Abstract
Objective:
To determine whether patients with cirrhosis and depressive symptoms have a different neuropsychological cognitive profile from patients with cirrhosis without depressive symptoms in order to show that cirrhosis may not be the only cause for cognitive decline in patients with cirrhosis.
Method:
Adult outpatients with a diagnosis of cirrhosis based on histologic findings and clinical characteristics, who did not have clinically overt hepatic encephalopathy and who were being treated in the advanced liver disease and liver transplant clinics, were recruited for the study from May 2003 to May 2006. Patients underwent neuropsychological testing and evaluation for depression using the Beck Depression Inventory-II (BDI-II). Age-adjusted standard neuropsychological domain scores were compared between depressed (BDI-II score ≥ 14) and nondepressed (BDI-II score < 14) patients.
Results:
Seventy-five subjects were included in the study. The 23 patients with depression were similar to the 52 nondepressed patients in level of education, age, and race; the laboratory parameters of international normalized ratio, bilirubin, creatinine, and albumin concentration; and Model for End-Stage Liver Disease scores. There was a higher percentage of women in the depressed group than in the nondepressed group, with a trend toward significance (52% vs 29%; P = .07). No etiology of liver disease was associated with depression. In linear regression analyses, decreases in cognitive function were associated with higher BDI-II scores for the domains of working memory (P = .026), with a trend toward significance for visual-perception (P = .056). Approximately 7% of the variability in working memory score was predicted using the BDI score.
Conclusions:
Depressive symptoms are associated with worsened cognitive function in cirrhosis.
In patients with cirrhosis, cognitive impairment may occur as a result of several mechanisms. A proposed pathophysiologic mechanism is through the accumulation of neurotoxic compounds and the synergistic actions of some of these compounds with proinflammatory cytokines in the brain that diminish the ability of astrocytes to detoxify ammonia in the brain, the accumulation of which may interfere with neurotransmitter transport and activity.1–6 Changes in neurotransmitter activity could also potentially lead to mood alterations, thereby creating an association between mood disorders, such as depression, and the cognitive impairment of cirrhosis. Because of this potential link, management of patients with hepatic encephalopathy may be best approached by addressing any mood disorder and also lowering levels of ammonia in the gastrointestinal tract. Recognition of the cognitive profile of depressed patients with cirrhosis could be helpful in raising awareness about mood status, which has a potential negative additive effect on cognition.
Depression, common in the general population, is frequent in patients with chronic liver disease and cirrhosis and is present in up to 15% of patients on the liver transplant waiting list and in up to 57% of patients with cirrhosis.7–11 Depression has been studied in the setting of liver disease caused by chronic hepatitis C virus (HCV) infection and primary biliary cirrhosis.10,12–15 Patients with chronic HCV frequently complain of depression and difficulty performing tasks7,9,10; furthermore, they have been found to have neuropsychological impairment in the domains of processing speed and working memory.11,16 However, fewer studies have been done in the general population with cirrhosis.
Depression can cause cognitive deficits that, fortunately, may respond to appropriate therapy. Whether the cognitive impairment due to depression in cirrhosis is different, or incremental, to the cognitive impairment of hepatic encephalopathy in patients with advanced liver disease has not been thoroughly studied. There is a risk that failure to recognize and treat depression in cirrhosis could lead clinicians to ascribe all cognitive deficits to hepatic encephalopathy.
The consensus from ongoing neuropsychological analysis of patients with minimal hepatic encephalopathy is that these patients show deficits mainly in attention and visuomotor abilities,17 whereas language and verbal intellect are usually found to be relatively spared.18 The results of neuropsychological tests aimed at evaluating memory in this population have been mixed. For instance, O'Carroll et al19 found memory dysfunction. However, others have found deficits in memory, but attributed this to deficits in attention and visuospatial functions.20 The visuospatial deficits have been ascribed to impairment in attention and fine-manual dexterity, not simply to motor speed.18 In this study, we used a comprehensive neuropsychological battery in order to capture the cognitive profile of patients with cirrhosis who had depressed symptoms and those without depressed symptoms. The overall goal of this study was to determine whether patients with cirrhosis and depressive symptoms had a different cognitive profile from patients with cirrhosis without depressive symptoms.
