroscopy (FCS). In addition, several of the widely used red and orange variants have also displayed photoconversion into species fluorescing at wavelengths other than their original (Kremers et al., 2009). Of the variants studied, Katusha, mKate, HcRed1, mPlum, and mRaspberry had blue shifts of their spectra to varying degrees, whereas mOrange1, mOrange2, and Kusabira-Orange displayed red spectral shifts after irradiation. While these suggest caution in using these molecules in photobleaching studies, the authors pointedly showed that these molecules can be used in highlighting experiments also (Kremers et al., 2009).
Summary and outlook
The key feature of optical highlighter molecules is that they provide the capability to contrast a sub-population of tagged molecules over the entire pool within a specimen. These sub-populations can be monitored temporally and spatially using conventional diffraction-limited imaging techniques (several applications reviewed in (Lippincott-Schwartz & Patterson, 2009)) or for several super-resolution imaging techniques (Patterson, 2009). Based on publications over the past few years, the development of fluorescent protein optical highlighters has for the most part followed that of conventional fluorescent proteins and the goals will likely continue to be to make them better and redder. For instance, users of just about any fluorescence technique have better molecular brightness of the probes as the feature at the top of their wish list. This is especially key for the optical highlighter fluorescent proteins since they often lack the brightness of their conventional fluorescent protein counterpart. (Table 1) Shifting the excitation and emission wavelengths toward the infrared part of the spectrum is another goal that is passed along from conventional fluorescent protein development. However, for optical highlighters requiring irradiation in the near UV wavelength spectrum, improvements to red-shift the photoactivation, photoconversion, or photoswitching wavelengths are additional goals.
Will the renewed interest in optical highlighters continue to drive the probe development and eventually evolve these techniques into commonly used approaches for biologists? The super-resolution techniques relying on these probes have produced a great deal of excitement from biologists whom wish only to observe their favorite subject with a little more clarity. In addition, commercial microscope companies have been implementing “photoactivation” modules into their systems for several years and some are even producing commercial versions of instruments and software used for (F)PALM and STORM. This combination of investigator and commercial interests will likely drive the field and make common the use of optical highlighters over the next few years.
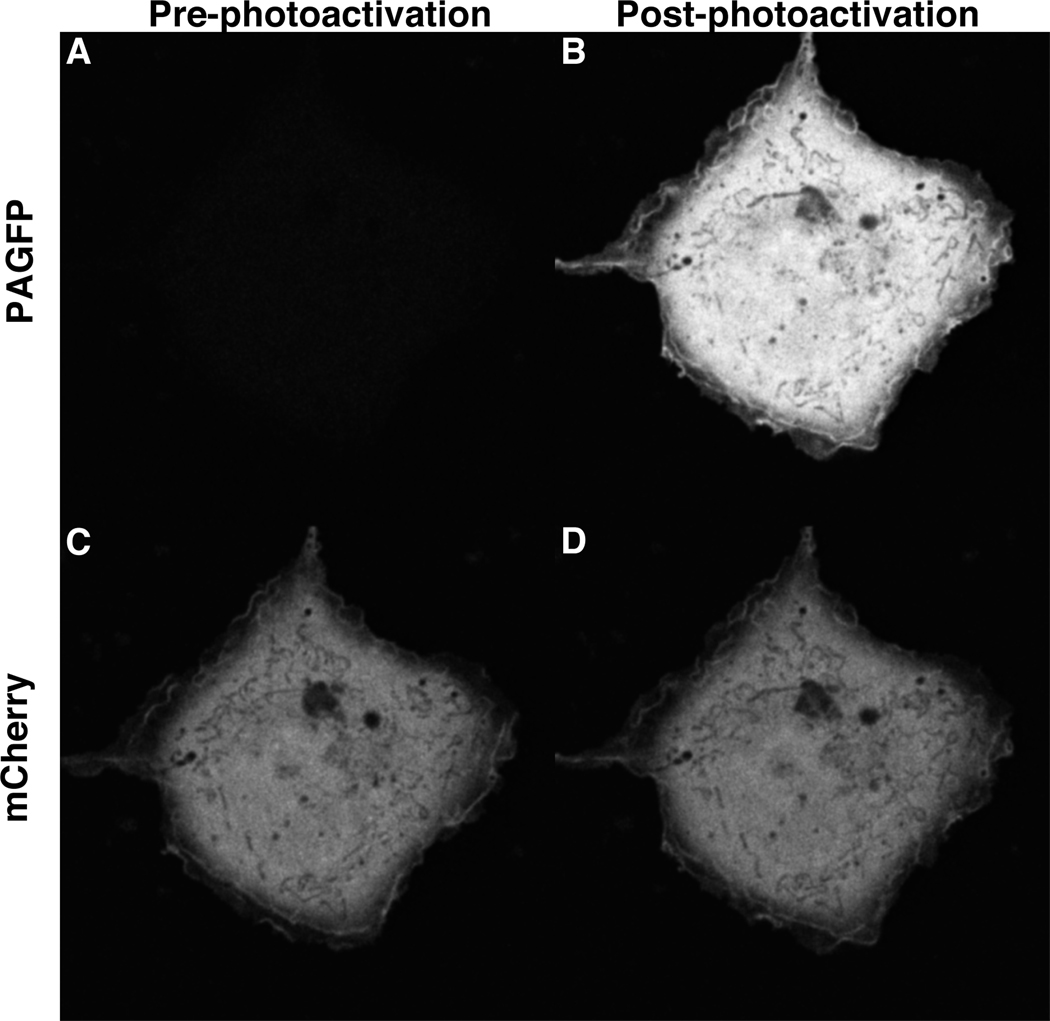
Figure 2.
Acknowledgements
This research was supported by the Intramural Research Program at the National Institutes of Health including the National Institute of Biomedical Imaging and Bioengineering.
References
