Abstract
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disease affecting thousands of people in the world and effective treatment is still not available. Over two decades of intense research using AD postmortem brains, transgenic mouse and cell models of amyloid precursor protein and tau revealed that amyloid beta (Aβ) and hyperphosphorylated tau are synergistically involved in triggering disease progression. Accumulating evidence also revealed that aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction initiates and contributes to the development and progression of the disease. The purpose of this article is to summarize the latest progress in aging and AD, with a special emphasis on the mitochondria, oxidative DNA damage including methods of its measurement. It also discusses the therapeutic potential of oxidative DNA damage and treatment strategies in AD.
Keywords: Amyloid-β, Oxidative stress, Antioxidant, Telomere, Stem cell, p53, DNA repair
1. Introduction
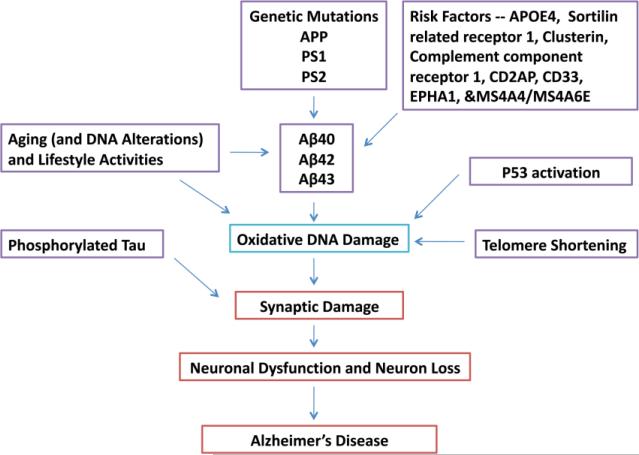
Alzheimer's disease (AD) is the most common progressive neurodegenerative disorder and the major cause for dementia [1]. As people age, memory and the ability to carry out tasks often decline and their risk for neuronal damage increases. AD is strongly age-associated disease. An estimated 5.4 million Americans have AD; approximately 200,000 people aged <65 years with AD comprise the younger-onset AD population, and over 50% of individuals with 85 years old will be affected by AD [2]. Pathologically, AD is associated with extracellular amyloid-beta (Aβ) deposits and intracellular aggregates of hyperphosphorylated tau, and neurofibrillary tangles, and also accompanied by oxidative stress/mitochondrial dysfunction and synaptic damage [1,3-7] (Figure 1). Aging is considered as major risk factor in AD. However, the mechanisms that underlie aging process in AD progression are not fully understood.
Figure 1.
Factors that are responsible for causing Alzheimer's disease (AD). Genetic mutations in amyloid precursor protein, presenilin 1 and presenilin 2 cause early-onset familial AD. Genetic variants in sortilin related receptor 1, clusterin, complement component receptor 1, CD2AP, CD33, EPHA1, &MS4A4/MS4A6E genes contribute to late-onset AD, in addition to ApoE 4/4 genotype. Aging, lifestyle activities and oxidative DNA damage are major contributing factors for the development of AD.
Several hypotheses have been proposed to explain the causes of aging. Mechanisms that govern genome integrity and stability are major guarantors of viability and longevity. 1) Telomere-shortening and its relationship with aging is extensively investigated and reported that changes in telomere structure and function are linked to aging [8-10]. 2) Activation of p53 is emerged as prime factor of a functional decline of a tissue in aging [11]. 3) Signaling that control energy production and metabolism, including insulin/IGF pathway is another important mechanism that contributes to aging [12,13]. 4) The mitochondrial oxidative damage is the strong cellular event involved in aging process [6,14-16]. 5) The latest evidence suggests a unifying mechanism that involves a combination of factors in ageing, including telomere shortening, and mitochondrial oxidative damage, p53 activation, and reduced peroxisome proliferator-activated receptor gamma, coactivator 1 alpha and beta (PGC-1α and PGC-1β) [17,18].
Among these, mitochondrial oxidative damage has been found excessively in the brains of aged people, especially AD patients and also AD-like transgenic animal models. The purpose of this article is to summarize the recent progress of aging and Aβ-induced oxidative DNA damage and related pathways in the pathogenesis of AD. Further, this article also discusses therapeutic approaches of oxidative DNA damage in treating patients with AD.
2. Cellular changes of Aging and oxidative DNA damage
Aging is a complex process that has been linked to accumulation of DNA damage. In mammals, including humans, an accumulation of oxidative DNA damage in different tissues including brain is observed during aging [19-21]. Recent observation from young, middle and old age mice groups further indicate that oxidative DNA damage is increased with ageing (Mao and Reddy unpublished observations). Telomeres, the ends of linear chromosomes, repetitive DNA regions of hexanucleotide repeats, protect chromosomal ends from deterioration during DNA replication. Telomerase (TERT), a reverse transcriptase, forms a complex with an RNA template and cofactors to extend telomeres. The shelterin protein complex then binds the 3' single-stranded end of telomeric DNA, protecting it from DNA damage responses [22,23]. Interestingly, TERT is also localized in mitochondria and regulates/modulates oxidative damage on the mtDNA [24].
Inhibition of TERT function and related process leads to short telomeres and premature age-related diseases. In fact, telomere shortening represents a cell-intrinsic mechanism leading to DNA damage accumulation and activation of DNA damage at checkpoints in aged cells. Activation of DNA damage checkpoints in response to telomere malfunction results in induction of cellular senescence -- a permanent cell cycle arrest. Senescence represents a tumor suppressor mechanism protecting cells from evolution of genomic instability and transformation [25]. As a drawback, telomere shortening may also limit tissue renewal and regenerative capacity of tissues in response to aging and chronic disease.
Short telomeres are a hallmark of dyskeratosis congenita, which is caused by mutations in genes (including TERT) controlling telomere homeostasis. Induced pluripotent stem cells from dyskeratosis congenita patients demonstrate that telomere shortening, induced by mutations of TERT and other related genes, eventually results in loss of self-renewal [26]. In aged organs, telomere shortening may also increase the risk of age-related diseases, including neurodegenerative diseases and cancer, by initiation of chromosomal instability, loss of proliferative competition of aging stem cells, and selection of aberrant growing clones. Consequently, aged individuals are more susceptible and vulnerable to various diseases. Recently, proteins were found in human serum, which are induced by telomere dysfunction and DNA damage. It was shown that these proteins (CRAMP, EF-1alpha, stathmin, n-acetyl-glucosaminidase and chitinase) increase in aged people and are further elevated in geriatric patients, representing new biomarkers of human aging and disease [21,27].
Interestingly, lifestyle factors (such as exercise, smoking, body mass) also affect on the expression of these serum markers of DNA damage. The expression of biomarkers of DNA damage correlated positively with other cellular aging marker p16 (INK4a) expression and negatively with telomere length in peripheral blood T-lymphocytes. Together, these data provide experimental evidence that both aging and lifestyle impact on the accumulation of DNA damage during human's aging [28].
Emerging evidence suggests that telomere shortening can limit the function and maintenance of adult stem cells, which are of utmost importance for organ maintenance and regeneration. In mouse models, telomere dysfunction leads to a depletion of adult stem cell compartments suggesting that stem cells are very sensitive to DNA damage [29].
Recent work reveals that additional effects of telomere shortening on neuronal differentiation, as adult multipotent progenitors with critically short telomeres yield reduced numbers of neurons that, furthermore, exhibit underdeveloped neuritic arbors. Genetic data indicate that the tumor suppressor protein p53 not only mediates the adverse effects of telomere attrition on proliferation and self-renewal but it is also involved in preventing normal neuronal differentiation of adult progenitors with dysfunctional telomeres. The effect of p53 on neuritogenesis is mechanistically linked to its cooperation with the Notch pathway in the upregulation of small GTPase RhoA kinases, Rock1 and Rock2, suggesting a potential link between DNA damage and the Notch signaling pathway in the control of neuritogenesis [30]. It has also been shown that telomerase expression is downregulated in the subependymal zone of aging mice leading to telomere length reductions in neurosphere-forming cells and deficient neurogenesis and neuritogenesis, it suggests that age-related deficits could be caused partly by dysfunctional telomeres and demonstrate that p53 is a central modulator of adult neurogenesis, regulating both the production and differentiation of postnatally generated olfactory neurons [30].
In addition, the transcription factor p53 protects neurons from transformation and DNA damage through the induction of cell-cycle arrest, DNA repair and apoptosis in a range of in vitro and in vivo conditions. Indeed, p53 has a crucial role in eliciting neuronal cell death during development and in adult organisms after exposure to a range of stressors and/or DNA damage [31].
p53 takes a critical part in a number of positive and negative feedback loops to regulate aging, carcinogenesis and other biological processes. Uncapped or dysfunctional telomeres are an endogenous DNA damage that activates ATM kinase and then p53 to induce cellular senescence or apoptosis. Recent studies have shown that p53, a downstream effector of the telomere damage signaling, also functions upstream of the telomere-capping protein complex by inhibiting one of its components, TRF2 (telomeric repeat binding factor 2). Since TRF2 inhibition leads to ATM activation, a novel positive feedback loop exists to amplify uncapped telomere-induced, p53-mediated cellular responses. Siah1 (seven in absentia homolog 1), a p53-inducible E3 ubiquitin ligase, plays a key role in this feedback regulation by targeting TRF2 for ubiquitination and proteasomal degradation [32].