Clinical Points
♦An assessment of mood disorders in patients with cirrhosis is important because of the association between cirrhosis and depressive symptoms.
♦Depressive symptoms may worsen cognitive impairment in patients with cirrhosis; therefore, treatment of depression should be considered for these patients.
METHOD
This study was approved by the Mayo Foundation Institutional Review Board, Rochester, Minnesota. We recruited consecutive adult outpatients from May 2003 to May 2006 with a diagnosis of cirrhosis based on histologic findings and clinical characteristics, who did not have clinically overt hepatic encephalopathy and who were being treated in the Mayo Clinic advanced liver disease and liver transplant clinics (Rochester, Minnesota). Non–English-speaking subjects and subjects with active gastrointestinal tract bleeding or other acute complications related to liver disease were excluded. Subjects included were required to be abstinent from alcohol and other substances of abuse for at least 6 months, to have no benzodiazepines or antipsychotic medications in their regimen, and to be free of a diagnosis of a primary neurologic disorder or an uncontrolled psychological disorder, such as schizoaffective or bipolar disorders.
All patients in the study were assessed with standardized neuropsychological tests and clinical evaluation during a single visit. Patients underwent neuropsychological testing and concurrent evaluation of depression using the Beck Depression Inventory-II (BDI-II). For our analysis, a score ≥ 14 on the BDI-II was considered diagnostic of depression.21 A BDI-II score is based on self-report,21 rather than a DSM-IV diagnosis.22 Data on antidepressant use were also collected using self-report and chart review, but depressed patients were grouped solely according to BDI-II score in order to capture only symptomatic depression. The grade of hepatic encephalopathy for each patient was determined by a clinician on the basis of the West Haven Criteria23,24 and that of Ferenci et al25 within 24 hours of the neuropsychological testing in most cases.
Methods of Neuropsychological Assessment
A standardized battery of pencil-and-paper neuropsychological tests was administered to all subjects by the same trained neuropsychometrist who was blinded to the results of the BDI-II and to the diagnosis of the patients. The tests included the Wechsler Adult Intelligence Scale-III (WAIS-III) block design, matrix reasoning, and picture completion (to obtain the perceptual Organization Index); arithmetic, digit span, and letter number sequencing subtests (to obtain the Working Memory Index); the digit symbol and symbol search subtests (for the Processing Speed Index)26; the Trailmaking A and B time subtest27; the California Verbal Learning Test (CVLT) total learning and long free delay subtests28; the Wechsler Memory Scale-III (WMS-III) logical memory I and II and verbal pairs I and II subtests29; the Rey-Osterrieth Complex Figure (copy only)30; and the Grooved Pegboard dominant and nondominant hands subtests31 (Table 1).
Table 1.
Neurocognitive Domains and Associated Neuropsychological Tests
| Domain | Test |
| Working memorya | WAIS-III working memory |
| Information processing speed | WAIS-III processing speed, Trailmaking A and B time |
| Learning | CVLT total learning, WMS-III logical memory I, verbal pairs I |
| Memory | CVLT long delay, WMS-III logical memory II and verbal pairs II |
| Visual-perceptual | WAIS-III perceptual organization, Rey-Osterrieth Complex Figure copy |
| Motor | Grooved Pegboard (dominant and nondominant hands) |
Also known as attention/concentration.
Abbreviations: CVLT = California Verbal Learning Test, WAIS-III = Wechsler Adult Intelligence Scale-III, WMS-III = Wechsler Memory Scale-III.
All scores were transformed to age-adjusted standard scores (placed on a common Z score metric), such that negative scores represented poorer performance. Age adjustment was based on appropriate normative samples (ie, standardization samples for WAIS-III/WMS-III, CVLT, Wide Range Achievement Test-III,32 and Conners’ Continuous Performance Test-II33; norms of Heaton et al,34 which also adjust for education, for Trailmaking A and B, Finger Tapping, and Grooved Pegboard; and norms of Meyers and Volbrecht35 for Rey-Osterrieth Complex Figure copy. These standard scores were then organized into pertinent cognitive domains (Table 1).
