Abstract
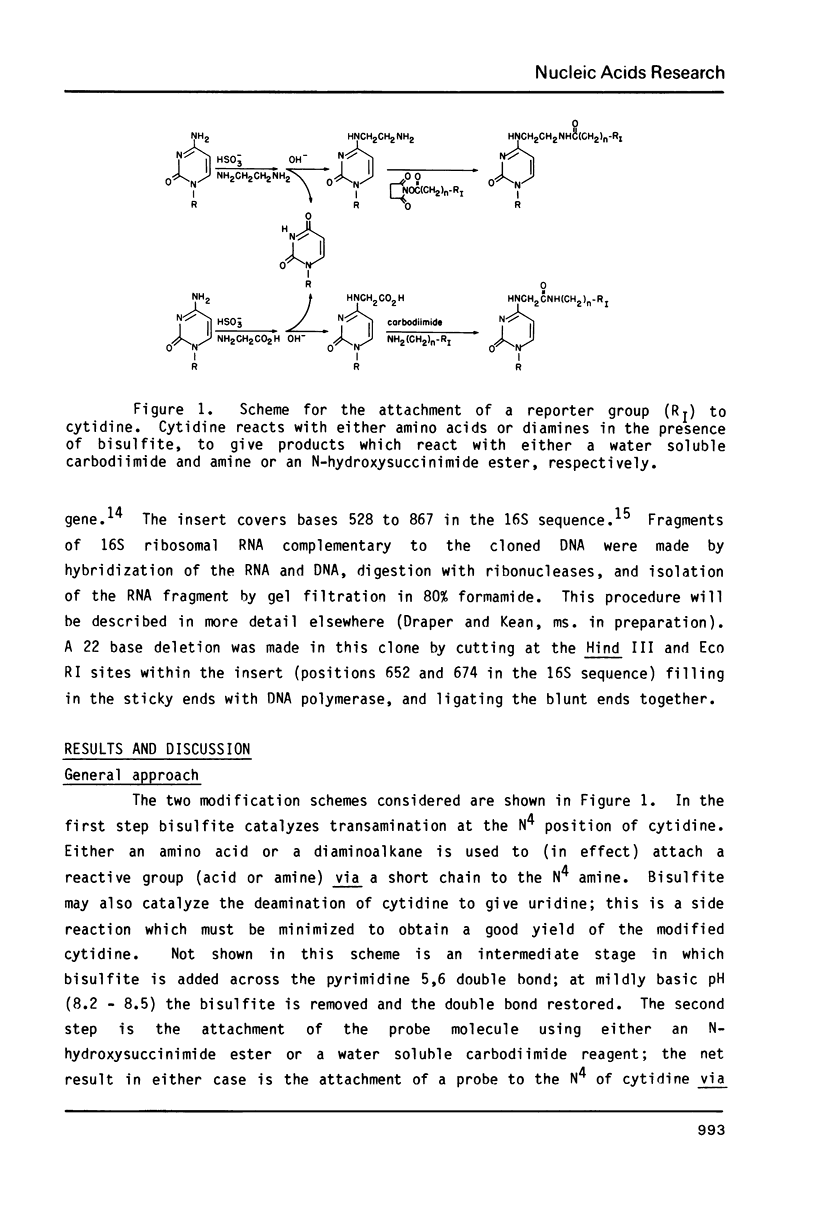
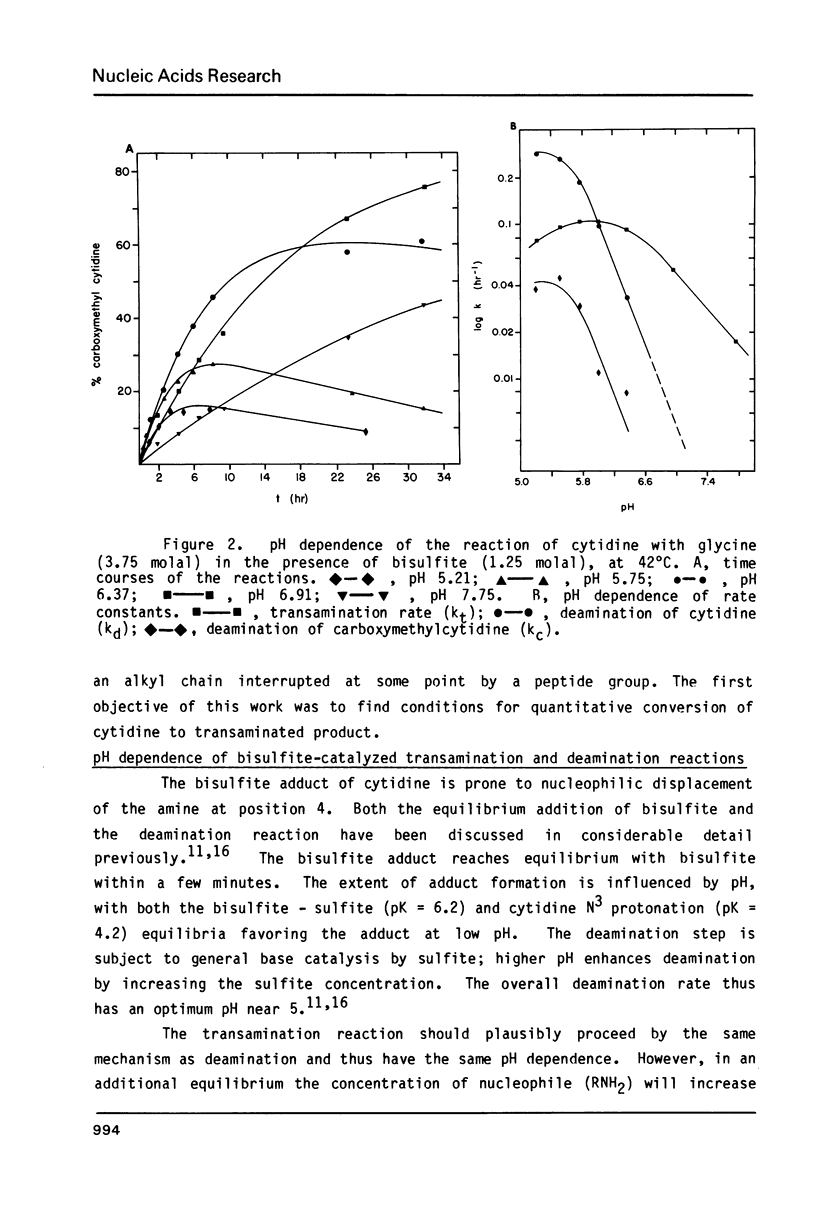
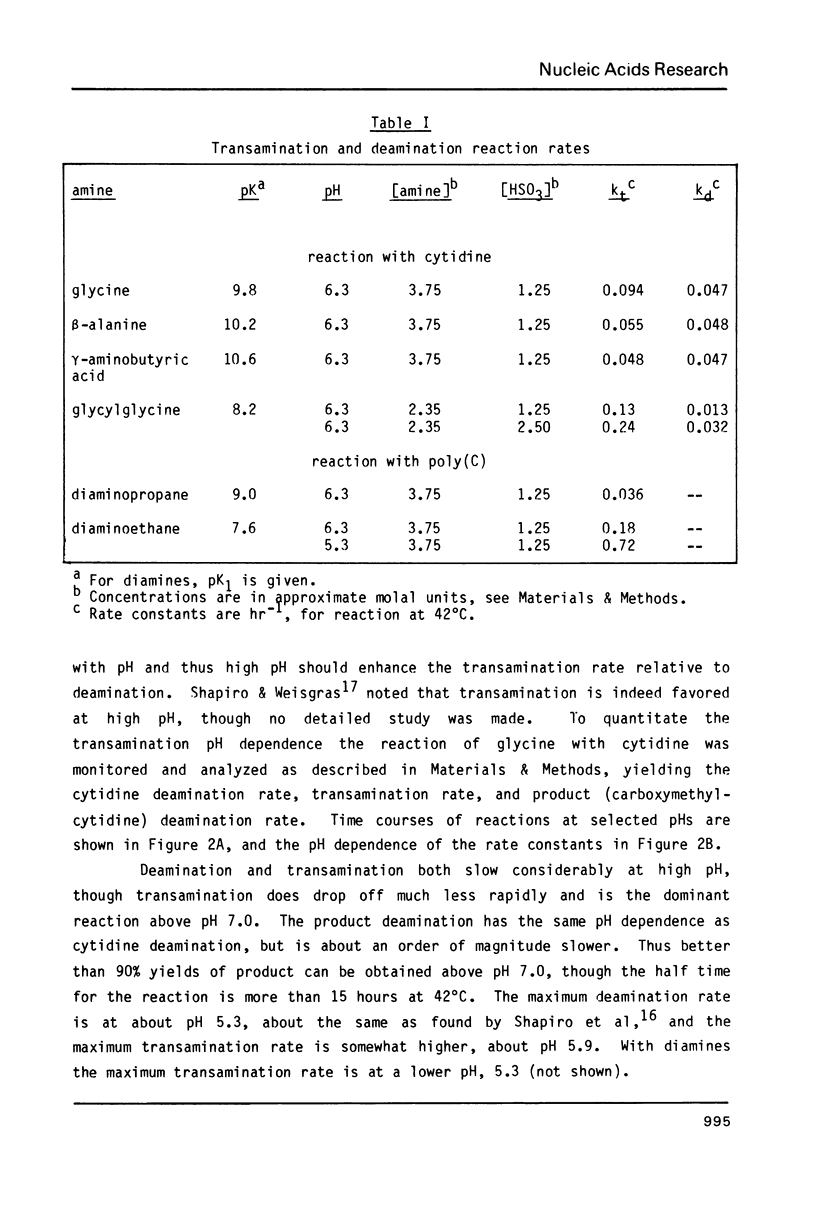
Bisulfite catalyzes transamination of cytidine at the N4 position; the suitability of this reaction for attaching reporter groups to selected cytidine residues in RNA molecules has been investigated. Poly(C) is nearly quantitatively converted to the poly (N4 aminoethyl-C) derivative after 3 hrs at 42 degrees C with ethylene diamine (pK1 = 7.6) and bisulfite. This derivative reacts quantitatively with N-hydroxysuccinimide esters; the linkage of a fluorescent dye, nitrobenzofurazan, to cytidine by this reaction is demonstrated. To direct the bisulfite reaction to selected cytidines within a large RNA molecule, the RNA is hybridized to complementary DNA containing a deletion. Only the cytidines in the single strand RNA loop (corresponding to the DNA deletion) are reactive. Two cytidines in the middle of a 340 base RNA fragment from 16S ribosomal RNA have been modified by this technique.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURR M., KOSHLAND D. E., Jr USE OF "REPORTER GROUPS" IN STRUCTURE-FUNCTION STUDIES OF PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:1017–1024. doi: 10.1073/pnas.52.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. W., Waring R. B., Ray J. A., Brown T. A., Scazzocchio C. Making ends meet: a model for RNA splicing in fungal mitochondria. Nature. 1982 Dec 23;300(5894):719–724. doi: 10.1038/300719a0. [DOI] [PubMed] [Google Scholar]
