Abstract
Humans are fundamentally social creatures who are ‘motivated’ to be with others. In this review we examine the role of oxytocin (OT) as it relates to social motivation. OT is synthesized in the brain and throughout the body, including in the heart, thymus, gastrointestinal tract, as well as reproductive organs. The distribution of the OT receptor (OTR) system in both the brain and periphery is even more far-reaching and its expression is subject to changes over the course of development. OTR expression is also sensitive to changes in the external environment and the internal somatic world. The OT system functions as an important element within a complex, developmentally sensitive biobehavioral system. Other elements include sensory inputs, the salience, reward, and threat detection pathways, the hypothalamic-pituitary-gonadal axis, and the hypothalamic-pituitary-adrenal stress response axis. Despite an ever expanding scientific literature, key unresolved questions remain concerning the interplay of the central and peripheral components of this complex biobehavioral system that dynamically engages the brain and the body as humans interact with social partners over the course of development.
Keywords: Motivation, Dyadic social interactions, Parenting, Sexual behavior, Oxytocin, Vasopressin, Salience, Reward, Dopamine, Estrogen, Testosterone, Stress, Cortisol, Epigenetics
Every variety of love…is born, lives, dies, or attains immortality in accordance with the same laws.
Henri Marie Beyle (Stendhal), 1822
1. Introduction
‘Motivation’ is defined in the Merriam Webster dictionary as being: “1.a: the act or process of giving someone a reason for doing something; b: the condition of being eager to act or work; 2: a force or influence that causes someone to do something.” In this review, we begin with the observation that humans are fundamentally social creatures who are ‘motivated’ to be with others. The various forms and contexts through which we can ‘be with others’ in today's world are ever expanding and include, for example, being ‘friends’ on Facebook and Twitter. More broadly, the concept of social motivation considers the basic human need to become a member of groups organized around one's familial, cultural, religious, national, community, political, occupational, scholastic, and/or recreational identity.
In this review, we first focus on the importance of monitoring dyadic social interactions. We then review the ever-expanding scientific literature concerning the biobehavioral processes that underlie the motivation to be with others. The complex neural and somatic systems that are influenced by the neuropeptide oxytocin (OT) in the emergence of intimate dyadic relationships over the course of development are a major focus of this review. Next, we present a conceptual model and close by posing questions for future research. Before reviewing any specific findings, we offer our evolutionary point-of-view concerning the likely existence of common biobehavioral processes in attachment and bonding among all mammals.
Humankind and especially the human brain are remarkable products of evolution. While the basic machinery of the vertebrate brain has been in place for more than 450 million years, our subspecies (Homo sapiens sapiens) emerged between 100,000 and 200,000 years ago. In the struggle for life, certain traits have come to predominate. It is likely that many of the elements in our mental and behavioral repertoire related to successful mammalian reproduction have been the focus of intense selective pressures ever since the first lactating proto-mammals emerged some 300 million years ago. The selection of a mate, bearing of viable offspring, and the formation of parental commitments that will sustain an infant through a period of dependency (especially lengthy for humans) are just a few of the developmentally sensitive, complex, interdependent processes needed for individual survival and species viability. Although most of our biological and behavioral potentialities are likely to be called upon at one point or another in the service of these goals, there must be highly conserved brain- and body-based systems that are specifically activated at developmentally appropriate moments to achieve and sustain these processes. We hypothesize that a thorough understanding of these “normal” processes will also lead to deeper insights into our vulnerability to develop a range of psychopathological outcomes (Leckman and Mayes, 1998).
This point of view is consistent with the work of Darwin (1872), Tinbergen (1963), Lorenz (1978), Hinde, 1970, Hinde, 1986, Bowlby, 1969, Bowlby, 1973, Bowlby, 1980, Bowlby, 1988 and Ainsworth et al. (1978), as well as scientists currently engaged in the study of bond formation (e.g., Carter et al., 2005) or reproductive strategies (e.g., Belsky et al., 1991) from an evolutionary perspective. As noted by Tinbergen (1963), this evolutionary perspective challenges us to examine both the “proximal” and “ultimate” causes of these conserved behaviors. Consequently, we will examine the biobehavioral mechanisms that stimulate and support social interactions as well as how these mechanisms change over the course of development. We will also consider how this complex biobehavioral system compares across species and how it directly impacts an individual's survival and ability to reproduce.
2. Characterization of dyadic social behaviors over the course of development
One important mechanism by which mammalian parents bond with their infants (Hrdy, 2005) and adults bond with each other (Sacher, 2005) involves approach and social engagement followed by a specific set of highly repetitive and predictable behaviors that are often species-specific. Consequently, this area of research depends upon the ability of investigators to record and empirically code the exact nature of these species-specific dyadic social interactions. Coding schemes to monitor dyadic interactions in rodents are well established (Myers et al., 1989, Hammock and Young, 2005). For example, Myers et al. (1989) developed a now-widely used intensive observation system in which dyadic interactions are scored 25 times for 3 min blocks five times per day for a total of 125 daily observation blocks per mother (Champagne et al., 2001, Francis et al., 1999). The coded dyadic repertoire includes such items as the mother licking and grooming (LG) any pup, nursing of pups in an arched-back posture, engaging in a “blanket” posture in which the mother lays over the pups or a passive posture while the pups nurse, as well as the absence of pup-mother contact. Similar paradigms are available for coding human behavior (Ainsworth et al., 1978, Feldman, 1998, Koos and Gergely, 2001, Tronick et al., 1980).
As with the animal work, these studies have begun to untangle the specific types of micro-level behaviors that typify different attachment relationships, such as interactions with mothers versus fathers, dyadic parent–child interactions versus whole family exchanges, interactions between parent and child as compared to those expressed by romantic partners at the initiation of pair bonding, as well as how the expression of these behaviors may differ by environment, e.g., in industrial versus more traditional societies. For example, using Coding Interactive Behavior (CIB) codes, Feldman and colleagues have been able to monitor minute, subtle, repetitive and spontaneous elements of dyadic and triadic behaviors across the life span (Feldman, 1998, Feldman, 2003, Feldman, 2007, Feldman et al., 2010c, Feldman et al., 2011). The codes used to characterize these dyadic and triadic interactions include mutual gaze, affect, vocalization, arousal indicators, lead-lag relationships, joint focus, exploratory behavior, proximity position, and type of touch. These behaviors are observed and coded throughout the interaction in multiple players within different contexts (for example, free play versus toy exploration paradigms, paternal versus maternal play, or dyadic versus triadic contexts) and can be consequently integrated into meaningful behavioral composites. By assessing the degree of coordination of various micro-level codes between partners, an exchange can further be classified as “synchronous” (interactions where partners coordinate their behavior in relation to the other's social signals) versus “intrusive” (interactions in which one partner over-stimulates and disregards the other's focus of interest or need for rest) (Feldman et al., 2010a).
3. Biological determinants of motivation in a social context during the course of development
There are several comprehensive reviews on this topic that focus either on animal studies, human studies, or both (Barrett and Fleming, 2011, Bartz et al., 2011, Bos et al., 2011, Goodson and Thompson, 2010, Heinrichs et al., 2009, Ross and Young, 2009). In this review, we have used a systems approach to survey important findings relevant to neural and somatic systems integral to social motivation. In addition to the biology and ontogeny of the OT and arginine vasopressin (AVP) system, we focus on how these nanopeptide systems interact with the salience and reward pathways, the hypothalamic-pituitary-gonadal axis, the hypothalamic-pituitary-adrenal stress response axis, the immune system and other peripheral organ systems.
3.1. Molecular and anatomical framework: the oxytocin (OT) and arginine vasopressin (AVP) axis
OT and AVP are nine amino acid peptides that are closely related structurally, differing at only two amino acids. Phylogenetically the genes regulating both OT and AVP can be traced to invertebrates. However, the specific amino acid sequences of OT and AVP are with few exceptions, mostly present only in placental mammals (Gainer and Wray, 1994). In the central nervous system (CNS), synthesis of OT and AVP occurs primarily in the supraoptic nuclei (SON) and paraventricular nuclei (PVN) of the hypothalamus, although the spinal cord, bed nucleus of the stria terminalis (BNST) and the anterior commissural nucleus are also central sources of OT (Sofroniew, 1983) and the suprachiasmatic nucleus, medial amygdala, BNST and other caudal brain stem areas are other central sources of AVP (Carter, 1998).
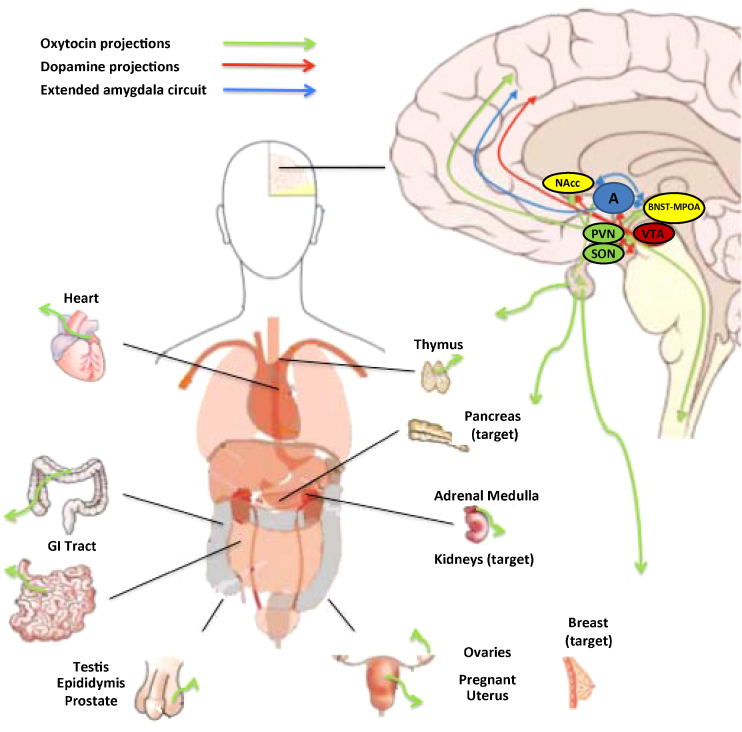
As depicted in Fig. 1, OT fibers are present in a number of brain regions including the medial preoptic area (MPOA), the BNST, the lateral septum, the nucleus accumbens (NAcc), the amgydala, the hippocampus, as well as caudally in the ventral tegmental area (VTA) of the midbrain and the spinal cord (Sofroniew, 1983, Wang et al., 1996). Magnocellular neurons in both the SON and PVN project to the posterior pituitary to release OT and AVP into the bloodstream, where they exert peripheral effects.
Fig. 1.
Central and peripheral sites of oxytocin (OT) release. Within the brain, OT is released from the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus, and to a lesser extent, the bed nucleus of the stria terminalis, spinal cord and anterior commissural nucleus. Central OT projections are pictured in green. Peripheral sources of OT include OT released into circulation via the posterior pituitary as well as numerous sites outside of the brain, including the heart, thymus, gastrointestinal tract, testis, epididymis, prostate, pregnant intrauterine tissue, ovaries, and adrenal medulla. The breast, pancreas and kidney are peripheral OT targets. NAcc = nucleus accumbens; MPOA = medial preoptic area; BNST = bed nucleus of stria terminalis; VTA = ventral tegmental area; A = amygdala.
Based on tract tracing studies performed in prairie voles (Microtus ochrogaster), Ross and Young (2009), argue that OT release is not limited to the synaptic cleft. They hypothesize that OT projections coursing through the forebrain could either be direct projections or be collaterals from magnocellular neurons projecting to the posterior pituitary from either the SON or the PVN or both (Ross et al., 2009). If the OT fibers seen in the forebrain are collaterals of magnocellular hypothalamic neurons, this would provide a direct mechanism for coordination of central release in the forebrain with peripheral release under specific physiological states, such as vaginocervical stimulation during mating or parturition, or sensory stimulation during breast feeding.
It also appears likely that neurons in the PVN and SON can release OT from their entire surface area (Pow and Morris, 1989) and that OT can diffuse through the extracellular space. Remarkably, dendritic release of OT has been well characterized and is independent of neuronal firing (for review see Ludwig and Leng, 2006) and likely contributes to brain OT concentrations. The complexities of this somatodendritic release are still only partially understood, but this mechanism could contribute to the ability of these compounds to communicate more dynamically and holistically with the body and brain (Bos et al., 2011, Landgraf and Neumann, 2004, Veening and Barendregt, 2010, Veening et al., 2010). Future research is needed to address the mechanisms that may underlie the synchronous release of OT in the brain and periphery.
OT receptors (OTRs) are widely distributed through the CNS in a highly species-specific fashion (Donaldson and Young, 2008, Goodson and Thompson, 2010). A large number of taxa have been examined. Within species, the both pattern of OTR distribution across brain regions and the density of OTRs within specific brain regions can be affected by rearing environments and developmental stage (Campbell et al., 2009, Leung et al., 2009).
3.1.1. Ontogeny of the OT and AVP systems: sensitive periods and the impact of environmental pertubations
From the fetal to adult stage, several developmental alterations take place in the OT and AVP systems. These changes presumably underlie the maturation of social behaviors and facilitate the various changing physiologic functions of these systems across the lifespan. For example, the weaning period is an important developmental transition in rodents, marked by reduced maternal investment, alterations in diet and increased independent socialization and exploration (Martin, 1984). The available evidence suggests that it is also a critical period in the function of the OT system (Higashida et al., 2010, Lopatina et al., 2011). Prior to weaning, exogenous sources of OT are provided to the young via the fetal placenta and maternal breast milk. Following weaning, the offspring become dependent upon the endogenous synthesis of OT, and consequently, deficits in the function of the OT system become more apparent and different pathological phenotypes may emerge. As reviewed below in the genetic section, Higashida and colleagues found that following weaning, mice pups need to be self sufficient in the production and release of OT in order to sustain maternal behavior and male social recognition (Higashida, 2010; Lopatina et al., 2011).
In adulthood, pregnancy and parturition mark another major transition point in the pattern of neuropeptide secretion and availability for females. During pregnancy, central basal release of OT is transiently suppressed by high levels of progesterone (Keverne and Kendrick, 1992). This is thought to sensitize the brain to the effects of OT released in the MPOA, BNST and olfactory bulb during parturition to facilitate the induction of maternal behavior (Kendrick et al., 1988a, Kendrick et al., 1988b, Kendrick et al., 1992).
In the brain, the expression of both the OTR and AVP (V1aR) receptor is sensitive to several important developmental transitions. The pre-weaning period in rats is characterized by reductions in OTR binding in the cingulate cortex and dorsal hippocampus, along with the appearance of OTR in the ventral hippocampus (Tribollet et al., 1989). At the onset of puberty in the rat, Tribollet et al. (1989) also reported increases in OTR binding in the olfactory tubercle and the ventromedial hypothalamic nucleus—an area with a high density of estrogen receptors in the adult brain—suggesting expression in this region may be contingent upon increased gonadal function accompanying the onset of puberty. In fact, estradiol-related and postpartum increases in OTR binding have been identified in several studies (Insel, 1986, De Kloet et al., 1986). Lukas et al. (2010) report decreases in OTR binding in the caudate-putamen and lateral septum and increases in OTR binding in the ventromedial hypothalamus and increased V1aR binding in the lateral septum with increasing age in male rats. In contrast, expression of OTR in some regions appears to be constant; for example, OTR binding in the dorsal motor nucleus of the vagus nerve is first identified during fetal life and persists into adulthood (Tribollet et al., 1989).
Studies suggest the developmental trajectory of the OT and AVP systems is not completely hardwired can be modulated by both early life experience and the rearing environment. For example, maternal separation induces dramatic and long-lasting changes in OT and AVP receptor binding in the juvenile, adolescent and adult brain. In male rats, 3 h of daily maternal separation between postnatal days 1 and 14 is associated with age-dependent alterations in receptor binding; specifically, increased V1aR binding in the lateral septum of juveniles and piriform cortex of adolescents and adults, decreased OTR binding in the agranular cortex of juveniles and adolescents and lateral septum and caudate putamen of adults and increased OTR binding in the ventromedial hypothalamus of adults (Lukas et al., 2010). The authors speculate that the altered expression of these receptor systems may underlie the long-term behavioral alterations associated with reduced maternal contact, including altered expression of olfaction-dependent behaviors such as social recognition (possibly related to changes in the piriform cortex or agranular cortex, regions associated with olfactory processing) or anxiety and aggression (presumably due to changes in the lateral septum). Similarly, in highly social prairie voles, differing amounts of manipulation from postnatal day 1 to 7 are also associated with alterations in the development of the OT system and social function. Compared to those not handled at all or handled once on postnatal day 7, early handling (once daily for the first 7 postnatal days or once on postnatal day 1) is associated with lower OTR binding in the BNST and NAcc, higher OT cell body density in the SON and behaviorally, increased time engaging in non-huddling contact and alloparental behavior (Bales et al., 2011).