All domains (except premorbid intelligence quotient estimate) included at least 2 measures to enhance the reliability of the estimated Z score in each domain. The score for each domain is the mean Z score for all subtests in that domain. The continuous neuropsychological domain scores of more than 1 SD (–Z) below the standardized mean of the population were defined as abnormal on the basis of published population means and standard deviations among a normal population for each test.
It should be noted that the neuropsychological tests and the BDI-II were administered at the same time; typically, the patients were asked to complete the BDI-II while waiting for the psychometrist to administer neuropsychological testing. Our patients had no clinically overt signs of hepatic encephalopathy; therefore, although they could be considered to have minimal hepatic encephalopathy, they were not labeled as such, since there are no standardized tests for minimal hepatic encephalopathy.
Statistical Methods
Depressed (BDI-II score ≥ 14) and nondepressed (BDI-II score < 14) patients were compared with respect to demographic characteristics, factors associated with liver disease, concomitant disease, and neuropsychological domain scores using the Fisher exact test for binary variables and the Wilcoxon rank sum test for continuous variables.
In addition, we used continuous BDI-II scores to predict neuropsychological domain scores using linear regression. Within the linear regression models, 4 models were planned for each neuropsychological domain score: BDI-II score, gender, HCV, and education level. In addition to assessing the overall effect of BDI-II score on neuropsychological score, presence of HCV and years of education were each used to adjust the overall effect, as each was thought to potentially confound the association of interest. If the unadjusted and adjusted coefficients for the BDI-II differed by 0.5 Z score units or more, then the added variable was considered a confounder. Age was not tested as a confounder, because neuropsychological scores were already adjusted for age for those tests in which an age effect is anticipated. Furthermore, gender was assessed as an effect modifier for the effect of BDI-II score on neuropsychological score. We also used the published population mean and standard deviation among a normal population to assess the overall neuropsychological abilities of this cirrhotic population for each test. Power was calculated using the correlation observed between BDI-II score and each neuropsychological domain score. Calculations were performed using SAS statistical software version 8.2,36 and the regression figures were produced using R version 2.0.1.37
RESULTS
Patient Characteristics
A total of 75 patients with cirrhosis were prospectively evaluated. All subjects had lack of clinically overt signs of hepatic encephalopathy according to our defined criteria as described above. Fifty-two patients were classified as nondepressed (BDI-II score < 14), with a mean (SD) BDI-II score of 6 (3.8). Twenty-three patients were classified as depressed (BDI-II score ≥ 14), with a mean (SD) BDI-II score of 21 (6.8). Nine of the 23 depressed patients were being treated with antidepressant medications at the time of evaluation. Four of the 9 patients had a diagnosis of depression from a psychiatrist. In contrast, only 1 of the 52 nondepressed patients (BDI-II score < 14) was being treated with an antidepressant (ie, symptoms were in remission); this patient was also diagnosed as having depression by a psychiatrist.
The depressed and nondepressed groups did not significantly differ in their demographic characteristics, liver disease characteristics, clinical tests of liver function, or presence of concomitant diseases (Table 2).
Table 2.
Characteristics of Patients With Cirrhosis
| Variable | Depressed (n = 23) | Nondepressed (n = 52) | P Value |
| Age, mean (interquartile range), y | 58.0 (44–78) | 58.5 (24–76) | .75 |
| Women, n (%) | 12 (52.2) | 15 (28.8) | .07 |
| Race, n (%) | 1.00 | ||
| White | 22 (95.7) | 49 (94.2) | |
| Other | 1 (4.1) | 3 (5.8) | |
| Employed, n (%) | 13 (56.5) | 40 (76.9) | .1 |
| Total education, mean (SD), y | 13.8 (2.15) | 14.6 (2.35) | .19 |
| Diagnosis, n (%) | |||
| Alcoholic | 6 (26.1) | 17 (32.7) | .79 |
| Hepatitis C virus | 5 (21.7) | 7 (13.5) | .50 |
| Choloestatic | 3 (13.0) | 13 (25.0) | .36 |
| Nonalcoholic steatohepatitis | 2 (8.7) | 3 (5.8) | .64 |
| Other | 11 (47.8) | 21 (40.4) | .62 |
| MELD score, median (interquartile range) | 10.1 (8.0–11.0) | 10. (8.0–12.0) | .75 |
| Clinical values, median (interquartile range)a | |||
| International normalized ratio | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | .54 |
| Bilirubin (mg/dL) | 1.3 (1.0–1.7) | 1.6 (1.0–3.1) | .10 |
| Creatinine (mg/dL) | 1.0 (1.0–1.2) | 1.0 (1.0–1.2) | .67 |
| Albumin (g/dL) | 3.5 (3.1–4.3) | 3.3 (2.9–3.9) | .24 |
| Concomitant diseases, n (%)b | |||
| Diabetes mellitus | 7 (30.4) | 9 (17.3) | .23 |
| Hypertension | 5 (21.7) | 9 (17.3) | .75 |
| Other | 3 (13.0) | 13 (25.0) | .36 |
Patients compared using the Wilcoxon rank sum test.
