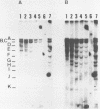
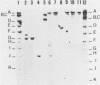
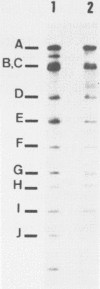
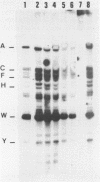
Abstract
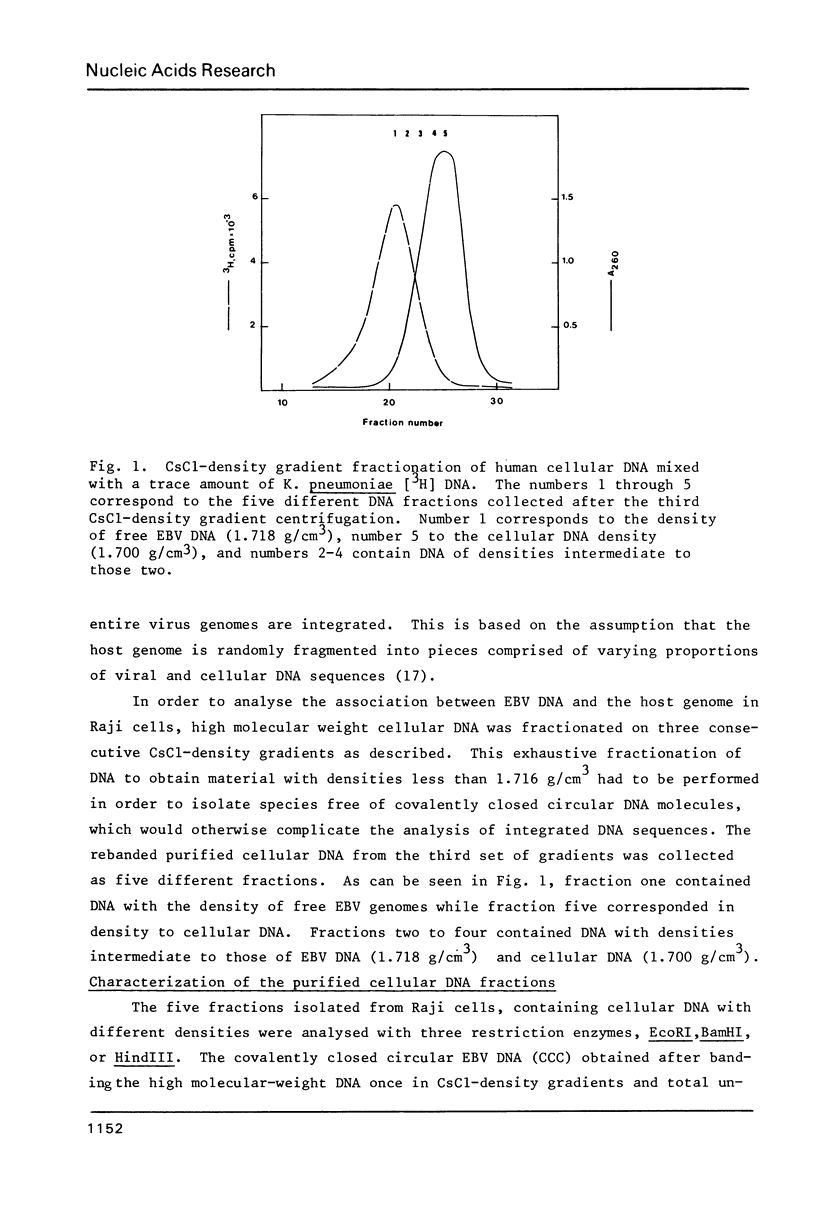
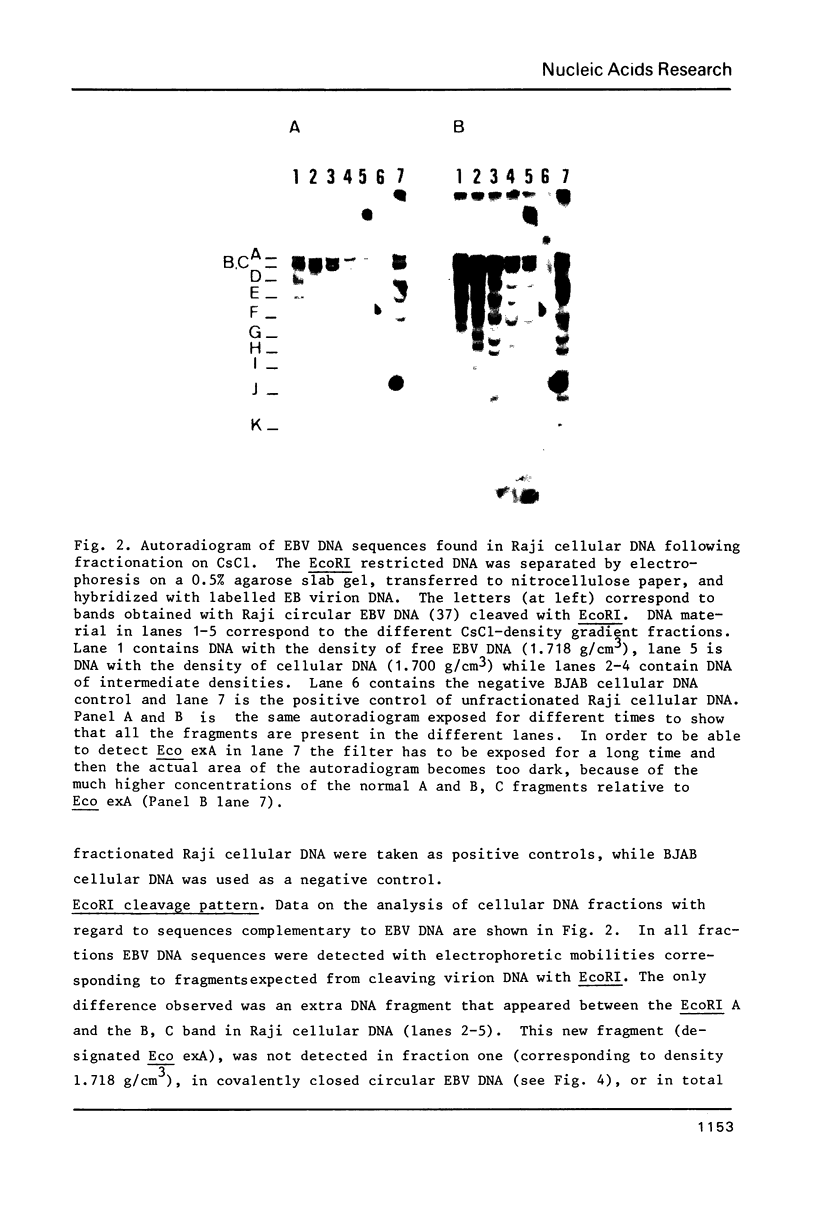
Human lymphoid cell lines cannot be grown in long-term tissue culture, as a rule, unless the cells have been transformed by Epstein-Barr virus (EBV). The latent EBV DNA in established cell lines, is mainly present as free covalently closed circles but viral DNA sequences with properties of integrated DNA also seem to be present. We have extended the studies on the physical state of the EB viral DNA sequences in the cell line Raji which appear at a lower density than that for free EB viral DNA during fractionation on CsCl density gradients. In such material a novel EcoRI EBV DNA fragment is present, which hybridizes to viral sequences homologous to EcoRI A. This fragment is not present in free covalently closed circular EBV DNA. When this EcoRI fragment is further analysed with HindIII a smaller fragment than expected, which contains BamHI W sequences, is detected. The demonstration of this HindIII fragment and its characteristics as a joint, viral-host chromosome fragment will be discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Lindahl T. Intracellular forms of Epstein-Barr virus DNA in Raji cells. IARC Sci Publ. 1975;(11 Pt 1):125–132. [PubMed] [Google Scholar]
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson-Anvret M., Lindahl T. Integrated viral DNA sequences in Epstein-Barr virus-converted human lymphoma lines. J Virol. 1978 Mar;25(3):710–718. doi: 10.1128/jvi.25.3.710-718.