Importantly, these effects seem to be sexually dimorphic and species-dependent. For example, early maternal separation is associated with decreased OT immunoreactivity in the PVN of lactating females but increased AVP immunoreactivity in the PVN of males of CB57BL/6 mice (Veenema et al., 2007). In this population, maternal separation is associated with decreased latency of maternal aggression but increased latency of inter-male aggression, whereas in rats, the opposite effects are reported for both adult inter-male aggression (Veenema et al., 2006) and maternal aggression (Boccia and Pedersen, 2001). Offspring age at weaning is also associated with sex-specific alterations in receptor expression and behavior. While no sex-related differences are noted for mice weaned on postnatal day 21, weaning on postnatal day 28 is associated with increased OTR binding in females and decreased OTR binding in males within the ventral medial hypothalamus (VMH; Curley et al., 2009). Given that the VMH is associated with estradiol-induced regulation of sociosexual behaviors, it is possible that these alterations underlie changes in reproductive strategies observed with varying parental investment, such as the increased lordosis response of females receiving low maternal care in the early postnatal period (Curley et al., 2009). These environmentally driven effects are likely driven by epigenetic mechanisms, discussed subsequently.
Other structural developmental changes in the brain have been identified, including the gradual emergence of projections between brain regions, such as AVP projections from the BNST and medial amygdala to the lateral habenula and septum (De Vries et al., 1981). Parturition, lactation and the induction of maternal behavior have also been associated with several morphological changes to oxytocinergic neurons in the rat SON, including dendritic bundling, the emergence of large areas of somatic appositions, increases in the number of presynaptic terminals making contact with multiple postsynaptic elements and dye coupling (an indicator of electrotonic coupling) (Hatton et al., 1992).
Outside of the brain, alterations in the peripheral OT system have also been noted; for example, OTR density in the porcine endometrium has been found to vary with the menstrual cycle, increasing during oestrus and decreasing during early pregnancy and the luteal phase (Okano et al., 1996). In humans, OTR density in the myometrium and decidua increase with pregnancy and peak with labor, presumably in association with OT's role in the stimulation of uterine contractions in the initiation of labor (Fuchs et al., 1982).
3.1.2. The role of oxytocin and vasopressin in parental behaviors
OT has long been considered a maternal hormone, traditionally known for its role in milk-let down during breastfeeding and uterine contraction during childbirth. A synthetic form of OT is used for birth induction and to prevent bleeding from uterine tissue after delivery. Breastfeeding, a fundamental maternal behavior, relies on OT secretion and OT is abundant in maternal milk and serves as an exogenous source of OT for the nursing infant (Carter, 2003).
Infant behaviors during breastfeeding (i.e., suckling and infant hand stimulation of the nipple) induce OT release in the mother (Amico and Finley, 1986) and this response is sensitive both to mechanisms of conditional learning and a range of infant reminders (McNeilly et al., 1983). Repeated assessments of maternal salivary OT around the time of breastfeeding show that OT concentrations peak a few minutes prior to breastfeeding in preparation for milk let-down (White-Traut et al., 2009).
Animal research describes a specific time-window most suitable for the initiation of bonding. For instance, during the estrous cycle in females, varying levels of estrogen sensitize the female brain to the effects of OT on pair bonding. Specifically, estradiol potentiates the OT system by increasing synthesis of the neuropeptide (Miller et al., 1989, Caldwell et al., 1989) and its receptor (Insel, 1986). In prairie voles, the removal of the ovary does not eliminate the capacity to form pair bonds (Williams et al., 1992); yet, females form bonds more quickly if they mate. This finding led researchers to the hypothesis that OT is the critical element in pair bonding (Carter, S., personal communication, 2011). The effect of OT on timing is also observed in the initiation of maternal behaviors; in virgin rats and sheep that are “primed” with estrogen, intracerebroventricular injections of OT quickly induce the full repertoire of maternal behavior in a dose-dependent fashion (Lévy et al., 1992, Pedersen et al., 1982). Importantly, this effect is facilitated by opioids, which are known to increase with parturition, act in synergy with OT in the induction of maternal behaviors (Keverne and Kendrick, 1992) and play a role in the development of maternal–infant touch and contact (Weller and Feldman, 2003). Conversely, administration of an OTR antagonist during this critical time supresses the onset of maternal behaviors (van Leengoed et al., 1987). In addition, in virgin female wild-type mice, central OT administration inhibits infanticide (McCarthy, 1990). It appears that timing creates both the willingness to partake in reward-seeking behaviors and marks a redefined motivational salience for participating in these bonding behaviors.
Production of OT during breastfeeding is associated with traits that support responsive and sensitive mothering, such as calmness and sociability (Uvnas-Moberg, 1998a, Uvnas-Moberg, 1998b), and a decrease in feelings that might hinder maternal function like anxiety, aggression, guilt and suspicion (Uvnas-Moberg et al., 1990, Uvnas-Moberg et al., 1993). Similarly, the amount of breast milk mothers expressed following premature birth was found to predict the amount of micro-level maternal behaviors during mother–infant interactions, especially affectionate touch (Feldman and Eidelman, 2003).
Numerous studies assessing the involvement of OT in human bonding examined the expression of micro-level social behavior in each partner during dyadic or triadic interactions along the dimensions of gaze, proximity, arousal, touch, affect, exploratory behavior, and vocalizations. The expression of these behaviors in various social contexts – such as face-to-face interactions, exploratory play, triadic contexts, interactions between children or adolescents with their best friends or a peer group, and exchanges between romantic partners – are assessed in relation to peripheral measures of OT. Such micro-level behaviors are integrated into meaningful behavioral constellations with distinct temporal patterns and can advance our understanding of the intricate relationships between the oxytocinergic system and attachment processes in humans. While human studies are limited to peripheral measurements (plasma, saliva, urine, or CSF) due to obvious constraints in the measurement of brain OT, studies testing the correlations between the behaviors of parents and infants and other social partners may show consistency with the animal studies and thus validate the use of peripheral measures. Neuroimaging studies of new mothers also point to the involvement of OT-related circuitry in sensitive care giving (Kim et al., 2011, Strathearn et al., 2009).
Measuring plasma OT in pregnant mothers throughout pregnancy and in the immediate postpartum period, Feldman and colleagues found that higher OT concentrations in the first trimester of pregnancy and the first postpartum month predicted maternal bonding behaviors, including gaze to infant, “motherese” high-pitched vocalizations, positive affect, and affectionate touch (Feldman et al., 2007). In this sample, the increase in OT concentrations from the first to the third trimester of pregnancy was associated with the mother's self-reported attachment to her fetus prior to birth (Levine et al., 2007). In contrast, Skrundz et al. (2011) reported that that expectant mothers during the third trimester of pregnancy at risk for postpartum depression have lower levels of plasma OT during pregnancy.
In a study of mothers and fathers during the first 6 months after the birth of their first child, maternal plasma OT concentrations were correlated with the mother's affectionate-style parenting, including maternal gaze, positive affect, “motherese” vocalizations, and affectionate touch (Gordon et al., 2010a). On the other hand, fathers’ OT levels were associated with their stimulatory play style, which included stimulatory and proprioceptive touch, high positive arousal, and object focus—measures thought to capture the father's tendency to induce positive arousal and orient the infant towards the external environment (Gordon et al., 2010a). In a social play context, paternal OT was also associated with affect synchrony, which was indexed by the coordination of paternal gaze, vocalizations, and positive affect during moments of infant positive vocalizations (Gordon et al., 2010c). Close proximity and warmth during mother–father–infant triadic family interactions were similarly linked with both maternal and paternal OT. At 6 months, the amount of triadic synchrony—defined as moments of physical proximity and affectionate touch between mother, father, and child while parents synchronize social gaze—was predicted by the peripheral levels of maternal and paternal OT (Gordon et al., 2010b).
To examine the effects of maternal and paternal touch patterns on the OT response, a group of 112 mothers and fathers (not couples) interacted in 15-min “play-and-touch” sessions with their 4–6 month old infants. Mothers who provided high levels of affectionate contact showed an increase in salivary OT following dyadic interactions, but mothers who provided minimal touch did not show an OT response. Among fathers, those who provided high levels of stimulatory contact also exhibited an OT response, whereas those displaying minimal contact showed no OT increase following the parent–infant session (Feldman et al., 2010b).
Another study tested the cross-generational transmission of peripheral OT levels from parents to infant. Mothers and fathers were observed in the “play-and-touch” paradigm and salivary OT was assessed in both parent and infant before and after play. Correlations were found between the OT levels of mothers and fathers and that of their infant in both the pre-play baseline and the post-contact period. Importantly, we found that the degree of affect synchrony moderated the cross-generational transmission of OT levels from parent to child. Dyads engaging in synchronous play also exhibited a significantly higher cross-generational link between the parent and infant's OT levels (Feldman et al., 2010c). Finally, we found that maternal and paternal plasma and salivary OT were inter-related and were associated with affect synchrony as well as with self-reported measures of romantic attachment and bonding to own parents, indicating that OT may mediate the individual's multiple attachments across the lifespan (Feldman et al., 2011). Interestingly, peripheral OT levels measured in young adults who were currently not involved in a romantic relationship were found to be correlated with the level of affectionate care they reported receiving as a child from their mothers as well as their fathers (Mother Care, r = 0.42, p < .01; Father Care, r = 0.36, p < .05) (Gordon et al., 2008, Parker et al., 1979).
OT reactivity measured in urine after a “cuddle play” session in which foster mother and child were asked to play together in an interaction that included a lot of warm physical contact was associated with the amount of “maternal delight” foster mothers displayed during the interactions (Bick and Dozier, 2010).
These findings linking parental behaviors with relatively stable peripheral levels of OT raise a number of issues that are as yet unresolved. Most notably, how are stable OT concentrations maintained in the periphery and how are they related to events in the brain? Is somatodendritic release and diffusion sufficient? Or is it due to release from axon collaterals extending into the forebrain from magnocellular hypothalamic neurons? Or, as we discuss in a later section of the review, are there, as yet unexplored mechanisms by which peripheral sources of OT contribute to this stability?
3.1.3. The role of oxytocin and vasopressin in sexual behavior and the formation and maintenance of adult pair bonds
Evidence from microinjection, double immunofluorescence and intra-cerebral microdialysis studies suggests CNS OT may play an important role in both the motivational and consummatory phases of sexual behavior by virtue of its connections with mesolimbic dopaminergic neurons (for a review, see Melis and Argiolas, 2011). OT injection into the PVN and extra-hypothalamic sites facilitates penile erections and improves male copulatory performance in monkeys, mice, rats, and rabbits (Melis and Argiolas, 2011, Argiolas and Gessa, 1991, Carter, 1992, Pedersen et al., 1992, Argiolas and Melis, 1995, Argiolas and Melis, 2004, Argiolas, 1999, Winslow and Insel, 1991, Arletti et al., 1985). In females, OT is thought to increase sexual receptivity. Intracerebroventricular and intraperitoneal injections of OT significantly increase lordosis behavior in estrogen-treated female rats in a dose-dependent manner (Arletti and Bertolini, 1985, Caldwell et al., 1986).
Consistent with these findings, OT is released during sexual activity in both men and women (Carmichael et al., 1987). Plasma OT levels measured in men during sexual climax were five times higher than at basal levels, but subsequently returned to basal levels within a 30 min period (Murphy et al., 1987, Uckert et al., 2003). Loving affectionate touch between romantic partners, such as hugs and provision of social support, are related to higher levels of OT (Grewen et al., 2005, Light et al., 2004, Light et al., 2005, Turner et al., 1999).
The effects of AVP on sexual behavior and partner preference formation seem to be more important in males, especially considering the fact that AVP has been found to be more abundant in males (De Vries et al., 1994, Insel and Hulihan, 1995). In the monogamous prairie vole, administration of a V1aR antagonist inhibits the formation of partner preference that normally follows mating, while AVP administration alone can induce partner preference in the absence of mating (Cho et al., 1999). Consistent with these findings, differences in the neuroanatomical distribution of V1aR correlate with differences in pair-bonding behaviors between different rodent species (Insel et al., 1994). In human males, AVP is secreted during sexual arousal (Murphy et al., 1987). Additionally, as in rodent species, the V1aR also seems to be important in regulation of sexual and bonding behaviors. Walum et al. (2008) report an association between a polymorphism in the gene encoding V1aR (AVPR1a) and various dimensions of male partner bonding, including perceived marital problems, marital status and marital quality.
Intranasal administration of OT and AVP is one mechanism whereby the central effects of these peptides can be manipulated and studied in humans. Intranasal administration can deliver AVP, and almost certainly OT, to the brain without uptake into the peripheral circulation (Riekkinen et al., 1987, Born et al., 2002). Two routes have been proposed for the passage of these peptides from the nose to the brain: an intraneuronal and an extraneuronal pathway (Chen et al., 1998, Illum, 2000). Intraneuronal transport requires the internalization of the peptide into olfactory neurons, followed by axonal transport. However, the likelihood of lysosomal degradation and lengthy period for delivery to the olfactory bulb (Illum, 2000) suggest this is an unlikely route. It therefore seems more plausible that AVP and OT travel by the extracellular route, passing through patent intercellular clefts in the olfactory epithelium to diffuse into the subarachnoid space (Illum, 2000).
Several studies suggest intranasal OT administration may increase prosocial behaviors and improve social cognition in humans under certain circumstances—an effect that would presumably enhance engagement in both parental and romantic affiliative behaviors. For example, intranasal OT administration is associated with increased perceptions of attractiveness (Theodoridou et al., 2009), trustworthiness (Baumgartner et al., 2008, Kosfeld et al., 2005, Theodoridou et al., 2009), and approachability (Rimmele et al., 2009). OT administration has also been found to enhance various dimensions of social cognition such as emotion recognition in both healthy adults (Domes et al., 2007) and individuals with autism (Guastella et al., 2010) and social memory (Rimmele et al., 2009, Savaskan et al., 2008). Importantly, not all studies of intranasal OT administration report a significant main effect of OT on the aforementioned dimensions, and a few studies actually report negative effects (Declerck et al., 2010, Bartz et al., 2010a, Bartz et al., 2010b) or an increase in behaviors that are not considered prosocial, like jealousy and gloating (Shamay-Tsoory et al., 2009). A review of the extant literature by Bartz et al. (2011) concludes that the prosocial effects of OT in humans are likely highly context- and person-specific and likely the result of OT's combined effects on anxiety reduction, affiliative motivation and perceptual selectivity.
Recent work from the Feldman laboratory, suggests that intranasal OT can dramatically increase peripheral levels of OT and may also influence the peripheral levels of OT of individuals involved in dyadic interactions (Weisman et al., 2011). If replicated, this finding again raises the question of the nature of the interrelationship between the factors that influence and co-determine central and peripheral OT levels.
Administration of intranasal AVP in humans appears to have sex-specific effects (Thompson et al., 2006). Specifically, in men, AVP stimulates agonistic facial motor patterns in response to the faces of unfamiliar men and decreases perceptions of the friendliness of those faces. In contrast, in women, AVP stimulates affiliative facial motor patterns in response to the faces of unfamiliar women and increases perceptions of the friendliness of those faces. More recently, intranasal AVP administration to men was also found to selectively prime cognitive processing towards sexual words over other types of words (Guastella et al., 2011) and to increase stress reactivity only in situations in which men experienced social evaluations (Shalev et al., 2011).
3.2. Interactions with dopamine and related salience and reward pathways
The dopaminergic system and its interactions with OT have been implicated in various aspects of motivated states and affiliative behavior. Central to dopamine's (DA) role in supporting affiliative behaviors is its involvement in the process of hedonic transformation, linking sensory cues from the object of recognition and bonding with the reward system to enable and sustain the formation of selective social bonds (Leckman et al., 2005).