Patients compared using the Fisher exact test.
Abbreviation: MELD = Model for End-Stage Liver Disease.
We included patients who were treated with antidepressants, whether or not they were exhibiting signs of depression in the analysis, without adjustments being made, because the effects of treatment on the cognitive domains were negligible.
Neuropsychological Profile of Patients With Cirrhosis Versus Normative Data
The neuropsychological profile of patients with cirrhosis was compared with normative data (population without cirrhosis), as shown in Table 3. Patients with cirrhosis, irrespective of depression status (data not shown), showed significant differences from norms in the neurocognitive domains of information processing speed (P = .0015), memory (P < .0001), visual-perceptual (P < .0001), and motor (P < .0001) functions.
Table 3.
Neurocognitive Function of Patients With Cirrhosis Compared With Normal Population Without Cirrhosis
| Cognitive Domain | Mean Z Score | 95% CI | P Value |
| Working memory | 0.07 | −0.15 to 0.29 | .54 |
| Information processing speed | −0.37 | −0.59 to −0.14 | .002 |
| Learning | 0.10 | −0.77 to 0.28 | .26 |
| Memory | −0.94 | −1.08 to −0.80 | < .0001 |
| Visual-perceptual | −0.91 | −1.22 to −0.60 | < .0001 |
| Motor | −1.17 | −1.43 to −0.91 | < .0001 |
Neuropsychological Profile According to BDI-II Scores
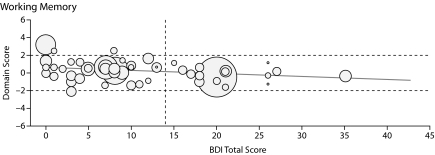
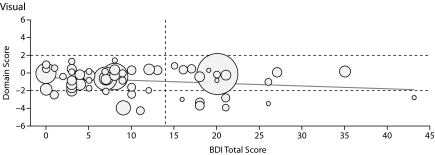
In linear regression analyses with BDI-II scores as a continuous variable, increasing depression score was associated with worsening cognitive performance in 2 domains: working memory and visual-perceptual functions. As reflected in the coefficients in Table 4, an increase of 10 points in the BDI-II score was associated with the following decreases in mean cognitive domain Z scores: –0.29 for working memory (P = .026) and –0.35 for visual-perceptual, which was not significant (P = .056). The effects of changes in BDI-II scores on neuropsychological domains are depicted in Figure 1.
Table 4.
Association of Beck Depression Inventory-II (BDI-II) Score With Neuropsychological Domainsa
| Cognitive Domain | Coefficient of BDI-II Scores | SE | P Value | R2 |
| Working memory | −0.029 | 0.013 | .026 | 0.066 |
| Information processing speed | −0.0192 | 0.0128 | .138 | 0.03 |
| Learning | −0.018 | 0.010 | .84 | 0.040 |
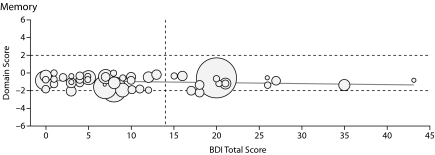
| Memory | −0.0104 | 0.008 | .196 | 0.023 |
| Visual-perceptual | −0.035 | 0.018 | .056 | 0.049 |
| Motor | 0.0016 | 0.016 | .92 | 0.0001 |
Each cognitive domain's standardized Z score was used as the outcome variable in a linear regression of BDI-II score. The BDI-II estimate may be interpreted as the mean change in the cognitive domain corresponding to a 1-unit change in BDI-II score; for example, an increase of 10 in the BDI-II score leads to a mean decrease of 0.29 in the working memory domain score.