In addition to telomere shortening mechanism, ROS and mitochondrial dysfunction as further described below, and environmental factors (e.g. toxic chemicals and irradiation), as well as DNA repair defects also contribute to accumulating DNA damage during aging [21,33,34] (Figure 1). Interestingly, recent findings suggest environmental genotoxins, specifically methylazoxymethanol (MAM), the genotoxic metabolite of the cycad azoxyglucoside cycasin, target common pathways involved in neurodegeneration and cancer, the outcome depending on whether the cell can divide (cancer) or not (neurodegeneration). Exposure to MAM-related environmental genotoxins may have relevance to the etiology of related tauopathies, notably, AD [35]. It also has been indicated that DNA damage is an initial and critical contributor for aging, and various factors that affect aging may all function ultimately through the accumulation of persistent DNA lesions containing unrepairable DNA double-strand breaks [20,36].
Mice deficient in the Polycomb transcriptional repressor Bmi1 develop numerous abnormalities including a severe defect in stem cell self-renewal, severe neurological abnormalities, alterations in thymocyte maturation and a shortened lifespan [37,38]. Cells derived from Bmi1-/- mice have impaired mitochondrial function, a marked increase in the intracellular levels of ROS and DNA damage, as well as subsequent engagement of the DNA damage response pathway. More interestingly, the antioxidant N-acetylcysteine or genetic disruption of the DNA damage response pathway by Checkpoint kinase (Chk) 2 deletion can rescue many deficits, and eventually elongates significantly lifespan [38]. These observations further indicate the important role of mitochondria, ROS and DNA damage in aging and neurodegenerative diseases.
Therefore oxidative DNA damage may be the center of aging and age-related diseases such as AD. Hence, protection from DNA damage presents a basic approach for elongation of healthy age and treatment of such age-related diseases.
3. Amyloid beta, particularly Aβ42 in Alzheimer's disease
Amyloid-beta peptide (Aβ) appears to play a pivotal role in the development of AD and amyloid plaques as the most important hallmark are routinely used for diagnosing AD in brain tissue [5,39,40]. Increasing studies demonstrated the toxicity of diverse Aβ species in vitro and in vivo, confirming the importance of age-dependent Aβ accumulation in AD pathogenesis [41-44]. A recent study revealed that area-specific vulnerability to Aβ deposition is associated with local levels of brain metabolism and neuronal activity, indicating that Aβ and glucose metabolism are linked to AD [45]. Transgenic mice (at least APP23 line), overexpressing mutant human amyloid precursor protein (APP), exhibit selective neuronal death in the brain regions such as hippocampus and cortex that are most affected in AD, suggesting that amyloid plaque formation is directly involved in AD neuron loss [46]. In APP/PS1 transgenic mice, global neocortical neuron loss is not apparent up to 8 months of age, but local neuron loss in the dentate gyrus is observed [47]. Recent stereological analysis of neocortical neuron number in subregions with high neuron density such as the granule cell layer of the dentate gyrus, modest but significant neuron loss is also found [48]. In addition, parallel studies using multiphoton microscopy and in vivo microdialysis revealed that pharmacological reduction of soluble extracellular Aβ by as little as 20-25% was associated with a dramatic decrease in plaque formation and growth. Interestingly this small reduction in Aβ synthesis was sufficient to reduce amyloid plaque load in 6-month-old but not 10-month-old mice [49]. These observations demonstrate that amyloid plaque effects first on some critical regions and vulnerable neurons in brain, and early interference that reduces the Aβ level and the plaques prior to neurons die is the key for treatment of this disease.
Furthermore, microinjection of plaque-equivalent concentrations of fibrillar Aβ in the aged rhesus monkey cerebral cortex results in profound neuronal loss, tau phosphorylation and microglial proliferation [50], indicating the both direct and indirect toxic roles to neuron of Aβ plaques. Recent evidence further indicates that the fibrillar conformation of Aβ deposited in compact plaques is associated with the pathologies observed in AD. Quantitative analysis revealed that the area adjacent to fibrillar Aβ, containing compact but not diffuse plaques in aged rhesus, aged human, and AD cortex, displays significant loss of neurons and small but statistically significant reduction in the density of cholinergic axons [51].
The Aβ peptide is a 38-43 amino acid in length and is generated by proteolytic processing of the APP, a cell-surface receptor via β-secretase (β-Amyloid cleavage enzyme, BACE1) and γ- secretase complex containing presenilins consequently [1,40,52-54]. Although several small Aβ molecules exist in the AD brain, the most common species are Aβ40 and Aβ42. Aβ40 is the major species in soluble Aβ, and is constitutively secreted into culture medium and cerebrospinal fluids [55,56]. However a number of studies have shown that Aβ42 is in fact the predominant species in the parenchymal amyloid deposits in AD brain, and it is an initially deposited species; furthermore, elevated Aβ42 levels, as well as particularly the elevation of the ratio of Aβ42 to the shorter major form Aβ40, has been identified as a key feature in early events in the pathogenesis of AD [57]. The specific pathological importance of Aβ42 has drawn attention to seeking drugs that will selectively lower the levels of this peptide through reduced production or increased clearance while allowing normal protein processing to remain substantially intact [52,57-60]. Furthermore mutations in APP and presenilin (PS1 and PS2) genes have been shown to increase the production of the longer Aβ form Aβ42 relative to Aβ40, and to cause the familial autosomal dominant form of AD [56, 61-63]. Notably it has been shown that expression levels of BACE1, the limiting enzyme of Aβ production, are increased by oxidative stress, ischemia and hypoxia [64,65].
Interestingly transgenic mice expressing high levels of Aβ40 do not develop overt amyloid pathology. However, mice expressing lower levels of Aβ42 accumulate insoluble Aβ1-42 and develop compact amyloid plaques, congophilic amyloid angiopathy, and diffuse Aβ deposits. Further, when mice expressing Aβ42 are crossed with mutant APP (Tg2576) mice, there is also a massive increase in amyloid deposition. These data further establish that Aβ42 is essential for amyloid deposition in the parenchyma and also in vessels [66].
Plasma Aβ levels are elevated in early-onset AD caused by autosomal dominant mutations. Recent findings showed that similar genetic elevations exist in late-onset AD (LOAD), providing strong evidence for the existence of novel, as yet unknown genetic factors that affect LOAD by increasing Aβ [67]. Further, higher plasma Aβ42 at baseline was a significant predictor for the development of probable or possible AD at 5 years [68]. Notably, in recent report of early AD (predementia) trial design, it has been proposed that CSF Aβ42 or amyloid PET imaging may be optimal biomarkers for selecting subjects for anti-amyloid interventions [69].
Based on these fundamental observations, immunization of AD patients with synthetic full length Aβ42 (AN1792, Elan Pharmaceuticals) has been studied in a randomized, double-blind, placebo-controlled phase 2a clinical trial [70,71]. Treatment was discontinued following reports of encephalitis, and in the 1 year analysis of the AN1792 data, vaccinated subjects did not show improvement and no single test revealed benefits of vaccination, although the Z-test was consistent with a potential slowing of decline. However, approximately 4.6 years after immunization with AN1792, patients defined as responders in the phase 2a study maintained low but detectable, sustained anti-AN1792 antibody titers and demonstrated significantly reduced functional decline compared with placebo-treated patients [70]. These data support that Aβ immunotherapy, even though some patients suffered side effects, may have long-term functional benefits, and further support the Aβ cascade hypothesis of AD. In addition, another anti-amyloid therapy that reduces amyloid accumulation as measured by PIB-PET in a recent clinical trial also supports the Aβ hypothesis as well as the rationale for treatment with an anti-amyloid intervention [72].
Overall, increasing evidence from human and animal models demonstrates amyloid plaque, especially derived from toxic Aβ42, is the main hallmark of the AD, it is also a predictive marker for the progression of preclinical to symptomatic AD, therefore treatments especially early treatments that reduce Aβ production or increase its clearance will have great promise as a potential therapeutic agent.
4. Hyperphosphorylated Tau in Alzheimer's Disease
The other hallmark of AD is intracellular neurofibrillary tangles, aggregated from hyper-phosphorylation of tau. Tau is a neuron-specific microtubule-associated protein and a critical component of the neuronal cytoskeleton. As one of the main microtubule-associated proteins, tau will lose the ability to bind microtubules when the homeostasis of phosphorylation and dephosphorylation is disturbed in neurons. Then hyperphospharylated tau eventually induces neurodegeneration and neuron loss by disrupting neuronal cytoskeleton. It has been shown that DNA damage-activated kinases such as Chk1 and Chk2 may be involved in tau phosphorylation and toxicity in the pathogenesis of AD [73]. Tau may also be a crucial partner of Aβ, enhance the pathology of Aβ, as well as Tau-Aβ interplay a role in AD. Research on the pathological changes in AD indicates that accumulated Aβ in vivo may initiate the hyperphosphorylation of tau. Also, the signal transduction pathways of tau hyperphosphorylation may be related to accumulated Aβ [74-77]. Fibrillar Aβ containing compact but not diffuse plaques in the aged rhesus cortex also contained activated microglia and clusters of phosphorylated tau-positive swollen neuritis [51]. On the other hand, recent study showed that peptides from structure-based designs can disrupt the Aβ fibril formation of original proteins, including those, such as tau protein, that lack fully ordered native structures. The specifically inhibiting peptides have been designed on structures of dual-β-sheet ‘steric zippers’, the successful inhibition of amyloid fibril formation not only shed light on new strategy of the development of therapeutics but also strengthens the hypothesis that amyloid spines contain steric zippers [78].