DA is synthesized from l-dihydroxyphenylalanine (l-DOPA) in the soma of neurons in the substantia nigra, hypothalamus, arcuate nucleus, VTA and zona incerta. The axons of these neurons project widely within the CNS via six main pathways with involvement in distinct functions: mesocortical (motivational and emotional responses), mesolimbic (reward and reinforcement behaviors), nigrostriatal (production of movement), tuberinfiundibular (regulation of prolactin secretion from the anterior pituitary), incertohypothalamic (innervation of the SON and PVN and related regions for control of endocrine function and sexual behavior) and diencephalospinal (spinal reflexes) (Baskerville and Douglas, 2010). Five DA receptor subtypes (D1, D2, D3, D4 and D5) have been identified. Of these receptors, D1 and D2 are the most abundant and are found in the striatum, cortex, hypothalamus, olfactory bulbs and substantia nigra.
There is substantial anatomical overlap between the OT and dopamine neuron populations. For example, OT is released from the MPOA, SON, and PVN of the hypothalamus (which mediates sociosexual behavior in rodents) and these regions are also rich in D2 receptors, suggesting DA released from the incertohypothalamic system may play a regulatory function in OT release in this region and consequently, in the regulation of associated sociosexual behaviors (Baskerville et al., 2009, Buijs et al., 1984, Decavel et al., 1987). Overlap in the OT–DA circuit also exists between the OT output from the PVN of the hypothalamus to the VTA, hippocampus and amygdala and the dopaminergic output from the VTA to the hippocampus, amygdala and NAcc, suggesting bidirectional interaction between the two systems (Baskerville and Douglas, 2010).
The prefrontal cortex and NAcc, which receive input from both OT and DA neurons and are rich in receptors for both systems, have been proposed as additional integrative sites that may underlie social attachment behaviors (Baskerville and Douglas, 2010, Gingrich et al., 1992, Smeltzer et al., 2006, Young, 1999). Dopaminergic stimulation has also been shown to induce OT secretion in both in vitro and in vivo studies (Argiolas, 1999, Bridges et al., 1976, Cameron et al., 1992, Melis et al., 1990, Melis et al., 1992, Succu et al., 2007) and stimulation of DA receptors in electrophysiological studies induces depolarization of OT hypothalamic cells (Mason, 1983, Yang et al., 1991).
Evidence suggests DA–OT interactions are involved in the neuromodulation of various aspects of mate-specific affiliative behavior, from the activation of the sexual response to the subsequent post-copulatory induction of bonding and partner preference. Both OT–DA and DA–OT pathways seem to be involved in the activation of penile erection. For example, intracerebroventricular administration of OT and DA independently facilitate penile erection (Arletti et al., 1985, Hull and Dominguez, 2007, Martino et al., 2005, Melis et al., 2005, Melis et al., 2006, Melis et al., 2007, Melis et al., 2009, Paredes and Agmo, 2004). Cross-deactivation of these two systems has also been observed, as penile erection following administration of OT or a DA agonist can be blocked by OTR blockade (Argiolas et al., 1987) or DA receptor antagonist (Martino et al., 2005), respectively.
Projections of OT neurons in the MPOA to the VTA (the origin of the mesocorticolimbic DA pathway that mediates behavioral responses to stimuli of salience) are important in the regulation and maintenance of social bonds, including human parenting. In the case of partner preference, mating-induced OT release may link sexual arousal and bonding by activating the mesolimbic dopamine circuit and causing release of DA from the NAcc (Aragona et al., 2003, Insel, 2003, Liu and Wang, 2003, Wang et al., 1999, Young and Wang, 2004). There is also evidence to suggest these effects may be reciprocal (Hammock and Young, 2006, Liu and Wang, 2003). Consistent with these suggestions, administration of OT or DA antagonists into the NAcc can block or attenuate the formation of partner preference (Waldherr and Neumann, 2007). Connections between the related neuropeptide, AVP, and DA in the NAcc may influence pair bonding perhaps in a gender specific fashion (Carter, 2007, Hammock and Young, 2006, Insel and Young, 2001).
The DA mesocorticolimbic pathway and its connections with the OT system are also important for the anticipatory (e.g., pup seeking) and consummatory (e.g., pup retrieval, pup LG) aspects of maternal behavior. Pups are an extremely salient stimuli for lactating dams – elevated DA in the NAcc is observed in response to pup suckling (Hansen et al., 1993) as well as in anticipation of LG (Champagne et al., 2004) and pup suckling elicits greater activation of the mesocorticolimbic DA system than either cocaine or food (Ferris et al., 2005, Turchan et al., 2001). Furthermore, following separation from pups, lactating dams will bar press many times for access to pups, but if the MPOA is lesioned, a significant reduction in bar-pressing behavior is observed (Lee et al., 2000). Human structural and functional neuroimaging studies also support the involvement of regions of the hypothalamus and the mesocorticolimbic DA system in perceived early attachment experience, specifically the quality of maternal care in childhood (Kim et al., 2010).
Local increases in DA levels within the NAcc are observed during nursing bouts (Champagne et al., 2004, Hansen et al., 1993) and variations in the magnitude of the NAcc DA signal correlate with the duration of pup LG (Champagne et al., 2004). Furthermore, injections of DA receptor antagonists result in deficits in maternal behavior (Keer and Stern, 1999, Numan et al., 2005), whereas improvements in dopaminergic signaling within the NAcc of dams with olfactory bulbectomies positively impacted their deficits in observed maternal behaviors (Sato et al., 2011). Evidence from Shahrokh et al. (2010) suggests this DA signal is OT-dependent. For example, infusions of OT into the VTA increases the DA signal observed in the NAcc, while infusion of an OT antagonist into the VTA obliterates differences in the magnitude of the NAcc DA signal observed between high and low LG dams. A significantly higher number of OT projections from the MPOA and PVN of the hypothalamus to VTA has been identified in high compared to low LG dams, suggesting individual differences in appetitive behaviors directed towards pups may at least in part be explained by differences in the number of MPOA-PVN projections and activity in the mesocorticolimbic pathway during interactions with pups (Shahrokh et al., 2010).
Along with DA, noradrenergic and serotonergic pathways are also involved in the salience and reward systems. The enzyme dopamine beta hydroxylase (Dbh) synthesizes two ligands for adrenergic receptors, epinephrine and norepinephrine. As reviewed below, Dbh knockout mice exhibit widespread deficits in maternal behavior (Thomas and Palmiter, 1997, Thomas et al., 1995, Thomas et al., 1998).
3.3. Interactions with sex hormones and the hypothalamic-pituitary-gonadal axis
The gonadal steroid hormones estradiol, the main estrogen in humans, and testosterone are likewise thought to figure prominently in the regulation of social behavior, in part through their developmental and direct effects on OT and AVP expression. Testosterone and estradiol are present in both sexes to varying degrees and synthesized from a common precursor, cholesterol. Testosterone is synthesized primarily in the Leydig cells of testes and to a lesser extent, in the adrenal gland. Estradiol is synthesized from the combined action of the theca (which produce testosterone) and granulosa (which convert testosterone to 17β-estradiol with the enzyme aromatase) cells of the ovaries. In men and postmenopausal women, extragonadal sites, including the brain, mesenchymal cells of adipose tissue, vascular endothelium and smooth muscle cells, as well as bone osteoblasts and chondrocytes, are more important sources of estradiol (Simpson, 2003).
Gonadal function, and consequently the synthesis of estradiol and testosterone, varies markedly over the lifespan and is driven by the release of hypothalamic Gonadotropin Releasing Hormone (GnRH), which in turn causes the anterior pituitary to release Follicle Stimulating Hormone (FSH) and Luteneizing Hormone (LH). In males, FSH induces spermatogenesis in Sertolli cells, while LH induces testosterone production by Leydig cells (which in turn also exerts positive feedback on the spermatogenic effects of Sertolli cells). The male HPG axis is regulated by negative inhibition at both the level of the anterior pituitary and hypothalamus by testosterone, as well as negative feedback on the anterior pituitary by Inhibin, a product of the Sertolli cells. In females, regulation of the HPG axis is cycle-dependent. During the follicular phase, FSH and LH secreted by the anterior pituitary induce estradiol secretion by follicular cells, which in turn exerts negative feedback on the anterior pituitary. Mid-cycle, rising estradiol levels exceed a critical concentration and subsequently begin to exert positive feedback on the anterior pituitary, ultimately leading to the FSH/LH surge associated with ovulation. During the luteal phase, LH and FSH primarily induce the secretion of progesterone from the ovaries, which then regulates the female HPG axis via negative feedback on the anterior pituitary. Variations in patterns and levels of gonadal hormonal secretions across the lifespan set into motion cascades of events with various important developmental consequences.
Gonadal steroids have profound biological and behavioral effects. In the periphery, prenatal and neonatal exposure to sex steroids guides key events in fetal phenotypic sexual differentiation, including differentiation of internal and external genitalia. Puberty is marked by the pulsatile and increased secretion of GnRH, which in turn drives greater FSH, LH, estradiol and testosterone secretion. These hormonal changes are responsible for the development and maintenance of secondary sex characteristics and later, ovum development, pregnancy maintenance and preparation of the female breast for lactation during the reproductive years. Finally, senescence is marked by increases in the levels and pulsatility of GnRH, LH and FSH, and decreases in estradiol.
In the brain, the conversion of testosterone into estradiol is catalyzed by the enzyme aromatase, whose expression in avian and mammalian species is restricted to the hypothalamic MPOA, limbic system and scattered neuronal populations in the cerebral cortex (Cornil et al., 2006). Sex hormones have important effects on the mammalian brain during development and can also alter brain function via both short and longer term pathways later on. In the short-term (seconds to minutes), estradiol and testosterone can exert transient non-genomic effects via induction of various second messenger signal transduction cascades (Balthazart et al., 2006, Heinlein and Chang, 2002). Binding to androgen or estrogen receptors can initiate transcription of target genes, resulting in a longer term genomic effect.
Unlike OT and AVP, estradiol and testosterone can both readily cross the blood–brain barrier to exert central effects. Prenatal exposure to gonadal steroids (from both the fetus’ circulating hormones and the hormones that enter fetal circulation via the placenta) guides patterns of connectivity between neurons in different brain regions and neuron death and survival, with the consequence that early exposure to androgens impacts sex-typical behavior across the lifespan (Arnold and Gorski, 1984, Collaer and Hines, 1995, De Vries and Simerly, 2002). One prominent example of the effects of prenatal androgen exposure is seen in the case of Congenital Adrenal Hyperplasia, whereby girls exposed to high levels of testosterone in the womb exhibit, for example, male-typical play behavior and drawings (Iijima et al., 2001, Nordenstrom et al., 2002, Swaab, 2007).
During adult life, estradiol and testosterone have been linked to the rapid activation of various socio-emotional behavioral profiles (many of which have also been linked to OT and AVP), including reproductive behaviors (sexual receptivity, frequency of copulatory behavior) and aggression (Balthazart and Ball, 2006, Mehta and Beer, 2010). Consistent with these findings, evidence suggests these steroid hormones directly modulate the function of the OT system, with estradiol potentiating the OT system, testosterone potentiating the AVP system and also affecting OT via aromatization of testosterone to estradiol. For example, estradiol administration is associated with increases in electrical excitability of OT-producing neurons in the PVN (Akaishi and Sakuma, 1985) and increases in the rate of transcription of the OTR gene (via the effect of estradiol on the gene's promoter region) (Quinones-Jenab et al., 1997). Fluctuations in estradiol levels during the estrous cycle produce parallel fluctuations in plasma OT and OTR mRNA levels (Bale et al., 1995, Ho and Lee, 1992, Sarkar et al., 1992; for a review, see Choleris et al., 2008).
3.3.1. Interactions between the OT/AVP and HPG axes and parental behaviors
It has been suggested that the pregnancy-related increases in estradiol and testosterone may help prime maternal behavior via the effects of these hormones on the OT system as steroid hormones have also been implicated in parental behaviors. In humans, administration of testosterone in women without children increases neural responsiveness towards infant crying (Bos et al., 2010) and increases in the levels of testosterone have been observed in pregnant women (Fleming et al., 1997). In fathers, infant crying is associated with increases in peripheral testosterone concentrations (Fleming et al., 2002).
Increased parental care in mice is also associated with increased aromatase activity (indicating higher conversion of testosterone to estradiol) in the brain (Trainor and Marler, 2002). In ewes, this parturition-induced increase in OT has also been shown to be potentiated by estradiol (Bridges, 1984, Keverne and Kendrick, 1992, Miller et al., 1989). Drawing from this evidence, it has been proposed that testosterone-mediated increases in AVP function and/or aromatization of testosterone to estradiol (which would increase central estradiol levels, in turn causing increases in OT synthesis) may activate caring behavior in the mother and father.
However, the most compelling evidence suggesting the HPG axis is crucial for maternal behavior comes from studies of mice that lack either aromatase (Ogawa et al., 1998, Spiteri et al., 2010a, Spiteri et al., 2010b) or estrogen receptor (ER) alpha (Pierman et al., 2008), which are discussed in the genetics section.
3.3.2. Interactions between the OT/AVP and HPG axes and sexual behavior and the formation and maintenance of adult pair bonds
Reduced expression of ER alpha in the ventro medial nucleus of the hypothalamus is associated with deficits in the entire repertoire of female rat sexual behavior, including absence of sexual incentive motivation (mate approach) as well as deficits in copulatory behaviors (failure to exhibit the lordosis response and proceptive behaviors such as ear wiggling, hopping and darting, and increased rejection of sexual advances by male stud mice) (Ogawa et al., 1998, Spiteri et al., 2010a, Spiteri et al., 2010b).
Bos et al. (2011) propose a model whereby gonadal steroids and neuropeptides jointly influence the motivation for social behavior via their action on the amygdala. Specifically, in contexts characterized by social challenge, testosterone acts in concert with AVP to reduce fear and increase sympathetic efference, amygdala output to the brainstem and motivation to act; in contrast, in contexts deemed to be safe, estradiol and OT increase parasympathetic efference and inhibit amygdala output to the brainstem, enabling greater prefrontal activity and OT-dopamine interactions, which would function to facilitate bonding (Bos et al., 2011). Consistent with this hypothesis, Riem et al. (2011) performed a randomized controlled trial to examine the influence of intranasally administered OT on maternal neural responses to infant crying. Intranasal OT significantly reduced activation in the right amygdala and increased activation in other limbic and frontal regions.
3.4. Arousal, anxiety, threat detection and the stress response in social bonding
The interaction between stress and affiliation is complex. Anatomically, the PVN – one of the nuclei where OT is produced in the brain – is also a key structure in the HPA axis. Specifically, the PVN is also the sole site of corticotropin releasing hormone (CRH) production. From the PVN, CRH is transported to the anterior pituitary, stimulating adrenocorticotropic hormone (ACTH) release, thereby activating the HPA axis and prompting the release of cortisol (CORT) from the adrenal gland. As in the case of the HPG axis, HPA axis function is regulated by negative feedback effects, in this case exerted by corticosteroids at the level of both the hypothalamus and pituitary. Indeed, Dabrowska et al. (2011) recently presented neuroanatomical evidence for reciprocal regulation of the CRH and OT systems in the hypothalamus and the bed nucleus of the stria terminalis in rats.
Elevated concentrations of stress hormones have been reported in humans during periods of falling in love and during the transition to parenthood (Carter, 1998, Marazziti and Canale, 2004). The HPA axis steroid CORT has been consistently implicated in human maternal behavior and responsiveness to the infant (Fleming et al., 1993, Fleming et al., 1997, Maestripieri, 2001, Stallings et al., 2001), however, the associations between CORT and parenting have been shown to be complex, depending on multiple factors including maternal age, prior experience, and feeding patterns (Krpan et al., 2005). OT has been reported to have negative (Altemus et al., 1995, Heinrichs and Domes, 2008, Heinrichs and Gaab, 2007, Meinlschmidt and Heim, 2007), positive (Hoge et al., 2008, Marazziti et al., 2006, Taylor et al., 2006, Tops et al., 2007) as well as non-significant (Gordon and Feldman, 2008, Levine et al., 2007) correlations with CORT.
