Abstract
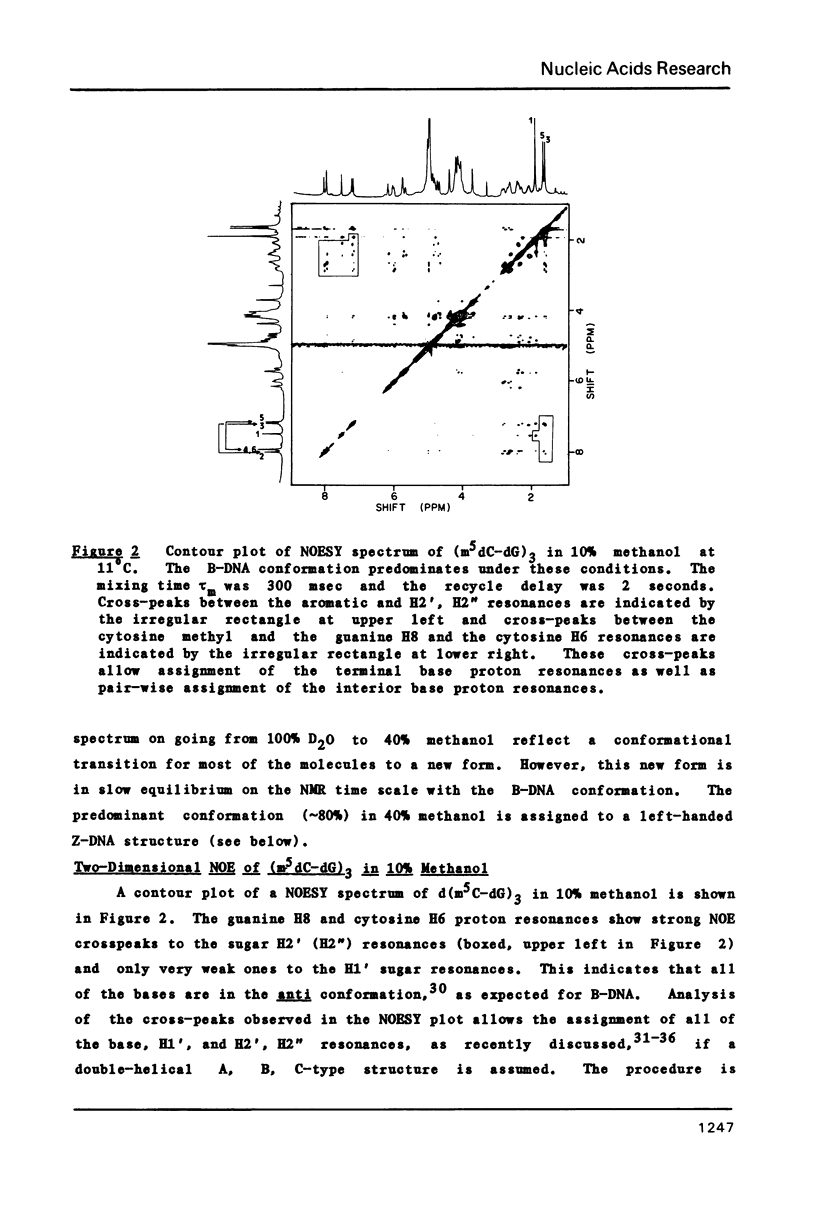
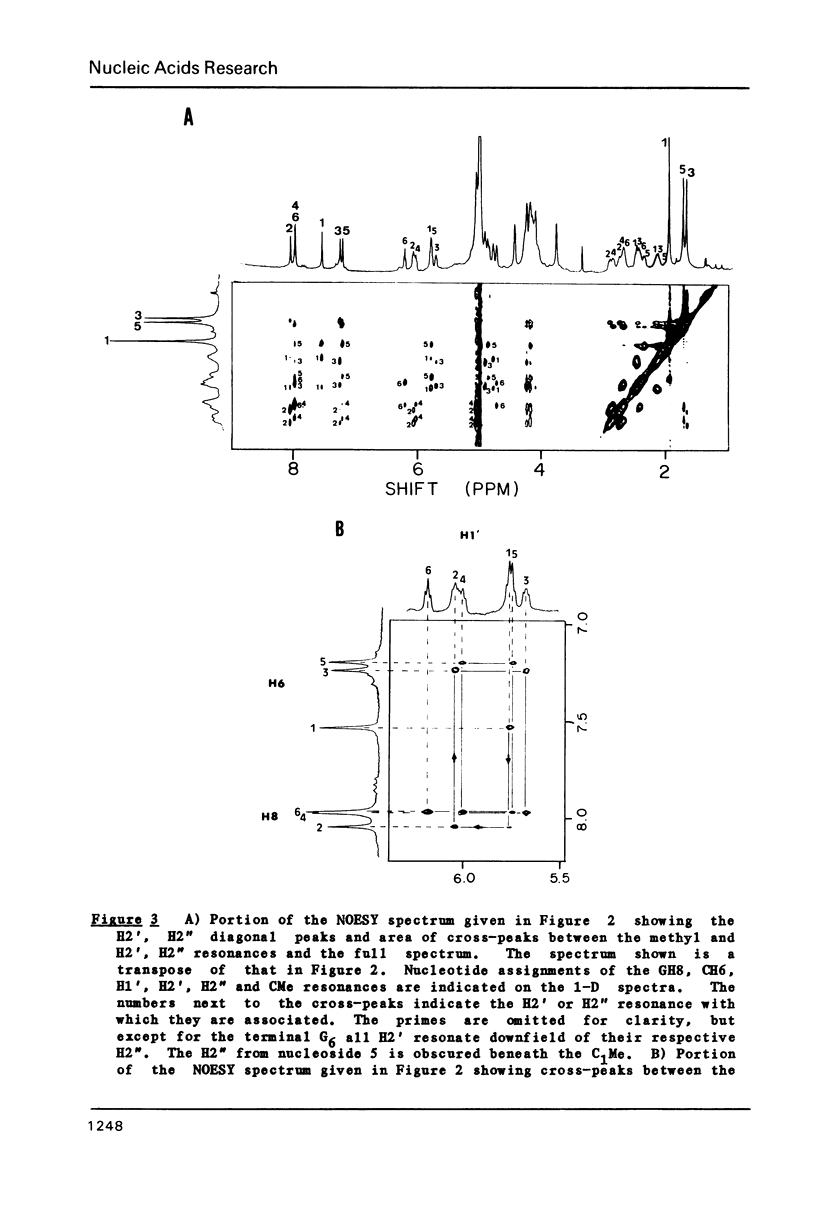