Tau, also known as axonal protein, has a dendritic function in postsynaptic targeting of the Src kinase Fyn, a substrate of which is the NMDA receptor (NR). Missorting of tau in transgenic mice expressing truncated tau (Delta-tau) and absence of tau in tau (-/-) mice both disrupt postsynaptic targeting of Fyn. This uncouples NR-mediated excitotoxicity and hence mitigates Aβ toxicity. Delta-tau expression and tau deficiency prevent memory deficits and improve survival in Aβ-forming APP23 mice, a model of AD. These deficits are also fully rescued with a peptide that uncouples the Fyn-mediated interaction of NR and PSD-95 in vivo. These findings suggest that this dendritic role of tau confers Aβ toxicity at the postsynapse with direct implications for pathogenesis and treatment of AD [77].
Furthermore, recent studies in several APP and tau transgenic mouse models revealed that mitochondrial dysfunction might be a possible link between Aβ and tau in AD pathology in vivo [79]. In a triple AD mouse model, a massive deregulation of 24 proteins, of which one-third were mitochondrial proteins mainly related to complexes I and IV of the oxidative phosphorylation (OXPHOS). Notably, deregulation of complex I was tau dependent, whereas deregulation of complex IV was Aβ dependent. Synergistic effects of Aβ and tau were evident in 8-month-old AD mice as only they showed a reduction of the mitochondrial membrane potential at this early age. At the age of 12 months, the strongest defects on OXPHOS, synthesis of ATP, and reactive oxygen species (ROS) were exhibited in the AD mice, again emphasizing synergistic, age-associated effects of Aβ and tau in perishing mitochondria [79].
Overall, these observations indicate the involvement of tau and Aβ synergistically, as well as the fundamental role of mitochondria in the AD pathology.
5. Mitochondria in Alzheimer's disease
5.1. Mitochondria are the main source of ROS
Mitochondria are the cytopasmic organelles essential for life and death. Mitochondria perform several cellular functions, including production of major part of cellular ATP, regulation of intracellular calcium, the release of proteins that activate the caspase family of proteases and free radical production and scavenging. The mitochondria contain the respiratory chain or electron transport chain (ETC) that is located in the inner mitochondrial membrane and consists of five complexes (complexes I–V); the fifth complex is directly involved in ATP synthesis. The complexes of the mitochondrial respiratory chain are made up of multiple subunits, and all contain proteins encoded by nuclear DNA and mtDNA, except for complex II, which is entirely encoded by nuclear DNA [16,80-82]. The mitochondria, also called the powerhouses, are the chief energy-producing organelles in the most cells, which provide most energy for our normal life. Usually, energy in the form of ATP is efficiently produced via OXPHOS in the mitochondrial respiratory chain. Several decades of research have firmly established that ROS production is inherent to mitochondrial oxidative metabolism, and mitochondria are believed to be the major intracellular source of ROS. Several years of research revealed that free radicals are produced at multiple sites in the mitochondria: Complex of I and III produces superoxide radicals via electron leaks, these radicals are dismutated by manganese superoxide dismutase, generating H2O2 and oxygen. H2O2 is converted into H2O by either glutathione peroxidase or catalase [83]. Complex II also produces ROS conditionally [82]. Components of tricarboxylic acid, including α- ketodehydrogenase and pyruvate dehydrogenae generate superoxide radicals in the matrix. In addition, mitochondrial outermembrane also produce free radicals via monoamine oxidase (localized on the outer mitochondrial membrane), by catalyzing the oxidative deamination of primary aromatic amines. This deamination is a quantitatively large source of H2O2.
A little ROS may not be toxic to cells, and may have some benefit roles to cells and homoeostasis. Recent data strongly suggest that ROS, and specifically mitochondria generated ROS, are involved in physiological signaling cascades regulating various cellular and organ functions [84,85]. However, excessive and/or sustained increase in ROS, may lead to oxidative stress, as aggravating or primary factors in numerous pathologies, including aging and neurodegenerative diseases, is widely recognized [6,82,85-87].
Mounting evidence suggests that Aβ cascade hypothesis remains the major cellular event in AD; increasing evidence also indicates a mitochondrial cascade hypothesis, which is for many of the biochemical, genetic, pathological as well as clinic features of AD, and mitochondrial dysfunction may initiate the disease, particularly sporadic AD [88]. This hypothesis, importantly, is supported by recent observations showing that early impairments of mitochondrial dysfunction and oxidative stress may precede Aβ overproduction and deposition [88-92].
5.2. Mitochondrial localization of Aβ
As described above Aβ deposit is the main hallmark of AD, and Aβ42 is the most toxic peptide and the predominant species in the parenchymal amyloid deposits in AD brain, and it is an initially deposited species [57-60]. Although the classical view is that Aβ is deposited extracellularly, both cellular and biochemical studies carried out in different models of AD and aging have provided evidence that this peptide can also accumulate inside neurons, target mitochondria, and contribute to disease progression [93-99]. By using in vivo and in vitro approaches, Hansson Peterson et al. demonstrated that Aβ is transported into rat mitochondria via the translocase of the outer membrane (TOM) and localizes within the mitochondrial cristae. A similar distribution pattern of Aβ in mitochondria has been shown by immunoelectron microscopy in human cortical brain biopsies [100].
It has been shown that Aβ42 can induce mitochondrial mislocalization, which contributes to Aβ42-induced neuronal dysfunction in a transgenic Drosophila model. In the Aβ42 fly brain, mitochondria were reduced in axons and dendrites, and accumulated in the somata without severe mitochondrial damage or neurodegeneration. In contrast, organization of microtubule or global axonal transport was not significantly altered at this stage. Aβ42-induced behavioral defects were exacerbated by genetic reductions in mitochondrial transport, and were modulated by cAMP levels and PKA activity. Levels of putative PKA substrate phosphoproteins were reduced in the Aβ42 fly brains. Importantly, perturbations in mitochondrial transport in neurons were sufficient to disrupt PKA signaling and induce late-onset behavioral deficits, suggesting a mechanism whereby mitochondrial mislocalization contributes to Aβ42-induced neuronal dysfunction. These results demonstrate that mislocalization of mitochondria underlies the pathogenic effects of Aβ42 in vivo [101].
5.3. Aβ induces ROS production
Oxidative stress and its sequelae are likely related to both apoptotic and necrotic mechanisms of neurotoxicity. There is evidence suggesting that tissues from both AD patients and individuals with mild cognitive impairment have elevated levels of oxidative DNA damage [33]. And post-mortem tissue provides strong evidence for significant increased levels of cellular oxidative stress in vulnerable regions (cortex and hippocampus) of AD brains compared to aged controls ([3,33,102,103]. Importantly, Aβ peptides directly initiate free radical/ROS formation, cellular dysfunction, and subsequent neuronal death [104-107]. Further mitochondria are thought to be the central target for oxidative stress induced damage [93,108]. Recent primary culture study has shown that oligomeric Aβ42 could induce reactive ROS production from cortical neurons through activation of NADPH oxidase [109]. Notably there is a defect in the antioxidant defense system, which may lead to oxidative damage in patients with AD. It has been found that erythrocyte antioxidant enzyme activities (catalase, glutathione peroxidase GPX and superoxide dismutase SOD) were significantly lower in patients with AD compared with controls. These results suggest that alterations in these enzymes may play a role in the etiopathogenesis of AD [110]. Therefore Aβ-associated oxidative stress and related antioxidant defense system may be of fundamental importance in AD etiology and pathogenesis.
5.4. Mitochondrial DNA changes in AD
The Aβ cascade hypothesis remains the main pathogenic model, as suggested by familiar AD, mainly associated to mutation in APP and presenilin genes. The remaining more than 98% of AD patients are mostly sporadic late-onset cases, with a complex etiology due to interactions between environmental conditions and genetic features of the individuals. Somatic mutations in mitochondrial DNA (mtDNA) could cause energy failure, increased oxidative stress and accumulation of Aβ, which in a vicious cycle reinforces mtDNA damage and oxidative stress. However, no clear causative mutations in the mtDNA have been linked to AD, even some variations have functional consequences, including changes in enzymatic activity [111]. Indeed, results of studies on the role of mtDNA polymorphisms or haplogroups in AD are controversial [91,112].
Recently, to investigate the possible association between mtDNA-inherited sequence variation, a high resolution analysis (sequencing of displacement loop and restriction analysis of specific markers in the coding region of mtDNA) in 936 AD patients and 776 cognitively assessed normal controls from central and northern Italy was performed. Among over 40 mtDNA sub-haplogroups analysed, subhaplogroup H5 is a risk factor for AD in particular for females and independently from the APOE genotype. Multivariate logistic regression revealed an interaction between H5 and age. When the whole sample is considered, the H5a subgroup of molecules, harboring the 4336 transition in the tRNAGln gene, already associated to AD in early studies, was about threefold more represented in AD patients than in controls (2.0% vs 0.8%; p=0.031), and it might account for the increased frequency of H5 in AD patients (4.2% vs 2.3%). The complete re-sequencing of the 56 mtDNAs belonging to H5 revealed that AD patients showed a trend towards a higher number of somatic mutations in tRNA and rRNA genes when compared with controls [113].
The ultrastructural features of vascular lesions and mitochondria in brain vascular wall cells from human AD, and APP transgenic positive (Tg+) mice have been studied. In situ hybridization using mtDNA probes for human wild and 5 kb deleted types and mouse types was performed along with immunocytochemistry have shown that there was a higher degree of mitochondria DNA deletion along with amyloid deposition, overexpression of oxidative stress markers, and mitochondrial structural abnormality in the vascular walls of the human AD, Tg (+) mice compared to age-matched controls. Therefore, selective pharmacological intervention, directed for abolishing the chronic hypoperfusion state, would possibly change the natural course of development of dementia [114].