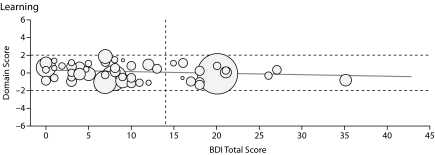
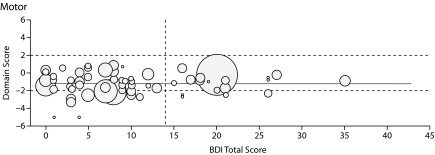
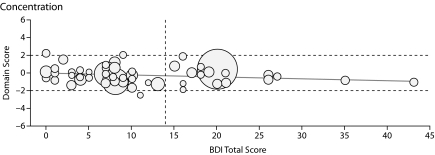
Figure 1.
Graphs Showing Domain Score Versus Total Beck Depression Inventory-II (BDI-II) Score for All Patients in the 6 Cognitive Domains Testeda
aThe boundaries for the abnormal region of the domain score (beyond 1 SD from the normal score) are shown by horizontal dashed lines, and the cutoff line for abnormal BDI-II score (> 14) is shown by the vertical dashed line. Each patient's Model for End-Stage Liver Disease score is depicted by the radius of the circle.
HCV did not confound the effect of BDI-II score on cognitive ability in any of the neuropsychological domains (data not shown). After adjusting for HCV, the association between depression and working memory remained similar and statistically significant (P = .027). Education also did not confound the association between depression and cognitive impairment in any domains (data not included). Gender modified the effect of depression on motor function, but not for the other neuropsychological domains. Among women, a 10-point increase in BDI-II score was associated with a mean decrease of 0.35 standard deviations in motor function. Among men, the 10-point increase in BDI-II score was associated with a mean decrease of 0.029 standard deviations in motor function (P = .039).
Power Calculation
Power was in the range of 41%–62% in the domains of working memory (also referred to as attention), learning, and visual-perceptual functions. Power in the domains of information processing speed, motor, and memory functions was lower (33%, 5%, and 25%, respectively). This may be because deficits in these domains are ubiquitous in cirrhosis, so that there is little observed variation within this diseased group.
DISCUSSION
The goal of this study was to examine the association between cognitive dysfunction and depression in patients with cirrhosis. As expected, the neurocognitive profile of patients with cirrhosis revealed general impairment, irrespective of whether they had depressive symptoms. In our sample, small, but statistically significant increases in depression score were associated with decreasing cognitive function in the domain of working memory. A trend toward significance was observed for the association between increasing depression scores and declines in the visual-perceptual performance domain.
In this clinical sample of consecutive patients with cirrhosis, clinically significant levels of depressive symptoms were common (31%). The high frequency of depression in patients with cirrhosis has also been shown in other studies wherein a rate up to 57% ranging from mild depression to extremely severe depression has been reported.38
Although our results require replication, the findings of this study are important because they suggest that depression is common in patients presenting to the clinic with cirrhosis, and that depression appears to have an additional effect on cognition, beyond that attributed to cirrhosis per se. It is unclear whether depression precedes the diagnosis of cirrhosis or vice versa in this population. However, it is known in general that when depression is treated in other populations, such as the elderly and patients with traumatic brain injury, cognition improves, including executive function, attention, and psychomotor function.39–41 Although the present results do not suggest a causal direction, it is plausible to hypothesize that cognitive function, specifically in working memory and visual perception, may worsen in patients with hepatic encephalopathy as depression scores increase or vice versa. These findings suggest that clinicians should be alert to monitor for signs of depression in cirrhotic patients with hepatic encephalopathy, especially since depression is often treatable.
It is well established that chronic disease overall is associated with neuropsychological deficits and high mortality,42–44 but only a few studies have examined the differences in cognitive profiles between depressed and nondepressed patients with cirrhosis and lack of overt hepatic encephalopathy. It is important to understand the factors that contribute to the variability in clinical presentation of patients with hepatic encephalopathy, including etiologies of liver disease. In general, of all the causes of liver disease, the effect of chronic HCV on cognition has been most extensively studied. Chronic HCV confers increased association with depression12,45–47 and added neuropsychological impairment. We found that when we adjusted for HCV, working memory was still impaired in patients with cirrhosis and depressive symptoms.