- Adam V, Lelimousin M, Boehme S, et al. Structural characterization of IrisFP, an optical highlighter undergoing multiple photo-induced transformations. Proc Natl Acad Sci U S A. 2008;105:18343–18348. doi: 10.1073/pnas.0805949105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando R, Flors C, Mizuno H, Hofkens J, Miyawaki A. Highlighted generation of fluorescence signals using simultaneous two-color irradiation on Dronpa mutants. Biophys J. 2007;92:L97–L99. doi: 10.1529/biophysj.107.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–1373. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- Andresen M, Stiel AC, Folling J, et al. Photoswitchable fluorescent proteins enable monochromatic multilabel imaging and dual color fluorescence nanoscopy. Nat Biotechnol. 2008;26:1035–1040. doi: 10.1038/nbt.1493. [DOI] [PubMed] [Google Scholar]
- Biteen JS, Thompson MA, Tselentis NK, Bowman GR, Shapiro L, Moerner WE. Super-resolution imaging in live Caulobacter crescentus cells using photoswitchable EYFP. Nat Methods. 2008;5:947–949. doi: 10.1038/NMETH.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudakov DM, Verkhusha VV, Staroverov DB, Souslova EA, Lukyanov S, Lukyanov KA. Photoswitchable cyan fluorescent protein for protein tracking. Nat Biotechnol. 2004;22:1435–1439. doi: 10.1038/nbt1025. [DOI] [PubMed] [Google Scholar]
- Dickson RM, Cubitt AB, Tsien RY, Moerner WE. On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature. 1997;388:355–358. doi: 10.1038/41048. [DOI] [PubMed] [Google Scholar]
- Ehrig T, O'Kane DJ, Prendergast FG. Green-fluorescent protein mutants with altered fluorescence excitation spectra. FEBS Lett. 1995;367:163–166. doi: 10.1016/0014-5793(95)00557-p. [DOI] [PubMed] [Google Scholar]
- Folling J, Bossi M, Bock H, et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat Methods. 2008;5:943–945. doi: 10.1038/nmeth.1257. [DOI] [PubMed] [Google Scholar]
- Gurskaya NG, Verkhusha VV, Shcheglov AS, et al. Engineering of amonomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol. 2006;24:461–465. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- Habuchi S, Tsutsui H, Kochaniak AB, Miyawaki A, van Oijen AM. mKikGR, a monomeric photoswitchable fluorescent protein. PLoS One. 2008;3:e3944. doi: 10.1371/journal.pone.0003944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupts U, Maiti S, Schwille P, Webb WW. Dynamics of fluorescence fluctuations in green fluorescent protein observed by fluorescence correlation spectroscopy. Proc Natl Acad Sci U S A. 1998;95:13573–13578. doi: 10.1073/pnas.95.23.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikal AA, Hess ST, Baird GS, Tsien RY, Webb WW. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: coral red (dsRed) and yellow (Citrine) Proc Natl Acad Sci U S A. 2000;97:11996–12001. doi: 10.1073/pnas.97.22.11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R, Prasher DC, Tsien RY. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci U S A. 1994;91:12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers GJ, Hazelwood KL, Murphy CS, Davidson MW, Piston DW. Photoconversion in orange and red fluorescent proteins. Nat Methods. 2009;6:355–358. doi: 10.1038/nmeth.