5.5. Mitochondrial dysfunction in AD
It has been shown that impairment occurs to all five of the mitochondrial OXPHOS complexes in the AD brain. Mitochondrial dysfunction, which is associated with metabolic dyshomeostasis and reduced ATP synthesis, occurs early in AD. Additionally, mitochondrial dysfunction is proposed to link amyloid deposition and neuronal synaptic loss. Thus, the existence of mitochondrial dysfunction is important in AD [96, 115].
Age-dependent accumulation of mutations in mtDNA and resulting increase in oxidative stress and impairment in mitochondrial respiratory chain, especially complex IV, gained attention as potential factors that could participate in the onset of sporadic AD [16, 88]. In fact, a reduced activity of the cytochrome c oxidase (COX, Complex IV of respiratory chain) has been reported in different brain regions of AD patients [116].
COX activity was also decreased in AD transgenic (Tg2576) mice, suggesting that mutant APP/Aβ impair mitochondrial metabolism in AD. Furthermore, an increase in hydrogen peroxide and a decrease in COX activity were found in young Tg2576 mice, prior to the appearance of Aβ plaques [93]. Also, in vitro study using primary neuron culture and confocal microscopy demonstrates that Aβ impairs the mitochondrial movement [117]. These findings indicate that mitochondria are the targets of Aβ, and mitochondrial dysfunction happens at early stage of the disease, suggesting that early mitochondria-targeted therapeutic interventions may be effective in delaying AD progression in treating AD patients.
The mechanism of the inhibitory potential of the Aβ42 on activity of electron transport chain enzyme complexes was investigated in human mitochondria. Synthetic Aβ1-42 specifically inhibited the terminal complex COX in a dose-dependent manner that was dependent on the presence of Cu2+ and specific “aging” of the Aβ42 solution. Maximal COX inhibition occurred when using Aβ42 solutions aged for 3-6 h at 30°C. The level of Aβ42-mediated COX inhibition increased with aging time up to approximately 6 h and then declined progressively with continued aging to 48 h [96]. Photo-induced cross-linking of unmodified proteins followed by SDS-PAGE analysis revealed dimeric Aβ as the only Aβ species to provide significant temporal correlation with the observed COX inhibition. Analysis of brain and liver from an AD model mouse (Tg2576) revealed abundant Aβ immunoreactivity within the brain mitochondria fraction. These data indicate that endogenous Aβ is associated with brain mitochondria and that Aβ1-42, possibly in its dimeric conformation, is a potent inhibitor of COX, but only when in the presence of Cu2+. Therefore, Cu2+-dependent Aβ-mediated inhibition of COX may be an important contributor to the neurodegeneration process in AD [96]. Increased copper concentration has been found in the AD brain that implies that copper may participate in the pathophysiology of AD [118]. In fact, copper can bind to APP and Aβ, then affects the structure and toxic of APP and Aβ, and then affect the formation of beta-sheet structure that is widely accepted as toxic secondary structure of Aβ [118].
To test the possibility that age-dependent decline in the mitochondrial respiratory function, especially COX activity, may participate in the formation and accumulation of Aβ, a neuron-specific COX-deficient mouse was first generated by the Cre-loxP system, in which the COX10 gene, which encodes the farnesyltransferase required for COX assembly and function, was deleted by a CamKIIα promoter-driven Cre-recombinase. These knockout (KO) mice showed an age-dependent COX deficiency in the cerebral cortex and hippocampus. Then AD-like double transgenic mice expressing mutants of APP and PS1 in a neuron-specific COX-deficient background were generated. Surprisingly, COX10 KO mice exhibited significantly fewer amyloid plaques in their brains compared with the COX-competent transgenic mice. This reduction in amyloid plaques in the KO mouse was accompanied by a reduction in Aβ42 level, BACE1 activity, and oxidative damage. Likewise, production of ROS from cells with partial COX activity was not elevated. Collectively, these results suggest that, contrary to previous models, a defect in neuronal COX does not increase oxidative damage nor predispose for the formation of amyloidogenic APP fragments [119,120]. On the other hand, this study also indicates that genetic modification of mitochondria can inhibit ROS overproduction, eventually reduce Aβ level, prevents the development and progress of AD, suggesting a useful approach for treatment of the disease.
6. DNA damage in Alzheimer's disease
6.1. Definition of oxidative DNA damage
Increased ROS/free radical production/NO, either produced from mitochondria or from inflammation process (macrophage/microglia in brain) can cause oxidation of nucleotide acids, especially DNA. The resulting DNA damages are present in most, if not all, human diseases, including AD, and aging [15,121]. The toxic hydroxyl radical is known to react with all components of the DNA molecule, damaging both the purine and pyrimidine bases and also the deoxyribose backbone [15,85]. Therefore DNA is a particularly important target for oxidation, as damage may lead to heritable alterations. Consequently, damage to DNA has been well studied, and several classes of product identified: base oxidation and fragmentation products (e.g. single- and double-strand breaks); inter/intra-strand cross-links; DNA–protein cross-links; and sugar fragmentation products [15,122]. The hydroxyl radical is perhaps the most frequently form of ROS, with over twenty different products formed from OH attack on the bases in DNA. The principal oxidative DNA damage products include 8-hydroxyadenine (8-OH-Ade), 8-hydroxyguanine (8-OH-Gua; and its deoxynucleoside equivalent, 8-OH-dG), 5,6-dihydroxy-5,6-dihydrothymine (thymine glycol, Tg) and ring-opened lesions: 4,6-diamino-5-formamidopyrimidine (FapyAde) and 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua). Overall, the most extensively studied DNA lesion is the formation of 8-OH-G and 8-OH-dG, which is widely accepted and used as an oxidative DNA biomarker. Permanent modification of genetic material resulting from these oxidative damage incidents represents the first step involved in mutagenesis, aging and age-related diseases, including cancer and neurodegenerative diseases including AD.
6.2. Methods for detection of oxidative DNA damage
Oxidized DNA can be detected by several ways: 1) high pressure liquid chromatography (HPLC) analysis for oxidized DNA from pure genomic DNA; 2) immunohistochemistry analysis using brain sections and antibody that is specific for 8-OHdG or 8-OHG; and 3) Comet assay that detects DNA damage in a single cell.
6.2.1HPLC analysis using genomic DNA
DNA can be isolated from tissues or blood either by traditional phenol extraction/ethanol precipitation (multiple) or a convenient kit, the DNA purity and concentration are determined spectrophotometrically. An OD of 1 at 260 nm corresponded to ~50 μg/ml DNA. Only samples with a high degree of DNA purity (such as OD 260/230 ratio above 2.0), could be used for HPLC analysis. To avoid any artificial DNA damage from phenol extraction, the salt methods have been developed [123-125]. It has been shown that the sodium iodide (NaI) isolation method is more sensitive and reliable assessment of 8-oxo-2-deoxyguanosine levels than the classical phenol method [126]. Relative commercial kits also developed by companies such as Qiagen, which may significantly save the timing course.
The measurement of 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxo-dG) (also called 8-hydroxy-2’-deoxyguanosine, 8-OHdG) is widely used method for determining oxidative DNA in DNA sample [127;128]. In brief, DNA will be digested and dephosphorylated by nuclease P1 followed by alkaline phosphatase. Digested DNA is analyzed by HPLC equipped with a reverse-phase analytical column and coupled with a photodiode array detector followed by an electrochemical (EC) detector. The quantification of nucleosides will be monitored by UV (at 254 nm) while the measurement of 8-oxo-dG was by EC detector (at 290mV). On the other hand, 8OHdG can also be determined in situ (brain or other organs, different region and sub-region) locally using immunohistochemistry with a specific 8OHdG antibody, following quantification with Stereologer software, Image J or other software analysis.
6.2.2. Immunostaining analysis is a qualitative method to measure 8-hydroxyguianosine (8-OHG)
a marker for oxidative DNA damage in brain sections of aging, AD mouse models and AD brains [93,129-131]. This method determines the accumulated oxidative DNA in cells. There are several good quality antibodies are available to detect DNA damage in the brain sections. Several studies quantified 8-OHG in the brains of neurodegenerative diseases ([132].
6.2.3. The Comet assay is another useful genotoxicity test for DNA damage [33,133,134]
In addition, purified DNA repair enzymes, applied to DNA during the course of the comet assay procedure, greatly increase the sensitivity and specificity of the assay. The kinetics of cellular repair after low doses of oxidative damage have been followed with this modified comet assay [135]. Therefore, the successful measurement of biomarkers of oxidative damage in human populations establishes the comet assay as a valuable tool in molecular epidemiology.
Overall these are useful methods to detect and quantify oxidized DNA in cells. However, HPLC based method yields more accurate oxidized DNA in cells.
6.3. Oxidative DNA damage in AD and therapeutic applications
In addition to Aβ stimulation of ROS production, which directly causes DNA damage, it has been shown that Aβ42 has DNA nicking activities similar to nucleases. Further, magnesium ion was shown to enhance the DNA nicking activity of Aβ, and Aβ oligomers showed more DNA nicking activity compared to monomers and fibrillar forms. These data support a role for Aβ in causing direct DNA damage [33,136].
In the Tg2576 mouse model of AD, at the age of three months the mice show no apparent signs of behavioral, neuroanatomical, cytological, or biochemical alterations. However, recent biochemical and morphological studies demonstrated that significant alterations in several antioxidant proteins are already detectable at this early stage of the disease and protein composition change concomitantly with early oxidative stress. Interestingly, the neocortex shows a compensatory response, consisting in an increase of ROS scavenging enzymes, while the hippocampus appears more prone to the oxidative insult [137].