There is a strong association between depression and chronic HCV.46,48 Hilsabeck et al47,49 and Forton et al50 described the neurocognitive deficits of patients with chronic HCV as being primarily in the areas of attention and concentration, working memory, and psychomotor speed. However, a limitation of the study by Hilsabeck et al47 was that the study population had a large proportion of patients with cirrhosis; therefore, it is difficult to differentiate the findings that are due to chronic HCV and those due to cirrhosis. The cognitive profiles of patients with cirrhosis and patients with chronic HCV without cirrhosis appear to differ; namely, the cognitive changes that have been ascribed to chronic HCV without cirrhosis and without hepatic encephalopathy are attention and slight memory deficits.51 In contrast, our subjects had biopsy-proven or clinically proven cirrhosis and represented liver disease from a wider spectrum of causes, and, in this study, correlations were made between neurocognitive impairment and depressive symptoms. In addition, the population studied by Hilsabeck et al47 was heterogeneous in the use of illicit drugs and psychiatric history. In contrast, Weissenborn et al51 and McAndrews et al52 excluded patients on interferon therapy and had more homogeneous populations and found deficits in attention and memory.
The observation of an association between depression and cirrhosis in our study confirms the findings of others.12,44–46 Although several studies have assessed the association between depression and chronic liver disease, especially HCV, most of these studies did not specifically examine patients with cirrhosis,53,54 and if patients with cirrhosis were evaluated, the effects of depression on cognition were not evaluated.46,55,56
Although significant differences associated with depression were only found in working memory function with a trend toward significance in visual-perceptual functions, it should be kept in mind that patients with cirrhosis can have profound and diffuse neurocognitive deficits in other domains, as shown by our prior work.57 The findings presented here indicate that the patients with cirrhosis represent a population with diffuse neurocognitive findings at baseline that is worsened by depression. Hence, the associated power calculations from this study do not reflect the clinically significant changes in cognition that may occur when cirrhosis is superimposed with depression.
Our findings indicate that more extensive research in the area of depression in patients with cirrhosis is needed. Neurotransmitter dysfunction has been identified as one of the pathogenic mechanisms of cognitive dysfunction of cirrhosis and hepatic encephalopathy, which could potentially lead to changes in mood. It is probable that the institution of treatment of depression in some patients with cirrhosis could reduce cognitive impairment, making them more productive in the workplace; improve their quality of life; and potentially address issues, such as driving. Although our results represent the findings from a small study with relatively low power, they suggest that larger studies are needed. The strengths of our study include use of a broad-based neuropsychological battery and a focus on patients with cirrhosis due to diverse etiologies of liver diseases. Furthermore, these results also indicate some potentially reversible components of hepatic encephalopathy. Hence, multimodal therapy aimed at decreasing ammonia concentration in the gastrointestinal tract and concurrent management of mood disorders, if present, may be the best approach.
Potential conflicts of interest: None reported.
Funding/support: Funded by National Institutes of Health grant DK60018 to Dr Stewart.
Acknowledgments
We thank Anne Marie Weber-Main, PhD (Department of Medicine, University of Minnesota, Minneapolis), for her critical review and editing of manuscript drafts. Dr Weber-Main reports no conflicts of interest related to the subject of this article.