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Lindahl T. Epstein-Barr virus DNA in human lymphoid cell lines: in vitro conversion. Virology. 1976 Aug;73(1):96–105. doi: 10.1016/0042-6822(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Rymo L., Walsh J. E., Björck E., Lindahl T., Griffin B. E. Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic Acids Res. 1981 Jul 10;9(13):2999–3014. doi: 10.1093/nar/9.13.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Rigby P. W. Cloning and characterization of the integrated viral DNA from three lines of SV40-transformed mouse cells. Cell. 1981 Aug;25(2):547–559. doi: 10.1016/0092-8674(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuring R., Winterhoff U., Tamanoi F., Stabel S., Doerfler W. Site of linkage between adenovirus type 12 and cell DNAs in hamster tumour line CLAC3. Nature. 1981 Sep 3;293(5827):81–84. doi: 10.1038/293081a0. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of viral DNA into the host genome. Curr Top Microbiol Immunol. 1975;71:1–78. doi: 10.1007/978-3-642-66193-8_1. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Björck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Bar virus DNA in transformed cells. J Virol. 1981 Oct;40(1):11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner T. L., Skalka A. M., Hanafusa H. Integration of Rous sarcoma virus DNA into chicken embryo fibroblasts: no preferred proviral acceptor site in the DNA of clones of singly infected transformed chicken cells. J Virol. 1981 Nov;40(2):421–430. doi: 10.1128/jvi.40.2.421-430.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Khoury G. Integration of DNA tumor virus genomes. Curr Top Microbiol Immunol. 