The accumulation of DNA damage can be particularly deleterious in postmitotic cells such as neurons, which are not self-renewed through cell proliferation. Therefore, the accumulation of oxidative DNA damage in nuclear DNA (nDNA) is believed to result in a slow build-up of DNA adducts in the genome able to trigger neuronal death, whereas its accumulation in the mitochondrial DNA (mtDNA) can result in base substitutions and deletions leading to the erroneous transcription of genes encoding important subunits of the electron transport chain, with subsequent mitochondrial dysfunction, increased oxidative damage, and neuronal death [33]. It has been shown that mtDNA is particularly sensitive to oxidative damage. In postmortem brain tissue there was a significant threefold increase in the amount of 8OHdG in mtDNA in parietal cortex of AD patients compared with controls. In the entire group of samples there was a small but significant increase in oxidative damage to nDNA and a highly significant threefold increase in oxidative damage to mtDNA in AD compared with age-matched controls [138]. Comparing no differences in levels of lipid peroxidation were found in any of the brain regions, and a significant difference was found only in the parietal lobe, increased levels of 8-hydroxyadenine, 8-hydroxyguanine, thymine glycol, Fapy-guanine, 5-hydroxyuracil, and Fapy-adenine, oxidized DNA bases were observed in parietal, temporal, occipital, and frontal lobe, superior temporal gyrus, and hippocampus. The baseline level of oxidative DNA damage in the temporal lobe was higher than in other brain regions in both control and AD brain [139]. Similarly, levels of multiple oxidized bases in AD brain specimens were significantly higher in frontal, parietal, and temporal lobes compared to control subjects, whereas cerebellum was only slightly affected in AD brains; mtDNA had approximately 10-fold higher levels of oxidized bases than nDNA. Oxidative DNA damage was also found in mild cognitive impairment (MCI), the phase between normal aging and early dementia [140-142]. These data are consistent with higher levels of oxidative stress in mitochondria, suggesting that oxidative damage to mitochondrial DNA may contribute to the neurodegeneration of AD, including the earliest detectable phase of AD. Interestingly, the fact that mtDNA has more oxidative damage also confirmed by the reliable assessment using the NaI isolation method, which showed that the levels of 8oxodG in mtDNA isolated from mouse liver, heart and brain were 6-, 16- and 23-fold higher than nDNA from these tissues. The steady-state levels of oxo8dG in mouse tissues range from 180 to 360 lesions in the nuclear genome and from one to two lesions in 100 mitochondrial genomes [19].
Interestingly, point mutation frequencies in mtDNA were 2 to 3-fold higher in the parietal gyrus, hippocampus, and cerebellum from AD subjects compared to normal controls. In contrast, levels of a commonly studied deletion mutation, mtDNA (4977), were not elevated in AD. The frequency of point mutations did not vary significantly among the three brain areas, whereas the frequency of mtDNA (4977) was 15- to 25-fold lower in the cerebellum in comparison to the cortex; this regional variation was seen in both the normal and AD brain [143]. It has also been shown that somatic mtDNA control region (CR) mutations accumulate with age in post-mitotic tissues including the brain and that the level of mtDNA mutations is markedly elevated in the brains of AD patients [144]. The elevated mtDNA CR mutations in AD brains are associated with a reduction in the mtDNA copy number and in the mtDNA L-strand transcript levels. Further, mtDNA CR mutations increase with age in control brains; that they are markedly elevated in the brains of AD, Down syndrome (DS) and dementia (DSAD) patients. The increased mtDNA CR mutation rate is also seen in peripheral blood DNA and in lymphoblastoid cell DNAs of AD and DSAD patients, and distinctive somatic mtDNA mutations, often at high heteroplasmy levels, are seen in AD and DSAD brain and blood cells DNA. In aging, DS, and DSAD, the mtDNA mutation level is positively correlated with BACE1 activity and mtDNA copy number is inversely correlated with insoluble Aβ40 and Aβ42 levels [145]. These data further indicate that increased Aβ, which produced by BACE1, causes the significant elevation of mtDNA mutation, and this mtDNA mutation may be responsible for both age-related dementia and the associated neuropathological changes observed in AD and DSAD.
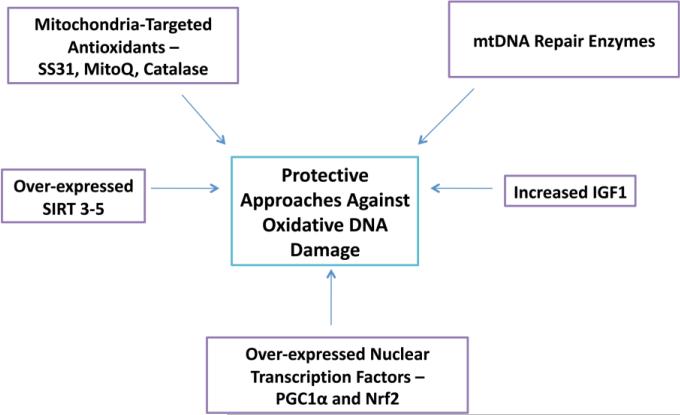
Overall oxidative DNA damage, especially mitochondria DNA damage in AD brain is a basic event during the initiation and development of the disease. Interestingly, increasing data including ours have shown that antioxidants such as CoQ and its variants, and that antioxidant enzymes and transcription factors such as NrF2, can significantly reduce ROS production and tissue damage, eventually improve cell survival and cellular function in vitro and in vivo [146-149] (Figure 2). Some of traditional Chinese medicines/herbal medicines may also works as antioxidants, neuroprotectants and/or mitochondrial boosters, such as Ginkgo biloba and Ginseng, therefore more studies in humans with strong methodology may provide useful information or effective medicine in the field [148,150].
Figure 2.
Protective therapeutic approaches that decrease oxidative DNA damage in aging and AD. 1. Mitochondria-targeted antioxidants, 2. Overexpression of mitochondria-localized sirtuins 3-5, 3. Mitochondria DNA repair enzymes, 4. Increased levels of insulin-like growth factor 1 and 5. Overexpression of nuclear transcription factors.
Recently we crossed APP transgenic mouse with mitochondria-targeted catalase mouse (MCAT) and produced MCAT/APP double transgenic mice. We found that MCAT not only significantly reduces DNA oxidative damage, but also dramatically inhibits Aβ deposits, eventually elongates the AD mouse lifespan (Mao and Reddy unpublished results). These observations suggest that mitochondria-targeted antioxidants may be a potential therapeutic approach for AD.
Notably, a placebo-controlled, double-blind, randomized trial showed that a safe and effective medicine, huperzine A (400 micro g/day for 12 weeks), which was originally isolated from the Chinese herb Lycopodium serratum, also known in China as Qian Ceng Ta or Jin Bu Huan, remarkably improves the cognition, behavior, activity of daily life, and mood of AD patients [151], confirmed early report, which concluded that Hup is a promising drug for symptomatic treatment of AD [152]. In a phase II trial of huperzine A in mild to moderate AD in the US, Huperzine A 200 μg BID did not influence change in ADAS-Cog at 16 weeks. In secondary analyses, huperzine A 400 μg BID showed a 2.27-point improvement in ADAS-Cog at 11 weeks vs 0.29-point decline in the placebo group (p = 0.001), and a 1.92-point improvement vs 0.34-point improvement in the placebo arm (p = 0.07) at week 16 [153]. Therefore, overall Huperzine A is a well-tolerated drug that could significantly improve cognitive performance in patients with AD, and it appears to be more effective than FDA approved acetylcholinesterase inhibitors, AChEIs [150, 154-156]. Considering the study design was not strong, sample sizes were still small, as well as individuals vary for an optimal effective dose, rigorous design, randomized, multi-centre, large-sample trials of Huperzine A and its derivatives for AD are needed to further assess the effects.
7. DNA repair as a therapeutic strategy in Alzheimer's disease
Despite oxidative DNA damage plays an important role in aging and diseases, including AD, as there are multiple pathways for its repair, clearly the cell does not want this damage to persist. Terminally differentiated neurons in the adult brain are able to re-enter the cell-division cycle under certain circumstances [157]. In humans, circumstantial evidence comes from diseases with DNA repair defects, such as Xeroderma Pigmentosum, which shows an accumulation of oxidative DNA damage and increased frequency of skin cancers and, in certain cases, characteristic neurological degeneration. There are several reviews summarized the recent progress on DNA repair, especially in neurodegenerative diseases, in both nDNA and mtDNA [33,158-161].
Among many repair pathways, the base excision repair (BER) pathway is the most important cellular protection mechanism responding to oxidative DNA damage. The BER pathway has been extensively studied in AD brains, and it is of particular importance in post mitotic tissues such as brain tissues, where simple base modifications are more likely to occur than is major DNA damage. The key enzymes in the BER process are DNA glycosylases, which remove different damaged bases by cleavage of the N-glycosylic bonds between the bases and the deoxyribose moieties of the nucleotide residues. To complete the repair after glycosylase action, the apurinic/apyrimidinic (AP) site is further processed by an incision step, DNA synthesis, an excision step, and DNA ligation through two alternative pathways. The short-patch BER (1-nucleotide patch size) and long-patch BER (2-6-nucleotide patch size) pathways need AP endonuclease to generate a 3' hydroxyl group but require different sets of enzymes for DNA synthesis and ligation. Protein-protein interactions have been reported among the enzymes involved in BER. It is proposed that the successive players in the repair pathway are assembled in a complex to perform concerted actions [33,158]. It has been shown that the activity of the most enzymes/proteins involved in DNA repair were down-regulated in AD brains [33,162], indicating a deficiency of the DNA repair system in such degenerative diseases.