One mechanism to explain the prosocial effects of OT is that the OT system reduces anxiety, especially social anxiety (McCarthy et al., 1996, Heinrichs and Domes, 2008). The transition to parenthood is known to be one of the most stressful periods in parents’ lives accompanied by worries and preoccupation around the newborn that is introduced into the emerging family system and requires substantial caretaking and resources from both parents (Cowan and Cowan, 1992, Cox and Paley, 1997). Similarly, bio-behavioral as well as mental aspects of stress come into play during periods of falling in love with a romantic partner, which includes preoccupations, worries about reciprocity, and increased stress and anxiety (Leckman et al., 1999). Activation of the stress response is not considered pathological during bond formation periods; in fact, lack of preoccupation or singular focus on the loved one during these times is considered abnormal. To some extent, stress may be an integral part of bonding and it may facilitate idealization of the other and increased focus and attention on the emerging attachment. Evidence in support of this hypothesis comes from work on male–female pair bond formation in male prairie voles (De Vries et al., 1996). Winnicott's (1956) notion of “primary maternal preoccupation” is a prime example of a theoretical construct that incorporates aspects of mental stress in normative maternal bonding to the infant. OT is also thought to mediate the calm state associated with breastfeeding (Neumann and Landgraf, 2008, Uvnas-Moberg, 1997, Uvnas-Moberg, 1998a, Uvnas-Moberg, 1998b).
An anxiety reduction hypothesis has also been proposed to explain some of the prosocial effects of intranasal OT, such as increasing trust and social approach (Heinrichs and Domes, 2008). This reduction in anxiety might also account for some of the selectively beneficial effects of intranasal OT for individuals on the autism spectum, given that social anxiety is frequently an important part of their clinical presentation (Amaral et al., 2008, Bartz et al., 2010a, Bartz et al., 2010b).
3.5. Interactions with the immune system
Some preliminary evidence suggests that the OT system may play a role in the modulation of immune function, perhaps by acting as a link between the neuroendocrine and immune systems (Hansenne, 2005, Macciò et al., 2010, Melis et al., 1993). Specifically, OT is produced in the thymus (Elands et al., 1990). Within the thymus, OT is expressed in thymic nurse cells and is thought to play a role in the maturation and differentiation of T-cell lymphocytes by acting as the self-antigen of the neurohypophysial family (Geenen et al., 1986, Geenen et al., 1988).
Administration of OT has been found to increase the mitogenic response of peripheral blood mononuclear cells to administered phytohemagglutinin (PHA), as well as the expression of CD25 (a transmembrane protein present on activated T-cells) and CD95 (a receptor protein that mediates apoptotic signaling). When administered in conjunction with estradiol, OT can counteract the immunosuppressive effects of estradiol on PHA-induced mitosis of peripheral blood mononuclear cells (Macciò et al., 2010). OT may also be involved in the regulation of T-cell lymphocytes. For example, Yamaguchi et al. (2004) found that OT can attenuate the neuroendocrine and cytokine response to a bacterial endotoxin (Clodi et al., 2008). Other investigators have reported that high levels of OT are associated with accelerated wound healing (Gouin et al., 2010). In addition, Ndiaye et al. (2008) identified both OT and the OTR on all major T-cell populations within the bovine corpus luteum and demonstrated that administration of OT induces calcium influxes in T-cells, suggesting OT may be involved in the regulation of immune cell activity within the corpus luteum.
3.6. Involvement of other peripheral organ systems
As depicted in Fig. 1, in addition to the thymus (Elands et al., 1990), OT and closely related peptides are synthesized in a number of somatic organ systems including the gastrointestinal tract (Ohlsson et al., 2006), heart (Danalache et al., 2010, Jankowski et al., 1998), pregnant intrauterine tissue (Chibbar et al., 1993, Mitchell et al., 1998), ovaries (Ivell and Richter, 1984), as well as the testis, epididymis and prostate (Ivell et al., 1997).
OT secreted from the posterior pituitary as well as these peripheral sources engage receptors in multiple sites, enabling the neuropeptide to play diverse roles in the maintenance of homeostasis. Peripheral OT targets include the mammary gland (Kimura and Ivell, 1999), ovary (Fuchs et al., 1990), uterine endometrium (Kimura et al., 1992) and myometrium (Fuchs et al., 1984), amnion, chorion and decidua (Chibbar et al., 1993), testis (Ivell et al., 1998), epididymis and prostate gland (Frayne and Nicholson, 1998), vascular endothelium (Thibonnier et al., 1999), heart (Gutkowska et al., 1997), and kidney (Schmidt et al., 1990).
Consistent with this pattern of distribution, OT exerts diverse peripheral effects, including milk ejection (Moos and Richard, 1989, Nishimori et al., 1996), initiation of parturition, contraction of ejaculatory tissues (Thackare et al., 2006), analgesia (Kordower and Bodnar, 1984), as well as trophic effects on myometrial cells (Devost et al., 2005). OT also regulates body fluid levels and cardiovascular homeostasis via its interactions with atrial natriuretic peptide (Gutkowska et al., 1997), influences pancreatic hormone secretions and circulating levels of glucagon, glucose and insulin (Bjorkstrand et al., 1996) and attenuates the synthesis or secretion of CORT by the adrenal cortex (Legros et al., 1988); for a review, see Gimpl and Fahrenholz (2001), Landgraf and Neumann (2004), and Neumann and Landgraf (2008). OT may also be involved in regulation of gastric motility (McCann and Rogers, 1990), and along with AVP, thermoregulation (Lipton and Glyn, 1980). AVP also has several peripheral targets and is involved in diverse homeostatic functions including regulation of water reabsorption by the kidney and peripheral vascular resistance.
4. Genetic and epigenetic influences
4.1. Genetic influences
The gene for the OT peptide is located in chromosome 20 in humans (Summar et al., 1990) and in chromosome 2 in the mouse (Hara et al., 1990). It consists of three exons, which together encode for the signal peptide, the nonapeptide, a tripeptide processing peptide and the carrier molecule for OT, neurophysin I (for a review, see Winslow and Insel, 2002).
The development of OT knockout (OTKO) mice (Gross et al., 1998, Nishimori et al., 1996, Young et al., 1996) was an important contribution to the existing pharmacologically based OT literature. While OTKO mice lack the milk ejection reflex and thus cannot lactate, other aspects of maternal behavior, associated with the OT system, such as parturition, initially appeared to be intact (Nishimori et al., 1996, Young et al., 1996). However, subsequent and more in depth studies of these mice revealed that nulliparous OTKO mice showed decreased levels of pup retrieval and LG (Pedersen et al., 2006) and were significantly more likely to engage in infanticidal behavior than wild-type mice in the same environment (Ragnauth et al., 2005). Compared to wildtype mice, OTKO mice also exhibit increased aggression in both isolation and resident-intruder paradigms (Winslow et al., 2000) and fail to recognize familiar conspecifics (Ferguson et al., 2000, Ferguson et al., 2001), an effect which may result from abnormal processing of olfactory social stimuli in the medial amygdala (Winslow and Insel, 2002).
It is also clear from the work of Higashida and colleagues that the endogenous production and proper secretion of OT is key to the maintenance of social recognition and maternal behavior (Higashida, 2010; Lopatina et al., 2011). They studied animals that lacked the gene for CD38, a transmembrane glycoprotein with ADP ribosyl cyclase activity, whose proper function is required for calcium-induced calcium release for OT secretion in hypothalamic neurons. Prior to the weaning period, no behavioral differences were noted between CD38 knockout and wildtype mice. However, post-weaning, CD38 knockout mice exhibited decreases in plasma OT and elevations in hypothalamic and pituitary OT concurrent with impaired social recognition (Higashida et al., 2010). In a subsequent study they also reported deficits in maternal behavior that were in part conditioned by whether the CD38 knockout dams were primiparous or multiparous (Lopatina et al., 2011).
The gene for the OTR is found on chromosome 3 in humans (Kimura et al., 1992) and chromosome 6 in mice. It consists of 3 introns and 4 exons and codes for a 388-amino acid polypeptide with seven transmembrane domains typical of G protein-coupled receptors. OTR knockout (OTRKO) mice demonstrate deficits in lactation. In the postpartum period, the OTRKO dams also display longer latencies to retrieve pups and spend less time crouching over their pups (Takayanagi et al., 2005). Interestingly, when the OTR gene was conditionally knocked out in the forebrain only, females were able to lactate (Lee et al., 2008); this may in part be due to the low selectivity of the OTR and the capacity of AVP to function as a partial agonist for the receptor in the absence of the preferred ligand (Chini et al., 1996, Kimura et al., 1994, Postina et al., 1996). The male adult OTRKO mice also display deficits in social discrimination and elevated aggressive behavior, obesity and dysfunctions in temperature regulation (Nishimori et al., 2008). Importantly, the OTRKO pups make fewer vocalizations in response to social isolation (Nishimori et al., 2008). Similar findings have been reported for pups lacking the mu-opioid receptor gene (Moles et al., 2004).
In human studies variations in the OTR gene have been associated with observed maternal sensitivity during a series of problem-solving tasks between mother and 2 year old toddlers (Bakermans-Kranenburg and Van IJzendoorn, 2008). Allelic variations in OTR gene have also been associated with women's tendency to give birth at a relatively earlier age (Prichard et al., 2007). Replication of these studies is needed as are studies to determine of these are indeed functional polymorphisms.
In addition to the genes coding for OT and the OTR, several other genes related to the OT circuitry have been identified as important for affiliative behaviors. For example, dams lacking the alpha-subunits of the two main members of the G-protein family needed for the OTR to function [Galpha(q/11) and Galpha(11)] also show profound deficits in maternal behavior in that they do not display nest building, pup retrieval, crouching or nursing (Wettschureck et al., 2004).
Concerning the HPG axis, gene knockout studies have found that mice lacking a key enzyme involved in estrogen synthesis, aromatase, display deficits in social recognition (essential for establishing the mother–infant bond) and AVP activation (Pierman et al., 2008). They also report that the exogenous administration of estrogen recovers social recognition and AVP activation. In addition, ER alpha knockout mice are less likely to engage in appropriate sexual behaviors (e.g., lordosis) and typical parental behavior (e.g., pup retrieval) (Ogawa et al., 1996, Ogawa et al., 1998). These dams were also more likely to engage in infanticide. In a subsequent study, Spiteri et al., 2010a, Spiteri et al., 2010b localized these effects, finding that decreased receptor expression in the posterodorsal amygdala is associated with deficits in social recognition and decreased anxiety, while decreased receptor expression in the ventromedial nucleus of the hypothalamus results in increased aggression towards novel juveniles.
With regard to the social salience pathway, mice lacking the dopamine transporter (DAT) gene have been characterized by high extracellular DA levels, spontaneous hyperlocomotion and marked deficits in maternal behavior (Spielewoy et al., 2000). These DAT knockout mice were fertile, but the percentage of pregnant females was lower and they had smaller litters compared to wildtype dams. Although there were no genotype differences in the first contact or first retrieval, the knockout mothers spent less time in the nest, took a longer time to regroup all the pups in the nest and spent more time between retrievals.
Similarly, the loss of a key enzyme in the synthesis of norepinephrine, Dbh, is also associated with deficits in maternal behavior (Thomas and Palmiter, 1997, Thomas et al., 1995, Thomas et al., 1998). Mice homozygous for the Dbh mutation die in utero of apparent cardiovascular failure (Thomas et al., 1995). However, these mice could be rescued by provision of adrenergic agonists or a synthetic precursor of norepinephrine, l-threo-3,4-dihydroxyphenylserine (DOPS), in the maternal drinking water (Thomas et al., 1995). The majority of these rescued animals became viable adults. In a subsequent study, Thomas and Palmiter (1997) demonstrated impaired maternal behavior across virtually all domains evaluated. Pups were observed scattered within the bedding around the nest. Often pups were not cleaned, and their placentas remained attached. Milk was not detected in the stomachs of most pups born to these knockout dams, which suggests that the pups were not nursing despite the presence of normal mammary gland tissue.
Timing is critical to the relationship between the presence of Dbh and the induction of maternal behaviors. Specifically, while administration of a norepinephrine precursor, l-threo-3,4-dihydroxyphenylserine (DOPS), in the evening prior to birth is associated with some recovery of maternal behavior, administration of DOPS in the morning after birth does not result in recovery. The greatest recovery is observed when DOPS is administered both before and after birth, suggesting norepinephrine may play a role in continuously realigning the dam's sense of what is salient as the object of salience evolves (Thomas and Palmiter, 1997). Interestingly, in 85% of the mutant females, the rescue of maternal behavior by DOPS extended to the mother's subsequent pregnancies even in the absence of DOPS injections (Thomas et al., 1998). This observation again reinforces the view that initial conditions are critically important and that once the neural processes associated with maternal behavior are initiated, they are, to some degree, self-sustaining.
Another gene crucial for the emergence of maternal behavior is FosB, an immediately early gene. Postpartum dams lacking FosB exhibit decreased pup retrieval, nursing and nest building, an effect presumably mediated at least in part by decreased FosB protein expression in the MPOA, an OT, estradiol and prolactin-sensitive region involved in maternal behavior (Brown et al., 1996). In addition genes coding for specific transcription factors (Peg3, Fkh5), enzymes (Mest/Peg1, neuronal nitric oxide synthase) and the prolactin receptor have also been shown to be important for diverse aspects of maternal behavior (Gammie and Nelson, 1999, Lefebvre et al., 1998, Li et al., 1999, Lucas et al., 1998, Ormandy et al., 1997, Wehr et al., 1997).
In sum, more than a dozen genes have been identified that disrupt various aspects of maternal behavior in rodents and there are at least two genes that reduce the level of ultrasonic vocalizations in response to maternal separation. They include the genes for OT and the OTR as well as genes that are essential for the function of the HPG axis and DA and noradrenergic central salience pathways. These findings offer compelling proof that the neurobiological systems that depend on the normal expression of these genes are, indeed, essential for normal patterns of dam-pup dyadic behavior to appear at a key point in development. Some of these findings from the DBH knockout studies also indicate that the circuitry required for the initiation of maternal behavior may not be crucial for its maintenance or re-initiation at the birth of subsequent litters (Thomas et al., 1998).
4.2. Epigenetic influences
Aspects of the intrauterine and early postnatal environments can have an enduring impact on the social development of mammals. One strand of the literature on epigenetic effects draws from studies on the effects of naturally occurring variations in pup LG, a measurable and stable maternal phenotype in female rodents (Francis et al., 1999). Pup LG is an important source of tactile stimulation and enhances brain development and plays roles in the regulation of endocrine and cardiovascular function in the neonatal rat (Schanberg et al., 1984). Patterns of LG exhibit intergenerational transmission so that the offspring of high LG mothers go on to become high LG mothers themselves and vice versa for low LG mothers (Francis et al., 1999). Cross-fostering studies further highlight the importance of the early environment, with pups born to low LG mothers but raised by high LG mothers exhibiting high LG behavior; conversely, high LG pups raised by low LG mothers exhibit low LG as mothers. Intergenerational transmission of maternal behavior is not unique to rodent models and has also been observed in various primate species including humans (Krpan et al., 2005, Maestripieri, 2005, Miller et al., 1997). Although the exact mechanisms whereby pup LG induces epigenetic effects remain to be fully elucidated, there is a growing literature documents the existence of maternally induced epigenetic effects, whereby differences in maternal care received in early life induce structural alternations in DNA that permanently alter gene expression in specific brain regions (for a review, see Kappeler and Meaney, 2010). This environmental programming of gene expression is thought to occur via the mechanism of DNA methylation. Presumably, this phenotypic plasticity is an adaptive response that has been shaped by natural selection, activating and matching different phenotypic programs to parental cues that are supposedly reflective of likely future environmental demands (Hinde, 1986).
Thus far, epigenetic changes have been documented in several genes that play key roles in the HPG and HPA axes. For example, Weaver et al. (2004) report that increased pup LG and arched-back nursing (ABN) by rat mothers altered the methylation of the promoter region of the glucocorticoid receptor (GR) gene in the hippocampus, such that significant differences in methylation patterns emerged between offspring of low versus high LG and ABN dams. Of note, these differences emerged over the first week of life and persisted into adulthood, but could be reversed with cross-fostering. They were associated with altered histone acetylation and transcription factor (NGFI-A) binding to the GR promoter. The exact mechanisms whereby pup LG induces epigenetic effects remain to be fully elucidated, but cytosine methylation in the pups of high LG mothers has been directly observed during the first week of life (Champagne and Curley, 2008, Champagne and Curley, 2009, Champagne et al., 2003).