REFERENCES
- 1.Mousseau DD, Baker GB, Butterworth RF. Increased density of catalytic sites and expression of brain monoamine oxidase A in humans with hepatic encephalopathy. J Neurochem. 1997;68(3):1200–1208. doi: 10.1046/j.1471-4159.1997.68031200.x. [DOI] [PubMed] [Google Scholar]
- 2.Rao VL, Giguère JF, Layrargues GP, et al. Increased activities of MAOA and MAOB in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. Brain Res. 1993;621(2):349–352. doi: 10.1016/0006-8993(93)90126-8. [DOI] [PubMed] [Google Scholar]
- 3.Jalan R, Turjanski N, Taylor-Robinson SD, et al. Increased availability of central benzodiazepine receptors in patients with chronic hepatic encephalopathy and alcohol related cirrhosis. Gut. 2000;46(4):546–552. doi: 10.1136/gut.46.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterworth RF. Alterations of neurotransmitter-related gene expression in human and experimental portal-systemic encephalopathy. Metab Brain Dis. 1998;13(4):337–349. doi: 10.1023/a:1020641009971. [DOI] [PubMed] [Google Scholar]
- 5.Shawcross DL, Wright G, Olde Damink SW, et al. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22(1):125–138. doi: 10.1007/s11011-006-9042-1. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth RF. Pathophysiology of hepatic encephalopathy: the concept of synergism. Hepatol Res. 2008;38(suppl 1):S116–S121. doi: 10.1111/j.1872-034X.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 7.Gutteling JJ, de Man RA, Busschbach JJ, et al. Health-related quality of life and psychological correlates in patients listed for liver transplantation. Hepatol Int. 2007;1(4):437–443. doi: 10.1007/s12072-007-9035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz E, Hammer J. Gastrointestinal symptoms in patients with liver cirrhosis are linked to impaired quality of life and psychological distress. Eur J Gastroenterol Hepatol. 2009;21(4):460–465. [PubMed] [Google Scholar]
- 9.Dan AA, Martin LM, Crone C, et al. Depression, anemia and health-related quality of life in chronic hepatitis C. J Hepatol. 2006;44(3):491–498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Bonkovsky HL, Snow KK, Malet PF, et al. HALT-C Trial Group. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46(3):420–431. doi: 10.1016/j.jhep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana RJ, Bieliauskas LA, Back-Madruga C, et al. HALT-C Trial Group. Cognitive function in hepatitis C patients with advanced fibrosis enrolled in the HALT-C trial. J Hepatol. 2005;43(4):614–622. doi: 10.1016/j.jhep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311–317. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 13.Huet PM, Deslauriers J, Tran A, et al. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol. 2000;95(3):760–767. doi: 10.1111/j.1572-0241.2000.01857.x. [DOI] [PubMed] [Google Scholar]
- 14.Goh J, Coughlan B, Quinn J, et al. Fatigue does not correlate with the degree of hepatitis or the presence of autoimmune disorders in chronic hepatitis C infection. Eur J Gastroenterol Hepatol. 1999;11(8):833–838. doi: 10.1097/00042737-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27(1):209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 16.Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35(2):433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 17.McCrea M, Cordoba J, Vessey G, et al. Neuropsychological characterization and detection of subclinical hepatic encephalopathy. Arch Neurol. 1996;53(8):758–763. doi: 10.1001/archneur.1996.00550080076015. [DOI] [PubMed] [Google Scholar]
- 18.Tarter RE, Hegedus AM, Van Thiel DH, et al. Nonalcoholic cirrhosis associated with neuropsychological dysfunction in the absence of overt evidence of hepatic encephalopathy. Gastroenterology. 1984;86(6):1421–1427. [PMC free article] [PubMed] [Google Scholar]
- 19.O'Carroll RE, Couston M, Cossar J, et al. Psychological outcome and quality of life following liver transplantation: a prospective, national, single-center study. Liver Transpl. 2003;9(7):712–720. doi: 10.1053/jlts.2003.50138. [DOI] [PubMed] [Google Scholar]
- 20.Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34(5):768–773. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 21.Beck A. Beck Depression Inventory-II (BDI-II) San Diego, CA: Harcourt; 1996. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy: a controlled, double-blind clinical trial. Am J Dig Dis. 1978;23(5):398–406. doi: 10.1007/BF01072921. [DOI] [PubMed] [Google Scholar]
- 24.Conn HL, Moilton M. The Hepatic Coma Syndromes and Lactulose. Baltimore, MD: Lippincott Williams & Wilkins; 1979. [Google Scholar]