For many years, the repair of most damage in mtDNA was thought limited to short-patch base excision repair (SP-BER), which replaces a single nucleotide by the sequential action of DNA glycosylases, an AP endonuclease, the mtDNA polymerase gamma, an abasic lyase activity, and mtDNA ligase. However, recent studies have considerably expanded our knowledge of mtDNA repair to include many mechanisms seen in nDNA repair, such as long-patch base excision repair (LP-BER), mismatch repair, and homologous recombination and nonhomologous end-joining [161,163]. In addition, elimination of mutagenic 8-oxodeoxyguanosine triphosphate helps prevent cell death due to the accumulation of this oxidation product in mtDNA. Also, recent evidence provided that irreparably damaged mtDNA might be targeted for degradation. Therefore, multiple DNA repair pathways and controlled degradation of mtDNA function together to maintain the integrity of mitochondrial genome [163].
The mammalian mitochondrial protein TFAM was originally identified as a transcription factor (A) but also appears to play a direct role in DNA repair. TFAM binds stronger to oxidatively damaged DNA than to intact DNA and shows higher affinity for 8-oxoG-containing base pairs than the relevant OGG1 and MYH DNA glycosylases [164,165]. Recent data suggest that TFAM indeed modulates the BER pathway in mitochondria by virtue of its DNA-binding activity and protein interactions [166]. TFAM also binds to p53, a tumor suppressor which localizes to mitochondria in response to death signals [167]. On the other hand, the 3′–5′ exonuclease activity of p53 can hydrolyze oxidized nucleotides like 8-oxodG at DNA 3′-ends, a reaction which is enhanced upon interaction with the mitochondrial single-stranded DNA-binding protein (mtSSB) [168].
In mammalian cells, oxidative damage to the mitochondrial DNA also correlates with the translocation of the tumor suppressor p53 to the organelles, which introduces a further regulation level, as p53 is thought to modulate DNA repair. Furthermore, p53 stimulates both removal of damaged bases and nucleotide re-insertion [169].
Although aging is clearly associated with modifications in mitochondrial DNA repair capacity, the situation is contrasted. This may also reflect a balance between DNA damage and repair, as mitochondrial DNA oxidative damage is inversely correlated with maximum life span in mammals [169] (Figure 3). On the other hand, changes in mitochondrial BER play a role in DNA damage accumulation and age-related functional decline [34]. In mammals, variations in organelle BER capacity with age appear to be organ and tissue-specific [170], for example, increased BER enzyme activities in mitochondria can be found in liver [158] or gradual decline in brain [171-173].
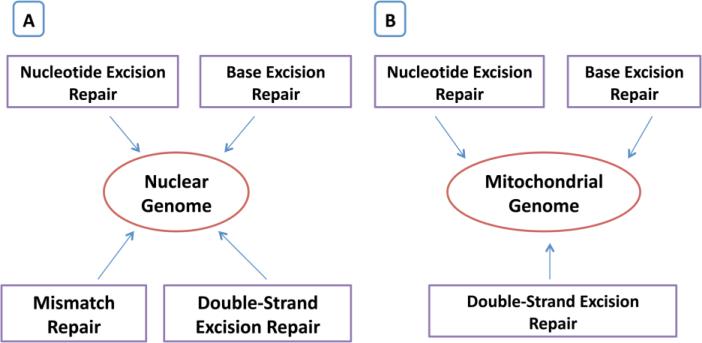
Figure 3.
DNA repair mechanisms of mitochondrial and nuclear genomes.
Base excision repair is thought to be the primary DNA repair pathway for small base modifications such as oxidation. Bohr's group employed a set of functional assays to measure BER activities in brain tissue from short post-mortem interval autopsies of sporadic AD patients and age-matched controls, as well as amnestic mild cognitive impairment (MCI) subjects. They found significant BER deficiencies in brains of AD patients due to limited DNA base damage processing by DNA glycosylases and reduced DNA synthesis capacity by DNA polymerase β. Interestingly the BER impairment was not restricted to damaged brain regions and was also detected in the brains of amnestic MCI patients, where it correlated with the abundance of neurofibrillary tangles. These findings suggest that BER dysfunction is a general feature of AD brains that could occur at the earliest stages of the disease. The results support that defective BER may play an important role in the progression of AD, and that mitochondrial BER might be critical in the development and maintenance of the central nervous system during aging [174].
As summarized in Figure 3, overall, these studies indicate that DNA repair in both mitochondrial and nuclear genomes are involved in AD, and recent research advancements may help develop strategies to repair DNA changes that are caused by aging, A , hyperphosphorylated and other oxidative insults.
8. Conclusions and future directions
Increasing evidence suggests that mitochondrial oxidative damage is early event and plays a key role in the progression and pathogenesis of AD. Recent AD postmortem brains, transgenic mouse models and cell models of AD revealed that Aβ significantly induces DNA damage in age-dependent manner in neurons from patients with AD or AD-like animal models. Aβ also induces oxidative mtDNA damage, in turn, generate excessive free radicals, cause more DNA damage, it likely forms a vicious cycle. On the other hand, antioxidant system, DNA repair and neurogenesis (including adult stem cells in brain) are functionally going down in aged humans and AD patients. All these factors and pathways likely work together induce synapse and neuron damage, eventually neuron loss in AD patients.
Given the complexities of AD, therapies that target the causes and different mechanisms simultaneously would be effective. Strategy using a multifaceted therapy that targets Aβ, hyperphosphorylated tau, and mitochondrial oxidative damage may be ideal approach. More importantly, evidence from clinical trials and studies of experimental therapeutics of AD mouse models indicate that early treatment is the key for cure or effective treatment of the disease. Similarly, prevention of the AD happening in our populations is another best way to fight this number one of the common progressive neurodegenerative diseases.
Highlights.
This article summarizes recent developments of aging and amyloid beta-induced oxidative DNA damage in Alzheimer's disease.
Discussed the factors that may be responsible for the development of Alzheimer's disease.
Methods of detection of oxidative DNA damage were discussed.
Discussed the therapeutic approaches of oxidative DNA damage.
Acknowledgements
This research was supported by NIH grants AG028072, RR00163, and Alzheimer Association grant IIRG-09-92429
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol.Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp.Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat.Rev.Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 6.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol.Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc.Natl.Acad.Sci.U.S.A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques FZ, Markus MA, Morris BJ. The molecular basis of longevity, and clinical implications. Maturitas. 2010;65:87–91. doi: 10.1016/j.maturitas.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr.Opin.Clin.Nutr.Metab.Care. 2011;14:28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg DT. An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am.J.Hum.Biol. 2011;23:149–167. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- 11.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ.Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 17.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J.Clin.Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton ML, Guo Z, Fuller CD, Van Remmen H, Ward WF, Austad SN, Troyer DA, Thompson I, Richardson A. A reliable assessment of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA. Nucleic Acids Res. 2001;29:2117–2126. doi: 10.1093/nar/29.10.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat.Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 21.von Figura G, Hartmann D, Song Z, Rudolph KL. Role of telomere dysfunction in aging and its detection by biomarkers. J.Mol.Med. 2009;87:1165–1171. doi: 10.1007/s00109-009-0509-5. [DOI] [PubMed] [Google Scholar]
- 22.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 23.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 24.Gordon DM, Santos JH. The emerging role of telomerase reverse transcriptase in mitochondrial DNA metabolism. J.Nucleic Acids. 2010;2010:390791. doi: 10.4061/2010/390791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 26.Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474:399–402. doi: 10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Schiffer E, Song Z, Wang J, Zurbig P, Thedieck K, Moes S, Bantel H, Saal N, Jantos J, Brecht M, Jeno P, Hall MN, Hager K, Manns MP, Hecker H, Ganser A, Dohner K, Bartke A, Meissner C, Mischak H, Ju Z, Rudolph KL. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc.Natl.Acad.Sci.U.S.A. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Z, von Figura G, Liu Y, Kraus JM, Torrice C, Dillon P, Rudolph-Watabe M, Ju Z, Kestler HA, Sanoff H, Lenhard Rudolph K. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9:607–615. doi: 10.1111/j.1474-9726.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalapareddy K, Jiang H, Guachalla Gutierrez LM, Rudolph KL. Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging: what markers can we use? Exp.Gerontol. 2008;43:998–1004. doi: 10.1016/j.exger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco MA, Farinas I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J.Neurosci. 2009;29:14394–14407. doi: 10.1523/JNEUROSCI.3836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tedeschi A, Di Giovanni S. The non-apoptotic role of p53 in neuronal biology: enlightening the dark side of the moon. EMBO Rep. 2009;10:576–583. doi: 10.1038/embor.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horikawa, Fujita K, Harris CC. p53 governs telomere regulation feedback too, via TRF2. Aging (Albany NY) 2011;3:26–32. doi: 10.18632/aging.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppede F, Migliore L. DNA damage and repair in Alzheimer's disease. Curr.Alzheimer Res. 2009;6:36–47. doi: 10.2174/156720509787313970. [DOI] [PubMed] [Google Scholar]
- 34.Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging--an update. Exp.Gerontol. 2010;45:478–488. doi: 10.1016/j.exger.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kisby GE, Fry RC, Lasarev MR, Bammler TK, Beyer RP, Churchwell M, Doerge DR, Meira LB, Palmer VS, Ramos-Crawford AL, Ren X, Sullivan RC, Kavanagh TJ, Samson LD, Zarbl H, Spencer PS. The Cycad Genotoxin MAM Modulates Brain Cellular Pathways Involved in Neurodegenerative Disease and Cancer in a DNA Damage-Linked Manner. PLoS One. 2011;6:e20911. doi: 10.1371/journal.pone.0020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat.Rev.Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 37.van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira II, Xu XL, van Lohuizen M, Motoyama N, Deng CX, Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 40.Selkoe DJ. Normal and abnormal biology of the beta-amyloid precursor protein. Annu.Rev.Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 41.Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J.Neurosci. 2000;20:1386–1392. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat.Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 43.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol.Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J.Biol.Chem. 2006;281:20315–20325. doi: 10.1074/jbc.M601016200. [DOI] [PubMed] [Google Scholar]