Similar epigenetic alterations have been reported in the promoter region of the GR gene in the hippocampus of humans who were suicide victims with a history of childhood abuse (McGowan et al., 2009). Compared to controls, these individuals exhibited increased methylation of the neuron-specific glucocorticoid receptor (NR3C1) promoter gene, along with decreased levels of GR mRNA and GR 1F splice variant mRNA transcripts. These findings bridge the gap between the animal and human literature, suggesting common epigenetic mechanisms mediate the effects of parental care on function of the HPA stress response system of the offspring.
Other epigenetic modifications may also have an impact on hippocampal function. For example, Zhang et al. (2010) reported changes in the methylation of the glutamic acid decarboxylase 1 (GAD1) gene in the hippocampus. Compared with the offspring of low-LG mothers, those reared by high-LG dams showed enhanced hippocampal GAD1 mRNA expression, decreased cytosine methylation, and increased histone 3-lysine acetylation of the GAD1 promoter.
Roth et al. (2009) have also reported that early-life adversity can leave lasting epigenetic marks in the brain-derived neurotrophic factor (BDNF) gene in the adult prefrontal cortex. They also observed a transgenerational effect such that altered BDNF DNA methylation was also observed in the offspring of females that had previously experienced the maltreatment regimen.
Epigenetic modifications have also been reported in some of same genes needed to initiate and sustain maternal behavior. Specifically, Champagne et al. (2006) found that the female offspring of high LG mothers showed increased ER alpha expression in the MPOA. Cross-fostering studies confirmed this association between maternal care and ER alpha expression in the MPOA. These findings suggest that maternal care is associated with cytosine methylation of the promoter, providing a potential mechanism for the programming of individual differences in ER alpha expression and maternal behavior in the female offspring.
These data from animal and human studies indicate that the interval surrounding birth is a critical period in the life of mammals – one that is likely to have enduring neurobiological and behavioral consequences, especially since these epigenetic changes can be passed on from generation to generation. If epigenetic programming of these genes does indeed take place, then the early epigenetic programming of many more genes is likely to occur some of which will influence the emergence of a wide variety of social behaviors. For example, there is now preliminary data to suggest that the OTR is differentially expressed and methylated in some individuals with autism. Specifically, Gregory et al. (2009) found statistically significant increases in the DNA methylation of the OTR in the peripheral blood cells and temporal cortex of individuals with autism as compared to age and sex matched controls. This increase in methylation was associated with reduced OTR mRNA levels in the temporal cortex tissue derived from these individuals. If replicated, this could be a major advance in our understanding of the biological factors underlying this disorder.
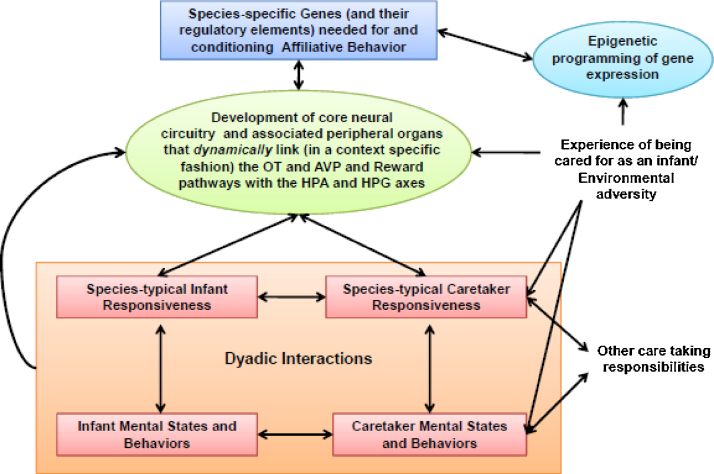
5. An emerging systems model
Drawing on the extant literature, we offer a tentative bio-behavioral model that focuses on the interface of several highly conserved neural systems involved in perception, arousal, maintenance of physiological homeostasis, and stress regulation, as well as reward and threat detection and response pathways (Fig. 2). Motivation and our OT systems are key ingredients in this model, but they do not work alone. We propose that in the course of evolution, natural selection has led to a species-specific integration of these systems producing the diversity of affiliative behaviors observable today. In our estimation, the utility of this integrative model comes in identification of which of these biobehavioral components are active in any one of a range of adaptive and maladaptive outcomes. Finally, we encourage investigators to explore how these systems are interacting with each other at each level of analysis, from the genome and epigenome to social behavior to metacognitive representations (Fig. 2). We believe this emerging model will provide a fruitful point of orientation for scientists from a range of disciplines to integrate their work and evaluate new experimental and therapeutic interventions as they develop this model and seek to inform social policy.
Fig. 2.
Integrative model. An integrative model of biobehavioral processes involved in the formation and maintenance of caretaker–child bonds and related attachment phenomena. “Responsiveness” includes: (a) a range of perceptual, autonomic, behavioral, emotional and cognitive responses conditioned by the absence, presence, or merely cues of the other; (b) responses conditioned by the caretaker's experience of being cared for as an infant; and (c) responses conditioned by perceived threats and adversity. By entering into a synchronous affective dyadic communication with the infant, the caregiver provides an external support for the infant's developing bioregulatory abilities and thereby conveys lifelong resilience to stress and enhanced coping ability. The experience of the caregiver and child's micro-level matching of affective states and level of arousal during face-to-face dyadic interactions beginning around the second month of life provides the basis for children's self awareness, social development, empathy, and moral internalization. Maternal gaze matching, facial expressions, vocalizations, and regulation of arousal states during face-to-face play provide critical environmental inputs during the sensitive period of maturation of the visual cortex. Furthermore, by synchronizing with infant arousal state, mothers entrain the infant's biological rhythms, providing a “resonance” of internal and external experience, self and other, brain and behavior. Metacognitive representations and mental states correspond to internal working models and are continually being updated and revised. However, one's earliest experiences with caregivers can powerfully shape the landscape of possible and expected interactions with others. For example, stranger anxiety appears when infants experience a mismatch between expected caregiver stimuli and stimuli that the infant encounters when exposed to an unfamiliar person. These metacognitive representations and mental states can be both conscious and unconscious and can directly influence both patterns of responsiveness in the self and others as well as the core neural circuitry and associated peripheral organs that dynamically link (in a context specific fashion) the oxytocin (OT) and arginine vasopressin (AVP) and the dopamine dependent reward pathways along with the hypothalamic-pituitary-adrenal (HPA) and the hypothalamic-pituitary-gonadal (HPG) axes (see text).
6. Critique of model and questions for future research
While the extant literature provides strong support for this model, many questions remain regarding the role of the OT system in social motivation. Moving forward, we must continue to disentangle the genomic and epigenomic mechanisms through which the HPA and HPG axes and the other systems that we examined interact with the OT/AVP system. We also need to examine how environmental conditions influence dyadic social interactions at key points in development and contribute to mold our social repertoires. Noting that these relationships are dynamic and may vary widely across species, it will be important to investigate these effects more thoroughly in humans and at different points in time in the formation and maturation of parental and romantic bonds. A clear understanding of the molecular events that underlie the various components of social motivation and affiliative behaviors, as well as how central and peripheral OT release are coordinated is also missing from the literature. Box 1 offers specific questions for future research. The various gaps in the literature and questions elicited by our model are challenges for future researchers and we hope that our emerging model will provide a framework from which new hypotheses can be formulated and evaluated. Eventually, an understanding of these processes could be instructive in the treatment of various disorders characterized by altered or absent social motivation including social phobia, post-traumatic stress disorder, depression, obsessive-compulsive disorder, schizophrenia and autism, and also in the development of preventative interventions and social policy (Leckman and Mayes, 2007).
Box 1. Questions for future research.
-
1.
How do the peripheral and central oxytocin (OT) systems interact moment-to-moment over the course of development to influence social motivation? Although several research groups are reporting relatively stable levels of peripheral levels of OT, what does this mean in specific social and developmental contexts (falling in love vs. early parenting)? To date, individual stability has been reported over the course of several months but can it be observed over several years and across developmental transitions? How does the OT system function during periods of rapid brain reorganization (e.g., puberty) versus those of relative developmental stability? Is the source of OT being measured in the plasma and saliva entirely derived from the posterior pituitary or do somatic sources such as the GI tract also contribute? If peripheral sources do contribute, what regulatory mechanisms guide this process and do they differ depending on gender, age or social context? What role do sensory afferents and the autonomic nervous system play as we seek to understand the brain–body interface and the role of OT in social motivation within dyadic relationships?
-
2.
Are the effects of OT on prosocial and affiliative behaviors causal or are they secondary to the anxiolytic effects of OT or the increased saliency of social cues related to OT? As reviewed by Bartz et al. (2011), the acute effects of OT on social cognition and prosocial behaviors are variable depending on context and the individual's relevant attributes. Additional research is needed to understand these individual differences.
-
3.
Are clinical trials of intranasal OT justified in children with autism or other neuropsychiatric disorders? Several studies are underway to examine the safety and efficacy of intranasal OT. Given the known risks and benefits, are there additional preliminary studies that should be performed? Are there any long-term risks associated with repeated OT exposure?
-
4.
Can OT administration be used to improve parenting in conditions associated with disruptions to the maternal–infant bonding, such as post-partum depression and premature birth? The numbers of women suffering postpartum depression and those of prematurely born infants increase significantly each decade and carry known risks to the initiation of parenting. Disruptions to OT functioning in these conditions have also been reported. What are the risks and benefits to using OT administration as a therapeutic agent in such conditions?
-
5.
What are the effects of trauma on the functioning of the OT system? Exposure to trauma carries a multi-dimensional effect on well-being and stress proneness but its impact on OT is still unknown. What are the differential effects of continuous versus acute trauma? Trauma experienced in early childhood versus that experienced at later stages?
-
6.
What are the effects of differing levels (central and peripheral) of OT on interpersonal interactions within the family and beyond? Recently, the Mother Child Education Foundation (Anne Çocuk Eğitim Vakfı), based in Turkey, suggested that family- and community-based programs designed to enhance early child development can alter patterns of decision making within the families and communities, so that there is greater “peace” and less conflict within families as well as within the larger community. Based on the preliminary report by Weisman et al. (2011), it may be reasonable to monitor peripheral levels of OT before and after such interventions
-
7.
We have described the involvement of OT in bond formation but what are the effects of bond dissolution on OT? If OT functions to maintain long-term affiliative bonds, what happens when we fall “out of love” or when individuals lose partners due to divorce or death? Alternatively, what happens if someone remains ‘in love’ despite being rejected by the other?
-
8.
How variable is the distribution and density of OT receptors in humans and do individual differences vary depending on gender, developmental stage and the initiation of intimate dyadic relationships? In various animal species the number and distribution varies by sex and developmental stage. To what extent is this true of our human species, particularly when new dyadic parent–child or adult romantic relationships are being formed?
-
9.
To what extent are central or peripheral OT receptors sensitive to epigenetic modification? Given the preliminary findings in the temporal cortices and peripheral blood of a small number of autistic subjects (Gregory et al., 2009), do epigenetic modifications of the OT receptor matter? Can these changes be transmitted from generation to generation? What is the impact of differing patterns of OT receptor methylation in specific brain regions and somatic tissues on social behaviors and motivations? Under what circumstances can these epigenetic changes be reversed?
7. Conclusions
As briefly summarized in Box 2, for over 100 years, a growing literature has implicated OT in a wide variety of physiological and behavioral functions that sustain mammalian survival and social behavior across all phases of the life cycle. Drawing from a vast literature that incorporates findings from both animal and human data and various investigative techniques, we propose a biobehavioral model whereby OT interfaces with various highly conserved neural systems – the dopaminergic reward pathway, the HPG and HPA axes, the social engagement system for arousal and threat detection, and various perceptual systems—to modulate social behavior and cognition in mammalian species. At birth and in early life, the evidence suggests that these interactions mediate key events in reproductive physiology (parturition, milk letdown) as well as the bonding and parenting behaviors necessary for our survival. As we emerge into adulthood, OT seems to underlie other critical elements of our sociobehavioral repertoire, including mate affiliation and bonding, social recognition, sexual incentive and copulatory behaviors, and aggression.
Box 2. Highlights.
The oxytocin (OT) system is a key element in social motivation.
-
•
OT is produced in the hypothalamus, the heart, thymus, GI tract, and reproductive organs.
-
•
It is implicated in sexual behavior, pair bonding, and parenting.
-
•
It interacts with the reward, sexual behavior, sensory, and stress response pathways.
-
•
It is sensitive to timing and is context-dependent and epigenetically programmed.
-
•
Peripheral measures of OT are individually stable and associated with dyadic affiliative behaviors.
Importantly, in addition to its involvement in the activation of these various timing-dependent and stage-specific behavioral profiles, the OT system also seems to play a central role in ongoing homeostatic processes, interacting with the HPG and HPA axes and the reward circuitry at multiple levels, and exerting its own effects in the periphery to maintain basal homeostasis in response to changing environmental demands. Literature on the genetics of the OT system suggests interspecies and inter-individual differences in the function of the OT system and affiliative behaviors may be to a large degree hardwired. However, it is also evident that the OTR system is highly nuanced and exhibits substantial plasticity, being responsive to early environment via epigenetic feedback effects that have long-lasting consequences.
What is the origin of the factors that motivate us to engage in social bonds? Apart from the evolutionary importance of successful reproduction, it is also important to reflect on our origins and the “oneness” that characterized the earliest periods of our biological and emotional development. It is possible that the forceful motivation and desire to bond lies in the longing to return in some ways to that initial state when we are a part of another, seen and experienced by our parents and under optimal conditions having our needs met, intimately and fully. Just as we are programmed to bond, an integral part of the human condition is also to try and understand the origins of our own motivation to engage in social behavior.
Behavioral, neurobiological, genetic, and epigenetic data relevant to social bonding in our human species and in model mammalian systems have the potential to inform our understanding of social motivation as well as to guide aspects of clinical practice, particularly early intervention programs for high-risk expectant parents. “Good enough” genes combined with “good enough” parental care are needed to ensure positive outcomes in childhood and beyond. Among these positive outcomes is the development of resiliency to subsequent adversities in life, the capacity to be a good enough parent for the next generation, and possibly improved physical health. Consequently, it is possible that effective early intervention programs may have enduring positive consequences extending to subsequent generations.
Close collaborations between clinicians and the designers of model intervention programs have been long standing. These collaborations are now beginning to include neuroimagers, developmental and behavioral neuroscientists, geneticists, and immunologists. Our capacity to study genes, the development of the brain and the determinants of immunological health has never been stronger. Future studies should permit the examination of how successful early intervention programs influence brain development, problem solving abilities, and stress responses and vulnerability to mental and physical illnesses.
While OT is a key element in this unfolding story, it is surely not the only one. Moving forward, we must continue to untangle the relationships between OT and other players in our highly complex internal biological and emotional worlds and external social environment, and how they produce the rich and highly nuanced, dynamic dyadic interactions that are characteristic of our species.
Acknowledgements
The authors thank C. Sue Carter for her detailed critique and suggested edits to this review. Lee Foundation, Singapore (IG); the National Institute of Mental Health (CM, JFL: R25MH077823; JFL: K05MH076273); the US-Israel Binational Science Foundation (2005-273, RF, JFL); the Institute for the Study of Unlimited Love (JFL); the National Alliance for Research on Schizophrenia and Depression (RF); and the Associates of the Yale Child Study Center.