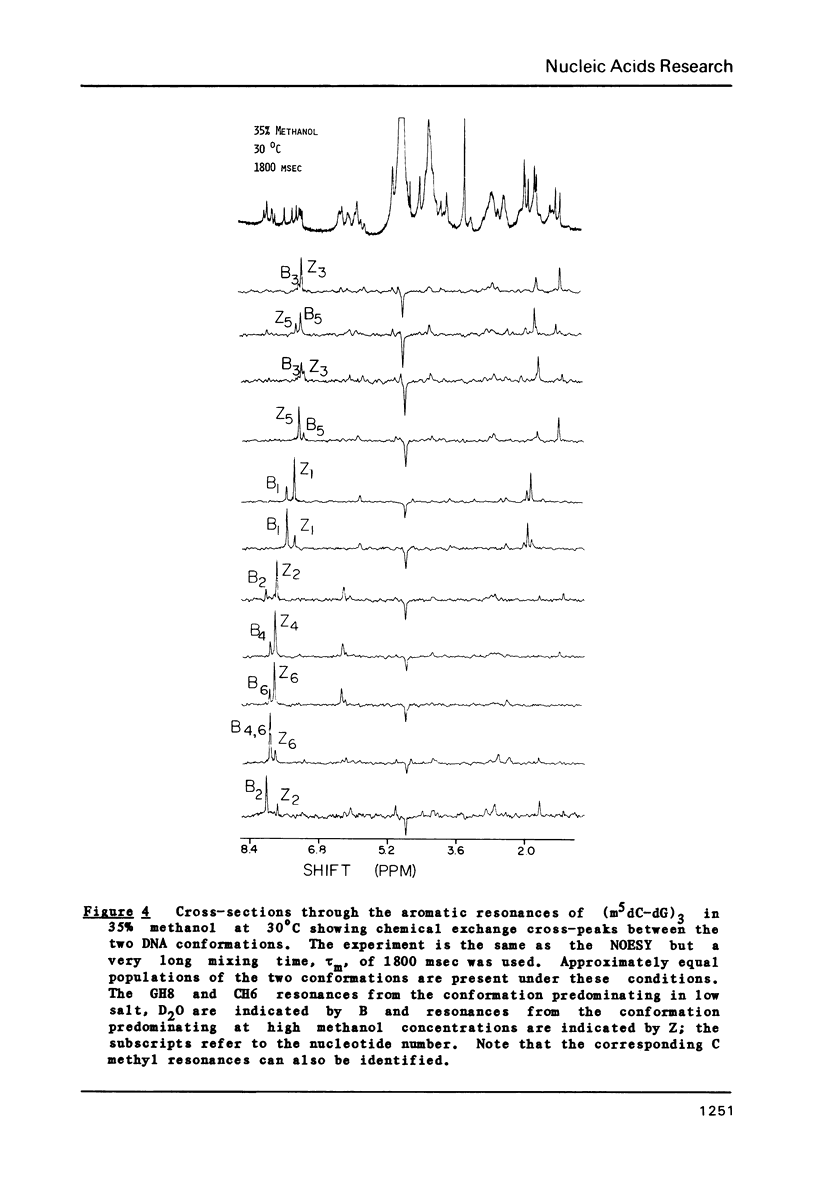