- 45.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat.Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 47.Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Holscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupp NJ, Wegenast-Braun BM, Radde R, Calhoun ME, Jucker M. Early onset amyloid lesions lead to severe neuritic abnormalities and local, but not global neuron loss in APPPS1 transgenic mice. Neurobiol.Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J.Neurosci. 2009;29:10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat.Med. 1998;4:827–831. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 51.Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, Geula C. Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex. Am.J.Pathol. 2010;177:325–333. doi: 10.2353/ajpath.2010.090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 53.Hardy J. Has the amyloid cascade hypothesis for Alzheimer's disease been proved? Curr.Alzheimer Res. 2006;3:71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 54.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer's disease: implications for synaptic damage and cognitive decline. J.Alzheimers Dis. 2010;20(Suppl 2):S499–512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 57.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 58.Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc.Natl.Acad.Sci.U.S.A. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller DL, Papayannopoulos IA, Styles J, Bobin SA, Lin YY, Biemann K, Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch.Biochem.Biophys. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- 60.Findeis MA. The role of amyloid beta peptide 42 in Alzheimer's disease. Pharmacol.Ther. 2007;116:266–286. doi: 10.1016/j.pharmthera.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Tamaoka A, Odaka A, Ishibashi Y, Usami M, Sahara N, Suzuki N, Nukina N, Mizusawa H, Shoji S, Kanazawa I. APP717 missense mutation affects the ratio of amyloid beta protein species (A beta 1-42/43 and a beta 1-40) in familial Alzheimer's disease brain. J.Biol.Chem. 1994;269:32721–32724. [PubMed] [Google Scholar]
- 62.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 63.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J.Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol.Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 65.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J.Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 66.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ertekin-Taner N, Younkin LH, Yager DM, Parfitt F, Baker MC, Asthana S, Hutton ML, Younkin SG, Graff-Radford NR. Plasma amyloid beta protein is elevated in late-onset Alzheimer disease families. Neurology. 2008;70:596–606. doi: 10.1212/01.WNL.0000278386.00035.21. [DOI] [PubMed] [Google Scholar]
- 68.Blasko G, Kemmler S, Jungwirth I, Wichart W, Krampla S, Weissgram K, Jellinger KH, Tragl, Fischer P. Plasma amyloid beta-42 independently predicts both late-onset depression and Alzheimer disease. Am.J.Geriatr.Psychiatry. 2010;18:973–982. doi: 10.1097/JGP.0b013e3181df48be. [DOI] [PubMed] [Google Scholar]
- 69.Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, Feldman HH, Petersen RC, Siemers E, Doody RS, Hendrix SB, Grundman M, Schneider LS, Schindler RJ, Salmon E, Potter WZ, Thomas RG, Salmon D, Donohue M, Bednar MM, Touchon J, Vellas B. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M, AN1792 (QS-21)-251 Study Team Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr.Alzheimer Res. 2009;6:144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta Neuropathol. 2010;120:369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 72.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 73.Iijima-Ando L, Zhao, Gatt A, Shenton C, Iijima K. A DNA damage-activated checkpoint kinase phosphorylates tau and enhances tau-induced neurodegeneration. Hum.Mol.Genet. 2010;19:1930–1938. doi: 10.1093/hmg/ddq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 75.Zheng WH, Bastianetto S, Mennicken F, Ma W, Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115:201–211. doi: 10.1016/s0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 76.Huang HC, Jiang ZF. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease. J.Alzheimers Dis. 2009;16:15–27. doi: 10.3233/JAD-2009-0960. [DOI] [PubMed] [Google Scholar]
- 77.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 78.Sievers SA, Karanicolas J, Chang HW, Zhao A, Jiang L, Zirafi O, Stevens JT, Munch J, Baker D, Eisenberg D. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature. 2011;475:96–100. doi: 10.1038/nature10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc.Natl.Acad.Sci.U.S.A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann.Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 81.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N.Engl.J.Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 82.Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? Biochim.Biophys.Acta. 2010;1802:66–79. doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer's disease: implications for mitochondrially targeted antioxidant therapeutics. J.Biomed.Biotechnol. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Droge W. Free radicals in the physiological control of cell function. Physiol.Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 85.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int.J.Biochem.Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 87.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab.Rev. 2007;39:443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- 88.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med. Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 89.Leuner, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid.Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 90.Gibson GE, Starkov A, Blass JP, Ratan RR, Beal MF. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim.Biophys.Acta. 2010;1802:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mancuso, Orsucci D, LoGerfo A, Calsolaro V, Siciliano G. Clinical features and pathogenesis of Alzheimer's disease: involvement of mitochondria and mitochondrial DNA. Adv.Exp.Med.Biol. 2010;685:34–44. doi: 10.1007/978-1-4419-6448-9_4. [DOI] [PubMed] [Google Scholar]
- 92.Young KJ, Bennett JP. The mitochondrial secret(ase) of Alzheimer's disease. J.Alzheimers Dis. 2010;20(Suppl 2):S381–400. doi: 10.3233/JAD-2010-100360. [DOI] [PubMed] [Google Scholar]
- 93.Manczak, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum.Mol.Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 94.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 95.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J.Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J.Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin MT, Beal MF. Alzheimer's APP mangles mitochondria. Nat.Med. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 98.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat.Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. Int.J.Alzheimers Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc.Natl.Acad.Sci.U.S.A. 2008;105:13145–50. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iijima-Ando K, Hearn SA, Shenton C, Gatt A, Zhao L, Iijima K. Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PLoS One. 2009;4:e8310. doi: 10.1371/journal.pone.0008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J.Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 103.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J.Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 104.Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc.Natl.Acad.Sci.U.S.A. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 106.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J.Struct.Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 107.Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic.Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 108.Aliev G, Seyidova D, Lamb BT, Obrenovich ME, Siedlak SL, Vinters HV, Friedland RP, LaManna JC, Smith MA, Perry G. Mitochondria and vascular lesions as a central target for the development of Alzheimer's disease and Alzheimer disease-like pathology in transgenic mice. Neurol.Res. 2003;25:665–674. doi: 10.1179/016164103101201977. [DOI] [PubMed] [Google Scholar]
- 109.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J.Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 110.Vural H, Demirin H, Kara Y, Eren I, Delibas N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer's disease. J.Trace Elem.Med.Biol. 2010;24:169–173. doi: 10.1016/j.jtemb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Lu J, Wang K, Rodova M, Esteves R, Berry DEL, Crafter A, Barrett M, Cardoso SM, Onyango I, Parker WD, Fontes J, Burns JM, Swerdlow RH. Polymorphic variation in cytochrome oxidase subunit genes. J Alzheimers Dis. 2010;21:141–54. doi: 10.3233/JAD-2010-100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mancuso, Calsolaro V, Orsucci D, Siciliano G, Murri L. Is there a primary role of the mitochondrial genome in Alzheimer's disease? J.Bioenerg.Biomembr. 2009;41:411–416. doi: 10.1007/s10863-009-9239-1. [DOI] [PubMed] [Google Scholar]
- 113.Santoro A, Balbi V, Balducci E, Pirazzini C, Rosini F, Tavano F, Achilli A, Siviero P, Minicuci N, Bellavista E, Mishto M, Salvioli S, Marchegiani F, Cardelli M, Olivieri F, Nacmias B, Chiamenti AM, Benussi L, Ghidoni R, Rose G, Gabelli C, Binetti G, Sorbi S, Crepaldi G, Passarino G, Torroni A, Franceschi C. Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset Alzheimer's disease. PLoS One. 2010;5:e12037. doi: 10.1371/journal.pone.0012037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aliyev A, Chen SG, Seyidova D, Smith MA, Perry G, de la Torre J, Aliev G. Mitochondria DNA deletions in atherosclerotic hypoperfused brain microvessels as a primary target for the development of Alzheimer's disease. J.Neurol.Sci. 2005;229-230:285–292. doi: 10.1016/j.jns.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 115.Murray IV, Proza JF, Sohrabji F, Lawler JM. Vascular and metabolic dysfunction in Alzheimer's disease: a review. Exp.Biol.Med.(Maywood) 2011 doi: 10.1258/ebm.2011.010355. [DOI] [PubMed] [Google Scholar]
- 116.Mancuso M, Coppede F, Murri L, Siciliano G. Mitochondrial cascade hypothesis of Alzheimer's disease: myth or reality? Antioxid.Redox Signal. 2007;9:1631–1646. doi: 10.1089/ars.2007.1761. [DOI] [PubMed] [Google Scholar]