- Draper D. E., Gold L. A method for linking fluorescent labels to polynucleotides: application to studies of ribosome-ribonucleic acid interactions. Biochemistry. 1980 Apr 29;19(9):1774–1781. doi: 10.1021/bi00550a008. [DOI] [PubMed] [Google Scholar]
- Goddard J. P., Schulman L. H. Conversion of exposed cytidine residues to uridine residues in Escherichia coli formylmethionine transfer ribonucleic acid. J Biol Chem. 1972 Jun 25;247(12):3864–3867. [PubMed] [Google Scholar]
- Hayatsu H. Reaction of cytidine with semicarbazide in the presence of bisulfite. A rapid modification specific for single-stranded polynucleotide. Biochemistry. 1976 Jun 15;15(12):2677–2682. doi: 10.1021/bi00657a030. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Pirkl E., Zentgraf H., Schaller H. Conversion of bacteriophage fd into an efficient single-stranded DNA vector system. Mol Gen Genet. 1980 Jan;177(2):231–242. doi: 10.1007/BF00267434. [DOI] [PubMed] [Google Scholar]
- Karpel R. L., Miller N. S., Lesk A. M., Fresco J. R. Stabilization of the native tertiary stucture of yeast tRNALeu3 by cationic metal complexes. J Mol Biol. 1975 Oct 5;97(4):519–532. doi: 10.1016/s0022-2836(75)80056-8. [DOI] [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Negishi K., Harada C., Ohara Y., Oohara K., Nitta N., Hayatsu H. N4-aminocytidine, a nucleoside analog that has an exceptionally high mutagenic activity. Nucleic Acids Res. 1983 Aug 11;11(15):5223–5233. doi: 10.1093/nar/11.15.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom O. W., Jr, Robbins D. J., Lynch J., Dottavio-Martin D., Kramer G., Hardesty B. Distances between 3' ends of ribosomal ribonucleic acids reassembled into Escherichia coli ribosomes. Biochemistry. 