- 25.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler DA. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 27.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 28.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test-Second Edition. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 29.Wechsler D. Wechsler Memory Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 30.Osterrieth P. Le test de copie d'une figure complexe. Arch Psychol. 1944;30:286–356. [Google Scholar]
- 31.Matthews CG, Klove H. Instruction Manual for Adult Neuropsychology Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 32.Jastak S, Wilkinson G. The Wide Range Achievement Test: Manual of Instructions. Wilmington, DE: Jastak Associates; 1984. [Google Scholar]
- 33.Conners CK, Staff MHS. Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. North Tonwanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- 34.Heaton R, Grant I, Matthews C. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 35.Meyers JE, Volbrecht M. Detection of malingerers using the Rey Complex Figure and Recognition Trial. Appl Neuropsychol. 1999;6(4):201–207. doi: 10.1207/s15324826an0604_2. [DOI] [PubMed] [Google Scholar]
- 36.Cary, NC: SAS Institute; 2004. SAS Statistical Software, Version 8.2. [Google Scholar]
- 37.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 38.Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37(8):593–600. doi: 10.1016/j.dld.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42(1):48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 40.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157(12):1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 41.Buyukdura J, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment [published online ahead of print October 31, 2010] ProgNeuropsychophaarmacol Biol Psychiatry. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270(15):1819–1825. [PubMed] [Google Scholar]
- 43.Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60(6):627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- 44.Zung WW, Magruder-Habib K, Velez R, et al. The comorbidity of anxiety and depression in general medical patients: a longitudinal study. J Clin Psychiatry. 1990;51(suppl 51):77–80. discussion 81. [PubMed] [Google Scholar]
- 45.Rocca P, Cocuzza E, Rasetti R, et al. Predictors of psychiatric disorders in liver transplantation candidates: logistic regression models. Liver Transpl. 2003;9(7):721–726. doi: 10.1053/jlts.2003.50133. [DOI] [PubMed] [Google Scholar]
- 46.Gallegos-Orozco JF, Fuentes AP, Gerardo Argueta J, et al. Health-related quality of life and depression in patients with chronic hepatitis C. Arch Med Res. 2003;34(2):124–129. doi: 10.1016/s0188-4409(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 47.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35(2):440–446. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- 48.Nelligan JA, Loftis JM, Matthews AM, et al. Depression comorbidity and antidepressant use in veterans with chronic hepatitis C: results from a retrospective chart review. J Clin Psychiatry. 2008;69(5):810–816. doi: 10.4088/jcp.v69n0514. [DOI] [PubMed] [Google Scholar]
- 49.Hilsabeck RC, Hassanein TI, Carlson MD, et al. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9(6):847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- 50.Forton DM, Allsop JM, Cox IJ, et al. A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection. AIDS. 2005;19(suppl 3):S53–S63. doi: 10.1097/01.aids.0000192071.72948.77. [DOI] [PubMed] [Google Scholar]
- 51.Weissenborn K, Krause J, Bokemeyer M, et al. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol. 2004;41(5):845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 52.McAndrews MP, Farcnik K, Carlen P, et al. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41(4):801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- 53.Gutteling JJ, de Man RA, van der Plas SM, et al. Determinants of quality of life in chronic liver patients. Aliment Pharmacol Ther. 2006;23(11):1629–1635. doi: 10.1111/j.1365-2036.2006.02934.x. [DOI] [PubMed] [Google Scholar]
- 54.Coughlan B, Sheehan J, Hickey A, et al. Psychological well-being and quality of life in women with an iatrogenic hepatitis C virus infection. Br J Health Psychol. 2002;7(pt 1):105–116. doi: 10.1348/135910702169394. [DOI] [PubMed] [Google Scholar]
- 55.Singh N, Gayowski T, Wagener MM, et al. Depression in patients with cirrhosis: impact on outcome. Dig Dis Sci. 1997;42(7):1421–1427. doi: 10.1023/a:1018898106656. [DOI] [PubMed] [Google Scholar]
- 56.Häuser W, Zimmer C, Schiedermaier P, et al. Biopsychosocial predictors of health-related quality of life in patients with chronic hepatitis C. Psychosom Med. 2004;66(6):954–958. doi: 10.1097/01.psy.0000145824.82125.a8. [DOI] [PubMed] [Google Scholar]
- 57.Stewart C, Cerhan J, Smith G, et al. Objective assessment of cognitive impairment in cirrhotics without overt encephalopathy: implications for diagnosis, therapy, and public policy. Gastroenterology. 2004;126(4):M1265. [Google Scholar]