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labas YA, Gurskaya NG, Yanushevich YG, Fradkov AF, Lukyanov KA, Lukyanov SA, Matz MV. Diversity and evolution of the green fluorescent protein family. Proc Natl Acad Sci U S A. 2002;99:4256–4261. doi: 10.1073/pnas.062552299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Patterson GH. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol. 2009;19:555–565. doi: 10.1016/j.tcb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanov KA, Chudakov DM, Lukyanov S, Verkhusha VV. Innovation: Photoactivatable fluorescent proteins. Nat Rev Mol Cell Biol. 2005;6:885–891. doi: 10.1038/nrm1741. [DOI] [PubMed] [Google Scholar]
- McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods. 2009;6:131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzlyak EM, Goedhart J, Shcherbo D, et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
- Patterson GH. Fluorescence microscopy below the diffraction limit. Semin Cell Dev Biol. 2009;20:886–893. doi: 10.1016/j.semcdb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- Schwille P, Kummer S, Heikal AA, Moerner WE, Webb WW. Fluorescence correlation spectroscopy reveals fast optical excitation-driven intramolecular dynamics of yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2000;97:151–156. doi: 10.1073/pnas.97.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiel AC, Andresen M, Bock H, et al. Generation of monomeric reversibly switchable red fluorescent proteins for far-field fluorescence nanoscopy. Biophys J. 2008;95:2989–2997. doi: 10.1529/biophysj.108.130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiel AC, Trowitzsch S, Weber G, et al. 1.8 A bright-state structure of the reversibly switchable fluorescent protein Dronpa guides the generation of fast switching variants. Biochem J. 2007;402:35–42. doi: 10.1042/BJ20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subach FV, Patterson GH, Manley S, Gillette JM, Lippincott-Schwartz J, Verkhusha VV. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat Methods. 2009;6:153–159. doi: 10.1038/nmeth.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subach FV, Patterson GH, Renz M, Lippincott-Schwartz J, Verkhusha VV. Bright monomeric photoactivatable red fluorescent protein for two-color super-resolution sptPALM of live cells. J Am Chem Soc. 2010;132:6481–6491. doi: 10.1021/ja100906g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Shimizu H, Nukina N, Miyawaki A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 2005;6:233–238. doi: 10.1038/sj.embor.7400361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhusha VV, Sorkin A. Conversion of the monomeric red fluorescent protein into a photoactivatable probe. Chem Biol. 2005;12:279–285. doi: 10.1016/j.chembiol.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Wiedenmann J, Ivanchenko S, Oswald F, et al. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc Natl Acad Sci U S A. 2004;101:15905–15910. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoe H, Meyer T. Spatial dynamics of GFP-tagged proteins investigated by local fluorescence enhancement. Nat Biotechnol. 1996;14:1252–1256. doi: 10.1038/nbt1096-1252. [DOI] [PubMed] [Google Scholar]