1980 Dec 23;19(26):5947–5954. doi: 10.1021/bi00567a001. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Nathans D. Local mutagenesis within deletion loops of DNA heteroduplexes. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7214–7217. doi: 10.1073/pnas.79.23.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines S. A., Schulman L. H. A new method for attachment of fluorescent probes to tRNA. Methods Enzymol. 1979;59:146–156. doi: 10.1016/0076-6879(79)59076-4. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Braverman B., Louis J. B., Servis R. E. Nucleic acid reactivity and conformation. II. Reaction of cytosine and uracil with sodium bisulfite. J Biol Chem. 1973 Jun 10;248(11):4060–4064. [PubMed] [Google Scholar]
- Shapiro R., Weisgras J. M. Bisulfite-catalyzed transamination of cytosine and cytidine. Biochem Biophys Res Commun. 1970 Aug 24;40(4):839–843. doi: 10.1016/0006-291x(70)90979-4. [DOI] [PubMed] [Google Scholar]
- Sono M., Wataya Y., Hayatsu H. Role of bisulfite in the deamination and the hydrogen isotope exchange of cytidylic acid. J Am Chem Soc. 1973 Jul 11;95(14):4745–4749. doi: 10.1021/ja00795a044. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Hearst J. E. Structure of E. coli 16S RNA elucidated by psoralen crosslinking. Cell. 1983 Apr;32(4):1355–1365. doi: 10.1016/0092-8674(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. Fluorescent derivatives of yeast tRNAPhe. Eur J Biochem. 1979 Aug 1;98(2):465–475. doi: 10.1111/j.1432-1033.1979.tb13207.x. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]