References
- Ainsworth M.D.S., Blehar M.C., Waters E., Wall S. Erlbaum; Hillsdale, NJ: 1978. Patterns of Attachment: A Psychological Study of the Strange Situation. [Google Scholar]
- Akaishi T., Sakuma Y. Estrogen excites oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Res. 1985;335(2):302–305. doi: 10.1016/0006-8993(85)90481-0. [DOI] [PubMed] [Google Scholar]
- Altemus M., Deuster P.A., Galliven E., Carter C.S., Gold P.W. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J. Clin. Endocrinol. Metab. 1995;80(10):2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Amico J.A., Finley B.E. Breast stimulation in cycling women, pregnant women and a woman with induced lactation: pattern of release of oxytocin, prolactin and luteinizing hormone. Clin. Endocrinol. 1986;25(2):97–106. doi: 10.1111/j.1365-2265.1986.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Aragona B.J., Liu Y., Curtis J.T., Stephan F.K., Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 2003;23(8):3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A. Neuropeptides and sexual behaviour. Neurosci. Biobehav. Rev. 1999;23(8):1127–1142. doi: 10.1016/s0149-7634(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Gessa G.L. Central functions of oxytocin. Neurosci. Biobehav. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Melis M.R. Neuromodulation of penile erection: an overview of the role of neurotransmitters and neuropeptides. Prog. Neurobiol. 1995;47:235–255. [PubMed] [Google Scholar]
- Argiolas A., Melis M.R. The role of oxytocin and the paraventricular nucleus in the sexual behavior of male mammals. Physiol. Behav. 2004;83:309–317. doi: 10.1016/j.physbeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Melis M.R., Vargiu L., Gessa G.L. d(CH2)5Tyr(Me)-[Orn8]vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH-(1–24) Eur. J. Pharmacol. 1987;134(2):221–224. doi: 10.1016/0014-2999(87)90168-3. [DOI] [PubMed] [Google Scholar]
- Arletti R., Bazzani C., Castelli M., Bertolini A. Oxytocin improves male copulatory performance in rats. Horm. Behav. 1985;19(1):14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Arletti R., Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6(3):247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Arnold A.P., Gorski R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., Van IJzendoorn M.H. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc. Cogn. Affect. Neurosci. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Dorsa D.M., Johnston C.A. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J. Neurosci. 1995;15(7 Pt 1):5058–5064. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K.L., Boone E., Epperson P., Hoffman G., Carter C.S. Are behavioral effects of early experience mediated by oxytocin? Front. Psych. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J., Ball G.F. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J., Cornil C.A., Taziaux M., Charlier T.D., Baillien M., Ball G.F. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138(3):783–791. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Barrett J., Fleming A.S. All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J. Child Psychol. Psychiatry. 2011;52(4):368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Bartz J., Simeon D., Hamilton H., Kim S., Crystal S., Braun A., Vicens V., Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc. Cogn. Affect. Neurosci. 2010;(November) doi: 10.1093/scan/nsq085. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Hollander E., Ludwig N.N., Kolevzon A., Ochsner K.N. Oxytocin selectively improves empathic accuracy. Psychol. Sci. 2010;21(10):1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. Social effects of oxytocin in humans context and person matter. Trends Cogn. Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baskerville T.A., Allard J., Wayman C., Douglas A.J. Dopamine–oxytocin interactions in penile erection. Eur. J. Neurosci. 2009;30(11):2151–2164. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- Baskerville T.A., Douglas A.J. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010;16(3):e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U., Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Bick J., Dozier M. Mothers’ and children's concentrations of oxytocin following close, physical interactions with biological and non-biological children. Dev. Psychobiol. 2010;52(1):100–107. doi: 10.1002/dev.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkstrand E., Eriksson M., Uvnas-Moberg K. Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology. 1996;63(4):377–383. doi: 10.1159/000126978. [DOI] [PubMed] [Google Scholar]
- Boccia M.L., Pedersen C.A. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26(7):572–657. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Born J., Lange T., Kern W., McGregor G.P., Bickel U., Fehm H.L. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bos P.A., Hermans E.J., Montoya E.R., Ramsey N.F., van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35(1):114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bos P.A., Panksepp J., Bluthe R.M., Honk J.V. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front. Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Basic Books; New York: 1969. Attachment and Loss. [Google Scholar]
- Bowlby J. vol. II. Basic Books; New York: 1973. (Separation. Anxiety and Anger. Attachment and Loss). [Google Scholar]
- Bowlby J. vol. III. Basic Books; New York: 1980. (Loss. Attachment and Loss). [Google Scholar]
- Bowlby J. Basic Books; New York: 1988. A Secure Base: Parent–Child Attachment and Healthy Human Development. [Google Scholar]
- Bridges R.S. A quantitative analysis of the roles of dosage, sequence and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Bridges T.E., Hillhouse E.W., Jones M.T. The effect of dopamine on neurohypophysial hormone release in vivo and from the rat neural lobe and hypothalamus in vitro. J. Physiol. 1976;260(3):647–666. doi: 10.1113/jphysiol.1976.sp011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.R., Ye H., Bronson R.T., Dikkes P., Greenberg M.E. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86(2):297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Buijs R.M., Geffard M., Pool C.W., Hoorneman E.M. The dopaminergic innervation of the supraoptic and paraventricular nucleus. A light and electron microscopical study. Brain Res. 1984;323(1):65–72. doi: 10.1016/0006-8993(84)90265-8. [DOI] [PubMed] [Google Scholar]
- Caldwell J.D., Brooks P.J., Jirkikowski G.R., Barakat A.S., Lund P.K., Pedersen C.A. Estrogen alters oxytocin mRNA levels in the preoptic area. J. Neuroendocrinol. 1989;1:273–278. doi: 10.1111/j.1365-2826.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Caldwell J.D., Prange A.J., Pedersen C.A. Oxytocin facilitates the sexual receptivity of oestrogen-treated female rats. Neuropeptides. 1986;7:175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Cameron J.L., Pomerantz S.M., Layden L.M., Amico J.A. Dopaminergic stimulation of oxytocin concentrations in the plasma of male and female monkeys by apomorphine and a D2 receptor agonist. J. Clin. Endocrinol. Metab. 1992;75(3):855–860. doi: 10.1210/jcem.75.3.1355489. [DOI] [PubMed] [Google Scholar]
- Campbell P., Ophir A.G., Phelps S.M. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J. Comp. Neurol. 2009;516:321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- Carmichael M.S., Humbert R., Dixen J., Palmisano G., Greenleaf W., Davidson J.M. Plasma oxytocin increases in the human sexual response. J. Clin. Endocrinol. Metab. 1987;64(1):27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Oxytocin and sexual behavior. Neurosci. Biobehav. Rev. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Developmental consequences of oxytocin. Physiol. Behav. 2003;79(3):383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav. Brain Res. 2007;176(1):170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Ahnert L., Grossmann K.E., Hrdy S.B., Lamb M.E., Porges S.W., Sachser N., editors. Attachment and Bonding: A New Synthesis. The MIT Press; Boston, MA: 2005. [Google Scholar]
- Champagne F., Diorio J., Sharma S., Meaney M.J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. U.S.A. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Chretien P., Stevenson C.W., Zhang T.Y., Gratton A., Meaney M.J. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 2004;24(17):4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Curley J.P. Maternal regulation of estrogen receptor alpha methylation. Curr. Opin. Pharmacol. 2008;8(6):735–739. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Curley J.P. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 2009;33(4):593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Champagne F.A., Weaver I.C., Diorio J., Dymov S., Szyf M., Meaney M.J. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne F.A., Weaver I.C., Diorio J., Sharma S., Meaney M.J. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144(11):4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Chen X.Q., Fawcett J.R., Rahman Y.E., Ala T.A., Frey W.H., II Delivery of nerve growth factor to the brain via the olfactory pathway. J. Alzheimers Dis. 1998;1(1):35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- Chibbar R., Miller F.D., Mitchell B.F. Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J. Clin. Invest. 1993;91(1):185–192. doi: 10.1172/JCI116169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B., Mouillac B., Balestre M.N., Trumpp-Kallmeyer S., Hoflack J., Hibert M., Andriolo M., Pupier S., Jard S., Barberts C. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Lett. 1996;397(2–3):201–206. doi: 10.1016/s0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- Cho M.M., DeVries A.C., Williams J.R., Carter C.S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Choleris E., Devidze N., Kavaliers M., Pfaff D.W. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog. Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- Clodi M., Vila G., Geyeregger R., Riedl M., Stulnig T.M., Struck J., Luger T.A., Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am. J. Physiol. Endocrinol. Metab. 2008;295(3):E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- Collaer M.L., Hines M. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol. Bull. 1995;118(1):55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Cornil C.A., Ball G.F., Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126(1):2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.P., Cowan P.A. US Basic Books; New York, NY: 1992. When Partners Become Parents: The Big Life Change for Couples. [Google Scholar]
- Cox M.J., Paley B. Families as systems. Annu. Rev. Psychol. 1997;48:243–267. doi: 10.1146/annurev.psych.48.1.243. [DOI] [PubMed] [Google Scholar]
- Curley J.P., Jordan E.R., Swaney W.T., Izraelit A., Kammel S., Champagne F.A. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev. Neurosci. 2009;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J., Hazra R., Ahern T.H., Guo J.D., McDonald A.J., Mascagni F., Muller J.F., Young L.J., Rainnie D.G. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat. Psychoneuroendocrinology. 2011;(April) doi: 10.1016/j.psyneuen.2011.03.003. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danalache B.A., Gutkowska J., Slusarz M.J., Berezowska I., Jankowski M. Oxytocin-Gly-Lys-Arg: a novel cardiomyogenic peptide. PLoS One. 2010;5(10):e13643. doi: 10.1371/journal.pone.0013643. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. John Murray; London: 1872. The Expression of the Emotions in Man and Animals. [Google Scholar]
- Declerck C.H., Boone C., Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm. Behav. 2010;57:368–371. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Voorhuis D.A.M., Boschma Y., Elands J. Estradiol modulates density of putative oxytocin sites for neurohypophysial hormones in the rat brain. Eur. J. Pharmacol. 1986;11:113–119. [Google Scholar]
- De Vries G.J., Buijs R.M., Swaab D.F. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain – presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries A.C., De Vries M.B., Taymans S.E., Carter C.S. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Natl. Acad. Sci. U.S.A. 1996;93(21):11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries G.J., Simerly R.B. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff D.W., Arnold A.P., Etgen A.M., Fahrbach S.E., Moss R.L., Rubin R.T., editors. vol. 4. Academic Press; San Diego: 2002. pp. 137–191. (Hormones, Brain, and Behavior). [Google Scholar]
- De Vries G.J., Wang Z., Bullock N.A., Neuman S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J. Neurosci. 1994;14(3 Pt 2):1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decavel C., Geffard M., Calas A. Comparative study of dopamine- and noradrenaline-immunoreactive terminals in the paraventricular and supraoptic nuclei of the rat. Neurosci. Lett. 1987;77(2):149–154. doi: 10.1016/0304-3940(87)90577-5. [DOI] [PubMed] [Google Scholar]
- Devost D., Girotti M., Carrier M.E., Russo C., Zingg H.H. Oxytocin induces dephosphorylation of eukaryotic elongation factor 2 in human myometrial cells. Endocrinology. 2005;146(5):2265–2270. doi: 10.1210/en.2004-1428. [DOI] [PubMed] [Google Scholar]
- Donaldson Z.R., Young L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves mind-reading in humans. Biol. Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Elands J., Resink A., De Kloet E.R. Neurohypophysial hormone receptors in the rat thymus, spleen, and lymphocytes. Endocrinology. 1990;126(5):2703–2710. doi: 10.1210/endo-126-5-2703. [DOI] [PubMed] [Google Scholar]
- Feldman, R., 1998. Coding Interactive Behavior (CIB) Manual. Unpublished manual. Bar-Ilan University.
- Feldman R. Infant–mother and infant–father synchrony: the coregulation of positive arousal. Infant Men. Hlth J. 2003;24:1–23. [Google Scholar]
- Feldman R. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R., Eidelman A.I. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Dev. Psychobiol. 2003;43(2):109–119. doi: 10.1002/dev.10126. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Schneiderman I., Weisman O., Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm. Behav. 2010;58(4):669–676. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Dev. Sci. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R., Singer M., Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 2010;13(2):271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Feldman R., Weller A., Zagoory-Sharon O., Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psychol. Sci. 2007;18(11):965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Ferguson J.N., Aldag J.M., Insel T.R., Young L.J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J.N., Young L.J., Hearn E.F., Matzuk M.M., Insel T.R., Winslow J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000;25(3):284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferris C.F., Kulkarni P., Sullivan J.M., Jr., Harder J.A., Messenger T.L., Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J. Neurosci. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A.S., Corter C., Franks P., Surbey M., Schneider B., Steiner M. Postpartum factors related to mother's attraction to newborn infant odors. Dev. Psychobiol. 1993;26(2):115–132. doi: 10.1002/dev.420260204. [DOI] [PubMed] [Google Scholar]
- Fleming A.S., Corter C., Stallings J., Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm. Behav. 2002;42(4):399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Fleming A.S., Steiner M., Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm. Behav. 1997;32(2):85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- Francis D., Diorio J., Liu D., Meaney M.J. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Frayne J., Nicholson H.D. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol. Hum. Reprod. 1998;4(6):527–532. doi: 10.1093/molehr/4.6.527. [DOI] [PubMed] [Google Scholar]
- Fuchs A.R., Behrens O., Helmer H., Vangsted A., Ivanisevic M., Grifo J., Barros C., Fields M. Oxytocin and vasopressin binding sites in human and bovine ovaries. Am. J. Obstet. Gynecol. 1990;163(6 Pt 1):1961–1967. doi: 10.1016/0002-9378(90)90781-2. [DOI] [PubMed] [Google Scholar]
- Fuchs A.R., Fuchs F., Husslein P., Soloff M.S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am. J. Obstet. Gynecol. 1984;150(6):734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- Fuchs A.R., Fuchs F., Husslein P., Soloff M.S., Fernstrom M.J. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982;215:1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- Gainer H., Wray S. Cellular and molecular biology of oxytocin and vasopressin. In: Knobil E., Neill J., editors. vol. 1. Raven Press; New York: 1994. pp. 1099–1130. (The Physiology of Reproduction). [Google Scholar]
- Gammie S.C., Nelson R.J. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J. Neurosci. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen V., Defresne M.P., Robert F., Legros J.J., Franchimont P., Boniver J. The neurohormonal thymic microenvironment: immunocytochemical evidence that thymic nurse cells are neuroendocrine cells. Neuroendocrinology. 1988;47(4):365–368. doi: 10.1159/000124938. [DOI] [PubMed] [Google Scholar]
- Geenen V., Legros J.J., Franchimont P., Baudrihaye M., Defresne M.P., Boniver J. The neuroendocrine thymus: coexistence of oxytocin and neurophysin in the human thymus. Science. 1986;232(4749):508–511. doi: 10.1126/science.3961493. [DOI] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gingrich J.A., Dearry A., Falardeau P., Bates M.D., Fremeau R.T., Jr., Caron M.G. Location and molecular cloning of D1 dopamine receptor. Neurochem. Int. Suppl. 1992;20:9S–15S. doi: 10.1016/0197-0186(92)90204-5. [DOI] [PubMed] [Google Scholar]