1976;73:35–65. doi: 10.1007/978-3-642-66306-2_2. [DOI] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar M. H., Campo M. S., Laird H., Jarrett W. F. Persistence of non-integrated viral DNA in bovine cells transformed in vitro by bovine papillomavirus type 2. Nature. 1981 Oct 29;293(5835):749–751. doi: 10.1038/293749a0. [DOI] [PubMed] [Google Scholar]
- Nazerian K., Lindahl T., Klein G., Lee L. F. Deoxyribonucleic acid of Marek's disease virus in virus-induced tumors. J Virol. 1973 Oct;12(4):841–846. doi: 10.1128/jvi.12.4.841-846.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Shank P. R., Varmus H. E. Mouse mammary tumor virus DNA in infected rat cells: characterization of unintegrated forms. Cell. 1977 Jan;10(1):19–26. doi: 10.1016/0092-8674(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Rymo L., Lindahl T., Adams A. Sites of sequence variability in Epstein-Barr virus DNA from different sources. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2794–2798. doi: 10.1073/pnas.76.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L., Lindahl T., Povey S., Klein G. Analysis of restriction endonuclease fragments of intracellular Epstein-Barr virus DNA and isoenzymes indicate a common origin of the Raji, NC-37, and F-265 human lymphoid cell lines. Virology. 1981 Nov;115(1):115–124. doi: 10.1016/0042-6822(81)90093-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- zur Hansen H., Diehl V., Wolf H., Schulte-Holthausen H., Schneider U. Occurrence of Epstein-Barr virus genomes in human lymphoblastoid cell lines. Nat New Biol. 1972 Jun 7;237(75):189–190. doi: 10.1038/newbio237189a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Oncogenic Herpes viruses. Biochim Biophys Acta. 1975 Mar 20;417(1):25–53. doi: 10.1016/0304-419x(75)90007-4. [DOI] [PubMed] [Google Scholar]