- 117.Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim.Biophys.Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin CJ, Huang HC, Jiang ZF. Cu(II) interaction with amyloid-beta peptide: a review of neuroactive mechanisms in AD brains. Brain Res.Bull. 2010;82:235–242. doi: 10.1016/j.brainresbull.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 119.Fukui H, Diaz F, Garcia S, Moraes CT. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer's disease. Proc.Natl.Acad.Sci.U.S.A. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pickrell AM, Fukui H, Moraes CT. The role of cytochrome c oxidase deficiency in ROS and amyloid plaque formation. J.Bioenerg.Biomembr. 2009;41:453–456. doi: 10.1007/s10863-009-9245-3. [DOI] [PubMed] [Google Scholar]
- 121.Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 122.Cooke MS, Olinski R, Evans MD. Does measurement of oxidative damage to DNA have clinical significance? Clin.Chim.Acta. 2006;365:30–49. doi: 10.1016/j.cca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 123.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang L, Hirayasu K, Ishizawa M, Kobayashi Y. Purification of genomic DNA from human whole blood by isopropanol-fractionation with concentrated Nal and SDS. Nucleic Acids Res. 1994;22:1774–1775. doi: 10.1093/nar/22.9.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Helbock HJ, Beckman KB, Ames BN. 8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999;300:156–166. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 126.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc.Natl.Acad.Sci.U.S.A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lau SS, Peters MM, Kleiner HE, Canales PL, Monks TJ. Linking the metabolism of hydroquinone to its nephrotoxicity and nephrocarcinogenicity. Adv.Exp.Med.Biol. 1996;387:267–273. doi: 10.1007/978-1-4757-9480-9_35. [DOI] [PubMed] [Google Scholar]
- 128.Laws GM, Adams SP. Measurement of 8-OHdG in DNA by HPLC/ECD: the importance of DNA purity. BioTechniques. 1996;20:36–38. doi: 10.2144/96201bm06. [DOI] [PubMed] [Google Scholar]
- 129.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum.Mol.Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 130.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 131.Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J.Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 132.Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage. Hum.Mol.Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piperakis SM, Visvardis EE, Tassiou AM. Comet assay for nuclear DNA damage. Methods Enzymol. 1999;300:184–194. doi: 10.1016/s0076-6879(99)00125-1. [DOI] [PubMed] [Google Scholar]
- 134.Collins AR. Investigating oxidative DNA damage and its repair using the comet assay. Mutat.Res. 2009;681:24–32. doi: 10.1016/j.mrrev.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 135.Collins AR, Dobson VL, Dusinska M, Kennedy G, Stetina R. The comet assay: what can it really tell us? Mutat.Res. 1997;375:183–193. doi: 10.1016/s0027-5107(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 136.Suram A, Hegde ML, Rao KS. A new evidence for DNA nicking property of amyloid beta-peptide (1-42): relevance to Alzheimer's disease. Arch.Biochem.Biophys. 2007;463:245–252. doi: 10.1016/j.abb.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 137.Cimini, Moreno S, D'Amelio M, Cristiano L, D'Angelo B, Falone S, Benedetti E, Carrara P, Fanelli F, Cecconi F, Amicarelli F, Ceru MP. Early biochemical and morphological modifications in the brain of a transgenic mouse model of Alzheimer's disease: a role for peroxisomes. J.Alzheimers Dis. 2009;18:935–952. doi: 10.3233/JAD-2009-1199. [DOI] [PubMed] [Google Scholar]
- 138.Mecocci, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann.Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 139.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer's disease. J.Neurochem. 1997;68:2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 140.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J.Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 141.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J.Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 142.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J.Neurochem. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 143.Chang SW, Zhang D, Chung HD, Zassenhaus HP. The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer's brain. Biochem.Biophys.Res.Commun. 2000;273:203–208. doi: 10.1006/bbrc.2000.2885. [DOI] [PubMed] [Google Scholar]
- 144.Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc.Natl.Acad.Sci.U.S.A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW, Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer's disease and down syndrome dementia. J.Alzheimers Dis. 2010;20(Suppl 2):S293–310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wadsworth TL, Bishop JA, Pappu AS, Woltjer RL, Quinn JF. Evaluation of coenzyme Q as an antioxidant strategy for Alzheimer's disease. J.Alzheimers Dis. 2008;14:225–234. doi: 10.3233/jad-2008-14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J.Alzheimers Dis. 2010;20(Suppl 2):S609–31. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Van Raamsdonk JM, Hekimi S. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship? Antioxid.Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 149.Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, Murphy MP, Pautler RG, Klann E. Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J.Neurosci. 2011;31:5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wollen KA. Alzheimer's disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern.Med.Rev. 2010;15:223–244. [PubMed] [Google Scholar]
- 151.Zhang Z, Wang X, Chen Q, Shu L, Wang J, Shan G. Clinical efficacy and safety of huperzine Alpha in treatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial. Zhonghua Yi Xue Za Zhi. 2002;82:941–944. [PubMed] [Google Scholar]
- 152.Xu SS, Gao ZX, Weng Z, Du ZM, Xu WA, Yang JS, Zhang ML, Tong ZH, Fang YS, Chai XS. Efficacy of tablet huperzine-A on memory, cognition, and behavior in Alzheimer's disease. Zhongguo Yao Li Xue Bao. 1995;16:391–395. [PubMed] [Google Scholar]
- 153.Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, Jin S, Thomas R, Aisen PS, Alzheimer's Disease Cooperative Study A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology. 2011;76:1389–1394. doi: 10.1212/WNL.0b013e318216eb7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li J, Wu HM, Zhou RL, Liu GJ, Dong BR. Huperzine A for Alzheimer's disease. Cochrane Database Syst.Rev. 2008;(2):CD005592. doi: 10.1002/14651858.CD005592.pub2. [DOI] [PubMed] [Google Scholar]
- 155.Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis. J.Neural Transm. 2009;116:457–465. doi: 10.1007/s00702-009-0189-x. [DOI] [PubMed] [Google Scholar]
- 156.Desilets AR, Gickas JJ, Dunican KC. Role of huperzine a in the treatment of Alzheimer's disease. Ann.Pharmacother. 2009;43:514–518. doi: 10.1345/aph.1L402. [DOI] [PubMed] [Google Scholar]
- 157.Nagy Z, Esiri MM, Smith AD. The cell division cycle and the pathophysiology of Alzheimer's disease. Neuroscience. 1998;87:731–739. doi: 10.1016/s0306-4522(98)00293-0. [DOI] [PubMed] [Google Scholar]
- 158.Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell Biochem.Biophys. 2001;35:141–170. doi: 10.1385/CBB:35:2:141. [DOI] [PubMed] [Google Scholar]
- 159.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don't divide what's to repair? Mutat.Res. 2007;614:24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 160.de Souza-Pinto NC, Hogue BA, Bohr VA. DNA repair and aging in mouse liver: 8-oxodG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radic.Biol.Med. 2001;30:916–923. doi: 10.1016/s0891-5849(01)00483-x. [DOI] [PubMed] [Google Scholar]
- 161.Jeppesen DK, Bohr VA, Stevnsner T. DNA repair deficiency in neurodegeneration. Prog.Neurobiol. 2011;94:166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lovell MA, Gabbita SP, Markesbery WR. Increased DNA oxidation and decreased levels of repair products in Alzheimer's disease ventricular CSF. J.Neurochem. 1999;72:771–776. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- 163.Liu, Demple B. DNA repair in mammalian mitochondria: Much more than we thought? Environ.Mol.Mutagen. 2010;51:417–426. doi: 10.1002/em.20576. [DOI] [PubMed] [Google Scholar]
- 164.Yoshida Y, Izumi H, Ise T, Uramoto H, Torigoe T, Ishiguchi H, Murakami T, Tanabe M, Nakayama Y, Itoh H, Kasai H, Kohno K. Human mitochondrial transcription factor A binds preferentially to oxidatively damaged DNA. Biochem.Biophys.Res.Commun. 2002;295:945–951. doi: 10.1016/s0006-291x(02)00757-x. [DOI] [PubMed] [Google Scholar]
- 165.Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann.N.Y.Acad.Sci. 2005;1042:101–108. doi: 10.1196/annals.1338.010. [DOI] [PubMed] [Google Scholar]
- 166.Canugovi, Maynard S, Bayne AC, Sykora P, Tian J, de Souza-Pinto NC, Croteau DL, Bohr VA. The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair (Amst) 2010;9:1080–1089. doi: 10.1016/j.dnarep.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yoshida Y, Izumi H, Torigoe T, Ishiguchi H, Itoh H, Kang D, Kohno K. P53 physically interacts with mitochondrial transcription factor A and differentially regulates binding to damaged DNA. Cancer Res. 2003;63:3729–3734. [PubMed] [Google Scholar]
- 168.Wong TS, Rajagopalan S, Townsley FM, Freund SM, Petrovich M, Loakes D, Fersht AR. Physical and functional interactions between human mitochondrial single-stranded DNA-binding protein and tumour suppressor p53. Nucleic Acids Res. 2009;37:568–581. doi: 10.1093/nar/gkn974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boesch, Weber-Lotfi F, Ibrahim N, Tarasenko V, Cosset A, Paulus F, Lightowlers RN, Dietrich A. DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim.Biophys.Acta. 2011;1813:186–200. doi: 10.1016/j.bbamcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 170.Karahalil, Hogue BA, de Souza-Pinto NC, Bohr VA. Base excision repair capacity in mitochondria and nuclei: tissue-specific variations. FASEB J. 2002;16:1895–1902. doi: 10.1096/fj.02-0463com. [DOI] [PubMed] [Google Scholar]
- 171.Chen, Cao G, Hastings T, Feng Y, Pei W, O'Horo C, Chen J. Age-dependent decline of DNA repair activity for oxidative lesions in rat brain mitochondria. J.Neurochem. 2002;81:1273–1284. doi: 10.1046/j.1471-4159.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- 172.Gredilla, Garm C, Holm R, Bohr VA, Stevnsner T. Differential age-related changes in mitochondrial DNA repair activities in mouse brain regions. Neurobiol.Aging. 2010;31:993–1002. doi: 10.1016/j.neurobiolaging.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA-repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol.Aging. 2006;27:1129–1136. doi: 10.1016/j.neurobiolaging.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 174.Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]