- Goodson J.L., Thompson R.R. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Gordon I., Feldman R. Synchrony in the triad: a microlevel process model of coparenting and parent–child interactions. Fam. Process. 2008;47(4):465–479. doi: 10.1111/j.1545-5300.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- Gordon I., Zagoory-Sharon O., Leckman J.F., Feldman R. Oxytocin and the development of parenting in humans. Biol. Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Zagoory-Sharon O., Leckman J.F., Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol. Behav. 2010;101(5):679–684. doi: 10.1016/j.physbeh.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Gordon I., Zagoory-Sharon O., Leckman J.F., Feldman R. Prolactin, oxytocin, and the development of paternal behavior across the first six months of fatherhood. Horm. Behav. 2010;58(3):513–518. doi: 10.1016/j.yhbeh.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Zagoory-Sharon O., Schneiderman I., Leckman J.F., Weller A., Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45(3):349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Gouin J.P., Carter C.S., Pournajafi-Nazarloo H., Glaser R., Malarkey W.B., Loving T.J., Stowell J., Kiecolt-Glaser J.K. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35(7):1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S.G., Connelly J.J., Towers A.J., Johnson J., Biscocho D., Markunas C.A., Lintas C., Abramson R.K., Wright H.H., Ellis P. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;22(7):62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen K.M., Girdler S.S., Amico J., Light K.C. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom. Med. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Gross G.A., Imamura T., Luedke C., Vogt S.K., Olson L.M., Nelson D.M., Sadovsky Y., Muglia J.J. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc. Natl. Acad. Sci. U.S.A. 1998;95(20):11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A.J., Kenyon A.R., Unkelbach C., Alvares G.A., Hickie I.B. Arginine vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology. 2011;36(2):294–297. doi: 10.1016/j.psyneuen.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Einfeld S.L., Gray K.M., Rinehart N.J., Tonge B.J., Lambert T.J., Hickie I.B. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Jankowski M., Lambert C., Mukaddam-Daher S., Zingg H.H., McCann S.M. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc. Natl. Acad. Sci. U.S.A. 1997;94(21):11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock E.A., Young L.J. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308(5728):1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hammock E.A., Young L.J. Oxytocin, vasopressin and pair bonding: implications for autism. Philos. Trans. R. Soc. Lond. B. 2006;361(1476):2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansenne I. Thymic transcription of neurohypophysial and insulin-related genes: impact upon T-cell differentiation and self-tolerance. J. Neuroendocrinol. 2005;17(5):321–327. doi: 10.1111/j.1365-2826.2005.01301.x. [DOI] [PubMed] [Google Scholar]
- Hansen S., Bergvall A.H., Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol. Biochem. Behav. 1993;45(3):673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hara Y., Battey J., Gainer H. Structure of mouse vasopressin and oxytocin genes. Brain Res. Mol. Brain Res. 1990;8(4):319–324. doi: 10.1016/0169-328x(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Heinlein C.A., Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hatton G.I., Modney B.K., Salm A.K. Increases in dendritic bundling and dye coupling of supraoptic neurons after the induction of maternal behavior. Ann. N. Y. Acad. Sci. 1992;652:142–155. doi: 10.1111/j.1749-6632.1992.tb34351.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr. Opin. Psychiatry. 2007;20(2):158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., von Dawans B., Domes G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Higashida H., Lopatina O., Yoshihara T., Pichugina Y.A., Soumarokov A.A., Munesue T., Minabe Y., Kikuchi M., Ono Y., Korshunova N., Salmina A.B. Oxytocin signal and social behaviour: comparison among adult and infant oxytocin, oxytocin receptor and CD38 gene knockout mice. J. Neuroendocrinol. 2010;22(5):373–379. doi: 10.1111/j.1365-2826.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- Hinde R.A. McGraw Hill; New York: 1970. Animal Behaviour. [Google Scholar]
- Hinde R.A. Some implications of evolutionary theory and comparative data for the study of human prosocial and aggressive behavior. In: Olweus D., Block J., Radke-Yarrow M., editors. Development of Anti-social and Prosocial Behavior. Academic Press; 1986. pp. 13–32. [Google Scholar]
- Ho M.L., Lee J.N. Ovarian and circulating levels of oxytocin and arginine vasopressin during the estrous cycle in the rat. Acta Endocrinol.-cop. 1992;126(6):530–534. doi: 10.1530/acta.0.1260530. [DOI] [PubMed] [Google Scholar]
- Hoge E.A., Pollack M.H., Kaufman R.E., Zak P.J., Simon N.M. Oxytocin levels in social anxiety disorder. CNS Neurosci. Ther. 2008;14(3):165–170. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy S.B. Evolutionary context of human development. In: Carter C.S., Ahnert L., Grossmann K.E., Hrdy S.B., Lamb M.E., Porges S.W., Sachser N., editors. Attachment and Bonding: A New Synthesis. The MIT Press; Boston, MA: 2005. pp. 9–32. [Google Scholar]
- Hull E.M., Dominguez J.M. Sexual behavior in male rodents. Horm. Behav. 2007;52(1):45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M., Arisaka O., Minamoto F., Arai Y. Sex differences in children's free drawings: a study on girls with congenital adrenal hyperplasia. Horm. Behav. 2001;40(2):99–104. doi: 10.1006/hbeh.2001.1670. [DOI] [PubMed] [Google Scholar]
- Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharmacol. Sci. 2000;11(1):1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Insel T.R. Postpartum increases in brain oxytocin binding. Neuroendocrinology. 1986;206:419–436. doi: 10.1159/000124694. [DOI] [PubMed] [Google Scholar]
- Insel T.R. Is social attachment an addictive disorder? Physiol. Behav. 2003;79(3):351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Insel T.R., Hulihan T.J. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 1995;109(4):782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel T.R., Wang Z.X., Ferris C.F. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci. 1994;14(9):5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Young L.J. The neurobiology of attachment. Nat. Rev. Neurosci. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ivell R., Balvers M., Rust W., Bathgate R., Einspanier A. Oxytocin and male reproductive function. Adv. Exp. Med. Biol. 1997;424:253–264. doi: 10.1007/978-1-4615-5913-9_47. [DOI] [PubMed] [Google Scholar]
- Ivell R., Bathgate R.A., Walther N., Kimura T. The molecular basis of oxytocin and oxytocin receptor gene expression in reproductive tissues. Adv. Exp. Med. Biol. 1998;449:297–306. doi: 10.1007/978-1-4615-4871-3_37. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. The gene for the hypothalamic peptide hormone oxytocin is highly expressed in the bovine corpus luteum: biosynthesis, structure and sequence analysis. EMBO J. 1984;3(10):2351–2354. doi: 10.1002/j.1460-2075.1984.tb02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M., Hajjar F., Kawas S.A., Mukaddam-Daher S., Hoffman G., McCann S.M., Gutkowska J. Rat heart: a site of oxytocin production and action. Proc. Natl. Acad. Sci. U.S.A. 1998;95(24):14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler L., Meaney M.J. Epigenetics and parental effects. Bioessays. 2010;32(9):818–827. doi: 10.1002/bies.201000015. [DOI] [PubMed] [Google Scholar]
- Keer S.E., Stern J.M. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol. Behav. 1999;67(5):659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Kendrick K.M., Keverne E.B., Chapmam C., Baldwin B.A. Intracranial dialysis measurement of oxytocin, monoamine and uric acid release from the olfactory bulb and substantia nigra of sheep during parturition, suckling, separation from lambs and eating. Brain Res. 1988;439:1–10. doi: 10.1016/0006-8993(88)91455-2. [DOI] [PubMed] [Google Scholar]
- Kendrick K.M., Keverne E.B., Chapman C., Baldwin B.A. Microdialysis measurement of oxytocin, aspartate, y-aminobutyric acid and glutamate release from the olfactory bulb of the sheep during vaginocervical stimulation. Brain Res. 1988;442:171–174. doi: 10.1016/0006-8993(88)91447-3. [DOI] [PubMed] [Google Scholar]
- Kendrick K.M., Keverne E.B., Hinton M.R., Goode J.A. Oxytocin, amino acids and monoamine release in the medial pre-optic area and bed nucleus of stria terminalis of the sheep during parturition and suckling. Brain Res. 1992;569:199–209. doi: 10.1016/0006-8993(92)90631-i. [DOI] [PubMed] [Google Scholar]
- Keverne E.B., Kendrick K.M. Oxytocin facilitation of maternal behavior in sheep. Ann. N. Y. Acad. Sci. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- Kim P., Feldman R., Mayes L.C., Eicher V., Thompson N., Leckman J.F., Swain J.E. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiatry. 2011 doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Leckman J.F., Mayes L.C., Newman M., Feldman R., Swain J.E. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev. Sci. 2010;13(4):662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Ivell R. The oxytocin receptor. Results Probl. Cell Differ. 1999;26:135–168. doi: 10.1007/978-3-540-49421-8_7. [DOI] [PubMed] [Google Scholar]
- Kimura T., Makino Y., Saji F., Takemura M., Inoue T., Kikuchi T., Kubota Y., Azuma C., Nobunaga T., Tokugawa Y., Tanizawa O. Molecular characterization of a cloned human oxytocin receptor. Eur. J. Endocrinol. 1994;131(4):385–390. doi: 10.1530/eje.0.1310385. [DOI] [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M.J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Koos O., Gergely G. A contingency-based approach to the etiology of ‘disorganized’ attachment: the ‘flickering switch’ hypothesis. Bull. Menninger Clin. 2001;65:397–410. doi: 10.1521/bumc.65.3.397.19851. [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Bodnar R.J. Vasopressin analgesia: specificity of action and non-opioid effects. Peptides. 1984;5(4):747–756. doi: 10.1016/0196-9781(84)90017-2. [DOI] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P.J., Fischbacher U., Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krpan K.M., Coombs R., Zinga D., Steiner M., Fleming A.S. Experiential and hormonal correlates of maternal behavior in teen and adult mothers. Horm. Behav. 2005;47(1):112–122. doi: 10.1016/j.yhbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leckman J.F., Carter C.S., Hennessy M.B., Hrdy S.B., Keverne E.B., Klann-Delius C., Schradin C., Todt D., von Holst D. Biobehavioral processes in attachment and bonding. In: Carter C.S., Ahnert L., Grossmann K.E., Hrdy S.B., Lamb M.E., Porges S.W., Sachser N., editors. Attachment and Bonding: A New Synthesis. The MIT Press; Boston, MA: 2005. pp. 301–347. [Google Scholar]
- Leckman J.F., Mayes L.C. Understanding developmental psychopathology: how useful are evolutionary perspectives? J. Am. Acad. Child Adolesc. Psychiatry. 1998;37:1011–1021. doi: 10.1097/00004583-199810000-00010. [DOI] [PubMed] [Google Scholar]
- Leckman J.F., Mayes L.C. Nurturing resilient children. J. Child Psychol. Psychiatry. 2007;48:221–223. doi: 10.1111/j.1469-7610.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Leckman J.F., Mayes L.C., Feldman R., Evans D.W., King R.A., Cohen D.J. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiat. Scand. Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Lee A., Clancy S., Fleming A.S. Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 2000;108(2):215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Caldwell H.K., Macbeth A.H., Young W.S., 3rd Behavioural studies using temporal and spatial inactivation of the oxytocin receptor. Prog. Brain Res. 2008;170:73–77. doi: 10.1016/S0079-6123(08)00407-X. [DOI] [PubMed] [Google Scholar]
- Lefebvre L., Viville S., Barton S.C., Ishino F., Keverne E.B., Surani M.A. Abnormal maternal behavior and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- Legros J.J., Chiodera P., Geenen V. Inhibitory action of exogenous oxytocin on plasma cortisol in normal human subjects: evidence of action at the adrenal level. Neuroendocrinology. 1988;48(2):204–206. doi: 10.1159/000125009. [DOI] [PubMed] [Google Scholar]
- Leung C.H., Goode C.T., Young L.J., Maney D.L. Neural distribution of nonapeptide binding sites in two species of songbird. J. Comp. Neurol. 2009;513:321–333. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Levine A., Zagoory-Sharon O., Feldman R., Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28(6):1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lévy F., Kendrick K.M., Keverne E.B., Piketty V., Poindron P. Intracerebral oxytocin is important for the onset of maternal behavior in inexperienced ewes delivered under peridural anesthesia. Behav. Neurosci. 1992;106(24):27–32. doi: 10.1037//0735-7044.106.2.427. [DOI] [PubMed] [Google Scholar]
- Li L.L., Keverne E.B., Aparicio S.A., Ishino F., Barton S.C., Surani M.A. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284:330–333. doi: 10.1126/science.284.5412.330. [DOI] [PubMed] [Google Scholar]
- Light K.C., Grewen K.M., Amico J.A. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol. Psychol. 2005;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Light K.C., Grewen K.M., Amico J.A., Boccia M., Brownley K.A., Johns J.M. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict. Behav. 2004;29(8):1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton J.M., Glyn J.R. Central administration of peptides alters thermoregulation in the rabbit. Peptides. 1980;1(1):15–18. doi: 10.1016/0196-9781(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang Z.X. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121(3):537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lopatina O., Inzhutova A., Pichugina Y.A., Okamoto H., Salmina A.B., Higashida H. Reproductive experience affects parental retrieval behaviour associated with increased plasma oxytocin levels in wild-type and Cd38-knockout mice. J. Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02136.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Lorenz K. Mariner Books; New York: 1978. Behind the Mirror. [Google Scholar]
- Lucas B.K., Ormandy C.J., Binart N., Bridges R.S., Kelly P.A. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- Lukas M., Bredewold R., Neumann I.D., Veenema A.H. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Ludwig M., Leng G. Dendritic peptide release and peptide-dependent behaviors. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Macciò A., Madeddu C., Chessa P., Panzone F., Lissoni P., Mantovani G. Oxytocin both increases proliferative response of peripheral blood lymphomonocytes to phytohemagglutinin and reverses immunosuppressive estrogen activity. In Vivo. 2010;24(2):157–163. [PubMed] [Google Scholar]
- Maestripieri D. Biological bases of maternal attachment. Curr. Direct. Psychol. Sci. 2001;10:79–83. [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc. Natl. Acad. Sci. U.S.A. 2005;102(27):9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D., Canale D. Hormonal changes when falling in love. Psychoneuroendocrinology. 2004;29(7):931–936. doi: 10.1016/j.psyneuen.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Marazziti D., Dell’Osso B., Baroni S., Mungai F., Catena M., Rucci P., Albanese F., Giannaccini G., Betti L., Fabbrini L., Italiani P., DelDebbio Al., Luccacchini A., Dell’Osso L. A relationship between oxytocin and anxiety of romantic attachment. Clin. Pract. Epidemol. Mental Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. The meaning of weaning. Anim. Behav. 1984;32:1257–1259. [Google Scholar]
- Martino B., Hsieh G.C., Hollingsworth P.R., Mikusa J.P., Moreland R.B., Bitner R.S. Central oxytocinergic and dopaminergic mechanisms regulating penile erection in conscious rats. Pharmacol. Biochem. Behav. 2005;81(4):797–804. doi: 10.1016/j.pbb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Mason W.T. Excitation by dopamine of putative oxytocinergic neurones in the rat supraoptic nucleus in vitro: evidence for two classes of continuously firing neurones. Brain Res. 1983;267(1):113–121. doi: 10.1016/0006-8993(83)91044-2. [DOI] [PubMed] [Google Scholar]
- McCann M.J., Rogers R.C. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J. Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M. Oxytocin inhibits infanticide in female house mice (Mus domesticus) Horm. Behav. 1990;24(3):365–375. doi: 10.1016/0018-506x(90)90015-p. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M., McDonald C.H., Brooks P.J., Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60(5):1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McGowan P.O., Sasaki A., D’Alessio A.C., Dymov S., Labonté B., Szyf M., Turecki G., Meaney M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly A.S., Robinson I.C., Houston M.J., Howie P.W. Release of oxytocin and prolactin in response to suckling. Br. Med. J. 1983;286(6361):257–259. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P.H., Beer J. Neural mechanisms of the testosterone-aggression relation: the role of orbitofrontal cortex. J. Cogn. Neurosci. 2010;22(10):2357–2368. doi: 10.1162/jocn.2009.21389. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G., Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol. Psychiatry. 2007;61(9):1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci. Biobehav. Rev. 2011;35:939–955. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Argiolas A., Stancampiano R., Gessa G.L. Effect of apomorphine on oxytocin concentrations in different brain areas and plasma of male rats. Eur. J. Pharmacol. 1990;182(1):101–107. doi: 10.1016/0014-2999(90)90497-t. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Melis T., Cocco C., Succu S., Sanna F., Pillolla G., Boi A., Ferri G.L., Argiolas A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 2007;26(4):1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Stancampiano R., Argiolas A. Hippocampal oxytocin mediates apomorphine-induced penile erection and yawning. Pharmacol. Biochem. Behav. 1992;42(1):61–66. doi: 10.1016/0091-3057(92)90447-n. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Stancampiano R., Argiolas A. Oxytocin- and vasopressin-like immunoreactivity in the rat thymus: characterization and possible involvement in the immune response. Regul. Pept. 1993;45(1-2):269–272. doi: 10.1016/0167-0115(93)90218-w. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Succu S., Mascia M.S., Argiolas A. PD-168077, a selective dopamine D4 receptor agonist, induces penile erection when injected into the paraventricular nucleus of male rats. Neurosci. Lett. 2005;379(1):59–62. doi: 10.1016/j.neulet.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Succu S., Sanna F., Boi A., Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur. J. Neurosci. 2009;30(7):1349–1357. doi: 10.1111/j.1460-9568.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- Melis M.R., Succu S., Sanna F., Melis T., Mascia M.S., Enguehard-Gueiffier C., Hugner H., Gmeiner P., Gueiffier A., Argiolas A. PIP3EA and PD-168077, two selective dopamine D4 receptor agonists, induce penile erection in male rats: site and mechanism of action in the brain. Eur. J. Neurosci. 2006;24(7):2021–2030. doi: 10.1111/j.1460-9568.2006.05043.x. [DOI] [PubMed] [Google Scholar]
- Miller F.D., Ozimek G., Milner R.J., Bloom F.E. Regulation of neuronal oxytocin mRNA by ovarian steroids in the mature and developing hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 1989;S6:2468–2472. doi: 10.1073/pnas.86.7.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Kramer R., Warner V., Wickramaratne P., Weissman M. Intergenerational transmission of parental bonding among women. J. Am. Acad. Psychiatry. 1997;36(8):1134–1139. doi: 10.1097/00004583-199708000-00022. [DOI] [PubMed] [Google Scholar]
- Mitchell B.F., Fang X., Wong S. Oxytocin: a paracrine hormone in the regulation of parturition? Rev. Reprod. 1998;3(2):113–122. doi: 10.1530/ror.0.0030113. [DOI] [PubMed] [Google Scholar]
- Moles A., Kieffer B.L., D’Amato F.R. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(5679):1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J. Physiol. 1989;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.R., Seckl J.R., Burton S., Checkley S.A., Lightman S.L. Changes in oxytocin and vasopressin secretion during sexual activity in men. J. Clin. Endocrinol. Metab. 1987;65(4):738–741. doi: 10.1210/jcem-65-4-738. [DOI] [PubMed] [Google Scholar]
- Myers M.M., Bruenelli S.A., Squire J.M., Shindeldecker R.D., Hofer M.A. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev. Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- Ndiaye K., Poole D.H., Pate J.L. Expression and regulation of functional oxytocin receptors in bovine T lymphocytes. Biol. Reprod. 2008;78(4):786–793. doi: 10.1095/biolreprod.107.065938. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Landgraf R. Advances in vasopressin and oxytocin—from genes to behaviour to disease. Preface. Prog. Brain Res. 2008;170:xi–xiii. doi: 10.1016/S0079-6123(08)00448-2. [DOI] [PubMed] [Google Scholar]
- Nishimori K., Takayanagi Y., Yoshida M., Kasahara Y., Young L.J., Kawamata M. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behavior and control of energy balance. Prog. Brain Res. 2008;170:79–90. doi: 10.1016/S0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- Nishimori K., Young L.J., Guo Q., Wang Z., Insel T.R., Matzuk M.M. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenstrom A., Servin A., Bohlin G., Larsson A., Wedell A. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2002;87(11):5119–5124. doi: 10.1210/jc.2001-011531. [DOI] [PubMed] [Google Scholar]
- Numan M., Numan M.J., Pliakou N., Stolzenberg D.S., Mullins O.J., Murphy J.M., Smith C.D. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav. Neurosci. 2005;119(6):1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Eng V., Taylor J., Lubahn D.B., Korach K.S., Pfaff D.W. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Taylor J.A., Lubahn D.B., Korach K.S., Pfaff D.W. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- Ohlsson B., Truedsson M., Djerf P., Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul. Pept. 2006;135(1-2):7–11. doi: 10.1016/j.regpep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Okano A., Okuda K., Takahashi M., Schams D. Oxytocin receptors in the porcine endometrium during the estrous cycle and early pregnancy. Anim. Reprod. Sci. 1996;41:61–70. [Google Scholar]
- Ormandy C.J., Camus A., Barra J., Damotte D., Lucas B., Buteau H., Edery M., Brousse N., Babinet C., Binart N., Kelly P.A. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Paredes R.G., Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog. Neurobiol. 2004;73(3):179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Parker G., Tulping H., Brown L.B. A parental bonding instrument. Br. J. Med. Psychol. 1979;52:1–10. [Google Scholar]
- Pedersen C.A., Ascher J.A., Monroe Y.L., Prange A.J., Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen C.A., Caldwell J.D., Jirikowski G.F., Insel T.R. vol. 652. The New York Academy of Sciences; New York: 1992. (Oxytocin in Maternal, Sexual and Social Behaviors. Annals of the New York Academy of Sciences). [PubMed] [Google Scholar]
- Pedersen C.A., Vadlamudi S.V., Boccia M.L., Amico J.A. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Pierman S., Sica M., Allieri F., Viglietti-Panzica C., Panzica G.C., Bakker J. Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm. Behav. 2008;54(1):98–106. doi: 10.1016/j.yhbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R., Kojro E., Fahrenholz F. Separate agonist and peptide antagonist binding sites of the oxytocin receptor defined by their transfer into the V2 vasopressin receptor. J. Biol. Chem. 1996;271(49):31593–31601. doi: 10.1074/jbc.271.49.31593. [DOI] [PubMed] [Google Scholar]
- Pow D.V., Morris J.F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Prichard, Z.M., Mackinnon, A.J., Jorm, A.F., Easteal, S., 2007. AVPR1A and OXTR polymorphisms are associated with sexual and reproductive behavioral phenotypes in humans. Mutation in brief no. 981. Hum. Mutat. 28(11), 1150 (Online). [DOI] [PubMed]
- Quinones-Jenab V., Jenab S., Ogawa S., Adan R.A., Burbach J.P., Pfaff D.W. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology. 1997;65(1):9–17. doi: 10.1159/000127160. [DOI] [PubMed] [Google Scholar]
- Ragnauth A.K., Devidze N., Mot V., Finley K., Goodwillie A., Kow L.M., Muglia L.J., Pfaff D.W. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Riekkinen P., Legros J.J., Sennef C., Jolkkonen J., Smitz S., Soininen H. Penetration of DGAVP (Org 5667) across the blood–brain barrier in human subjects. Peptides. 1987;8(2):261–265. doi: 10.1016/0196-9781(87)90101-x. [DOI] [PubMed] [Google Scholar]
- Riem M.M., Bakermans-Kranenburg M.J., Pieper S., Tops M., Boksem M.A., Vermeiren R.R., van Ijzendoorn M.H., Rombouts S.A. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol. Psychiatry. 2011;70(3):291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Hediger K., Heinrichs M., Klaver P. Oxytocin makes a face in memory familiar. J. Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H.E., Young L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H.E., Cole C.D., Smith Y., Neumann I.D., Landgraf R., Murphy A.Z., Young L.J. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.L., Lubin F.D., Funk A.J., Sweatt J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher N. Adult social bonding: insights from studies of nonhuman mammals. In: Carter C.S., Ahnert L., Grossmann K.E., Hrdy S.B., Lamb M.E., Porges S.W., Sachser N., editors. Attachment and Bonding: A New Synthesis. The MIT Press; Boston, MA: 2005. pp. 119–135. [Google Scholar]
- Sarkar D.K., Frautschy S.A., Mitsugi N. Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactinemia. Ann. N. Y. Acad. Sci. 1992;652:397–410. doi: 10.1111/j.1749-6632.1992.tb34370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Nakagawasai O., Tan-No K., Onogi H., Niijima F., Tadano T. Influence of olfactory bulbectomy on maternal behavior and dopaminergic function in nucleus accumbens in mice. Behav. Brain Res. 2011;215(1):141–145. doi: 10.1016/j.bbr.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Savaskan E., Ehrhardt R., Schulz A., Walter M., Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–377. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schanberg S.M., Evoniuk G., Kuhn C.M. Tactile and nutritional aspects of maternal care: specific regulators of neuroendocrine function and cellular development. Proc. Soc. Exp. Biol. Med. 1984;175(2):135–146. doi: 10.3181/00379727-175-41779. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Jard S., Dreifuss J.J., Tribollet E. Oxytocin receptors in rat kidney during development. Am. J. Physiol. 1990;259(6 Pt 2):F872–F881. doi: 10.1152/ajprenal.1990.259.6.F872. [DOI] [PubMed] [Google Scholar]
- Shahrokh D.K., Zhang T.Y., Diorio J., Gratton A., Meaney M.J. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I., Israel S., Uzefovsky F., Gritsenko I., Kaitz M., Ebstein R.P. Vasopressin needs an audience: neuropeptide elicited stress responses are contingent upon perceived social evaluative threats. Horm. Behav. 2011;60:121–127. doi: 10.1016/j.yhbeh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Fischer M., Dvash J., Harari H., Perach-Bloom N., Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol. Psychiatry. 2009;66(9):864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Simpson E.R. Sources of estrogen and their importance. J. Steroid Biochem. 2003;86(3-5):225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Skrundz M., Bolten M., Nast I., Hellhammer D.H., Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer M.D., Curtis J.T., Aragona B.J., Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci. Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Vasopressin and oxytocin in the mammalian brain and spinal cord. Trends Neurosci. 1983;6:467–472. [Google Scholar]
- Spielewoy C., Roubert C., Hamon M., Nosten-Bertrand M., Betancur C., Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 2000;11(3-4):279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T., Musatov S., Ogawa S., Ribeiro A., Pfaff D.W., Agmo A. Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology. 2010;91(2):142–154. doi: 10.1159/000255766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T., Musatov S., Ogawa S., Ribeiro A., Pfaff D.W., Agmo A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res. 2010;210(2):211–220. doi: 10.1016/j.bbr.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Stallings J., Fleming A.S., Corter C., Worthman C., Steiner M. The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpostpartum. Women Parent. 2001;1(1):71–100. [Google Scholar]
- Strathearn L., Fonagy P., Amico J., Montague P.R. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succu S., Sanna F., Melis T., Boi A., Argiolas A., Melis M.R. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: involvement of central oxytocin. Neuropharmacology. 2007;52(3):1034–1043. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Summar M.L., Phillips J.A., 3rd, Battey J., Castiglione C.M., Kidd K.K., Maness K.J., Weiffenbach B., Gravius T.C. Linkage relationships of human arginine vasopressin-neurophysin-II and oxytocin-neurophysin-I to prodynorphin and other loci on chromosome 20. Mol. Endocrinol. 1990;4(6):947–950. doi: 10.1210/mend-4-6-947. [DOI] [PubMed] [Google Scholar]
- Swaab D.F. Sexual differentiation of the brain and behavior. Best Pract. Res. Clin. Endocrinol. Metabol. 2007;21(3):431–444. doi: 10.1016/j.beem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y., Yoshida M., Bielsky I.F., Ross H.E., Kawamata M., Onaka T., Yanagisawa T., Kimura T., Matzuk M.M., Young L.J., Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.E., Gonzaga G.C., Klein L.C., Hu P., Greendale G.A., Seeman T.E. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006;68(2):238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Thackare H., Nicholson H.D., Whittington K. Oxytocin—its role in male reproduction and new potential therapeutic uses. Hum. Reprod. Update. 2006;12(4):437–448. doi: 10.1093/humupd/dmk002. [DOI] [PubMed] [Google Scholar]
- Theodoridou A., Rowe A.C., Penton-Voak I.S., Rogers P.J. Oxytocin and social perception: oxytocin increases perceived facial trust and attractiveness. Horm. Behav. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Thibonnier M., Conarty D.M., Preston J.A., Plesnicher C.L., Dweik R.A., Erzurum S.C. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140(3):1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- Thomas S.A., Matsumoto A.M., Palmiter R.D. Noradrenaline is essential for mouse fetal development. Nature. 1995;374(6523):643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Thomas S.A., Marck B.T., Palmiter R.D., Matsumoto A.M. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J. Neurochem. 1998;70(6):2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Thomas S.A., Palmiter R.D. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Thompson R.R., George K., Walton J.C., Orr S.P., Benson J. Sex-specific influences of vasopressin on human social communication. Proc. Natl. Acad. Sci. U.S.A. 2006;103(20):7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Z. Tierpsychol. 1963;20:410–433. [Google Scholar]
- Tops M., van Peer J.M., Korf J., Wijers A.A., Tucker D.M. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44(3):444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Trainor B.C., Marler C.A. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. Biol. Sci./R. Soc. 2002;269(1493):823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E., Charpak S., Schmidt A., Dubois-Dauphin M., Dreifuss J.J. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J. Neurosci. 1989;9(5):1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E.Z., Als H., Brazelton T.B. Monadic phases: a structural descriptive analysis of infant–mother normal interaction. Merrill-Palmer Q. 1980;26:3–24. [Google Scholar]
- Turchan J., Anderson C., Hauser K.F., Sun Q., Zhang J., Liu Y., Wise P.M., Kruman I., Maragos W., Mattson M.P., Booze R., Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.A., Altemus M., Enos T., Cooper B., McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry. 1999;62(2):97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Uckert S., Becker A.J., Ness B.O., Stief C.G., Scheller F., Knapp W.H., Jonas U. Oxytocin plasma levels in the systemic and cavernous blood of healthy males during different penile conditions. World J. Urol. 2003;20(6):323–326. doi: 10.1007/s00345-002-0300-5. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin linked antistress effects—the relaxation and growth response. Acta Physiol. Scan. Suppl. 1997;640:38–42. [PubMed] [Google Scholar]
- Uvnas-Moberg K. Antistress pattern induced by oxytocin. News Physiol. Sci. 1998;13:22–25. doi: 10.1152/physiologyonline.1998.13.1.22. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K., Arn I., Jonsson C.O., Ek S., Nilsonne A. The relationships between personality traits and plasma gastrin, cholecystokinin, somatostatin, insulin, and oxytocin levels in healthy women. J. Psychosom. Res. 1993;37(6):581–588. doi: 10.1016/0022-3999(93)90052-h. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K., Widstrom A.M., Werner S., Matthiesen A.S., Winberg J. Oxytocin and prolactin levels in breast-feeding women. Correlation with milk yield and duration of breast-feeding. Acta Obstet. Gyn. Scand. 1990;69(4):301–306. doi: 10.3109/00016349009036151. [DOI] [PubMed] [Google Scholar]
- van Leengoed E., Kerker E., Swanson H.H. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J. Endocrinol. 1987;112(2):275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Blume A., Niederie D., Buwalda B., Neumann I.D. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Bredewold R., Neumann I.D. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice. Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Veening J.G., Barendregt H.P. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7 doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening J.G., de Jong T., Barendregt H.P. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiol. Behav. 2010;101(2):193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Waldherr M., Neumann I.D. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc. Natl. Acad. Sci. U.S.A. 2007;104(42):16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H., Westberg L., Henningsson S., Neiderhiser J.M., Reiss D., Igl W., Ganiban J.M., Spotts E.L., Pedersen N.L., Eriksson E., Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc. Natl. Acad. Sci. U.S.A. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yu G., Cascio C., Liu Y., Gingrich B., Insel T.R. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav. Neurosci. 1999;113(3):602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhou L., Hulihan T.J., Insel T.R. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J. Comp. Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Weaver I.C., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wehr R., Mansouri A., de Maeyer T., Gruss P. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development. 1997;124:4447–4456. doi: 10.1242/dev.124.22.4447. [DOI] [PubMed] [Google Scholar]
- Weisman, O., Zagoory-Sharon, O., Feldman R., 2011. Intranasal oxytocin improves the autonomic, hormonal and social basis of fatherhood. Poster presentation at the 10th World Congress of Biological Psychiatry, Prague, Czech Republic.
- Weller A., Feldman R. Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides. 2003;24:779–780. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- Wettschureck N., Moers A., Hamalainen T., Lemberger T., Schutz G., Offermanns S. Heterotrimeric G proteins of the Gq/11 family are crucial for the induction of maternal behavior in mice. Mol. Cell. Biol. 2004;24:8048–8054. doi: 10.1128/MCB.24.18.8048-8054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut R., Watanabe K., Pournajafi-Nazarloo H., Schwertz D., Bell A., Carter C.S. Detection of salivary oxytocin levels in lactating women. Dev. Psychobiol. 2009;51(4):367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.R., Catania K.C., Carter S.C. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 1992;26(3):339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Winnicott D. Hogarth; London: 1956. Primary Maternal Preoccupation. [Google Scholar]
- Winslow J.T., Hearn E.F., Ferguson J., Young L.J., Matzuk M.M., Insel T.R. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm. Behav. 2000;37(2):145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow J.T., Insel T.R. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36(2–3):221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Winslow J.T., Insel T.R. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J. Neurosci. 1991;11:2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Yamada K., Suzuki T., Wu Y.P., Kita K., Takahashi S., Ichinose M., Suzuki N. Induction of uPA release in human peripheral blood lymphocytes by [deamino-cysl,d-Arg8]-vasopressin (dDAVP) Am. J. Phys. Endocrinol. Metab. 2004;287:E970–E976. doi: 10.1152/ajpendo.00027.2003. [DOI] [PubMed] [Google Scholar]
- Yang C.R., Bourque C.W., Renaud L.P. Dopamine D2 receptor activation depolarizes rat supraoptic neurones in hypothalamic explants. J. Physiol. 1991;443:405–419. doi: 10.1113/jphysiol.1991.sp018840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm. Behav. 1999;36(3):212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young L.J., Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young W.S., 3rd, Shepard E., Amico J., Hennighausen L., Wagner K.U., LaMarca M.E., McKinney C., Ginns E.I. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J. Neuroendocrinol. 1996;8(11):847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- Zhang T.Y., Hellstrom I.C., Bagot R.C., Wen X., Diorio J., Meaney M.J. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J. Neurosci. 2010;30(39):13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]