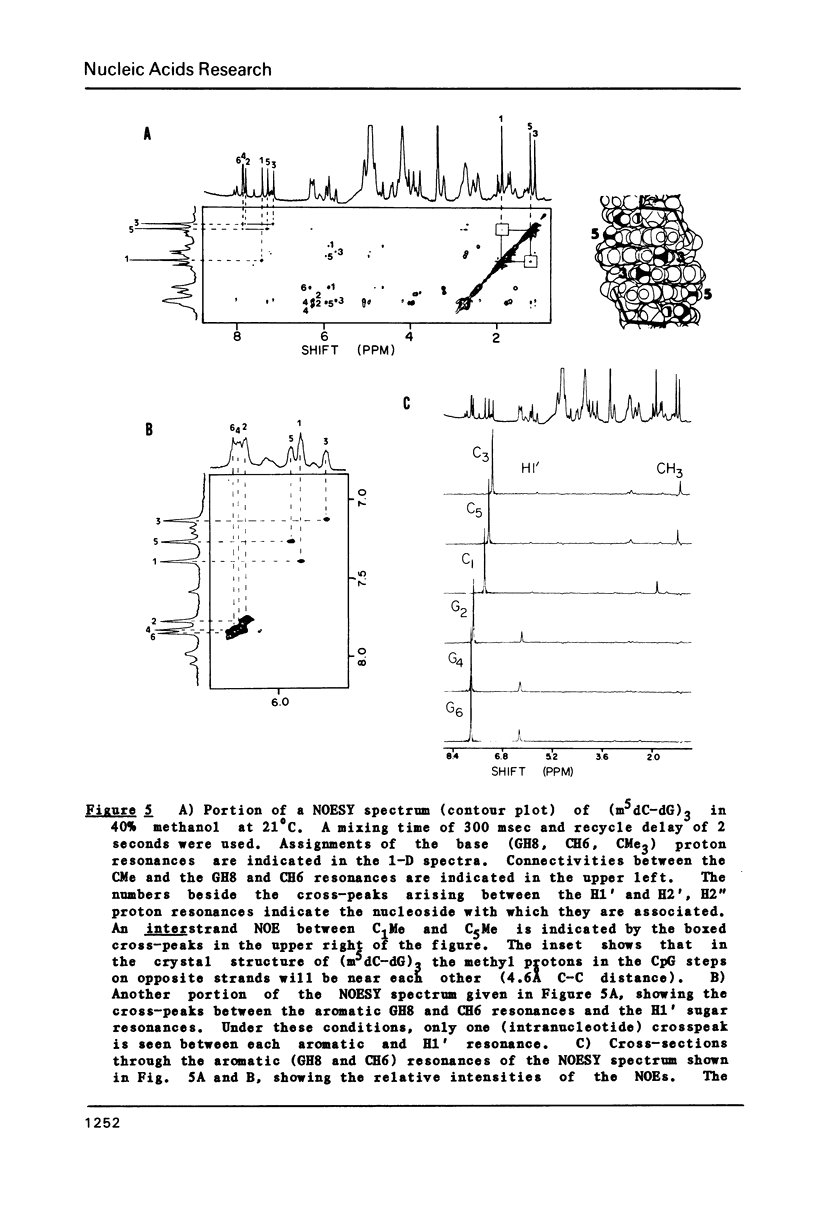
The deoxyribose hexanucleoside pentaphosphate (m5dC-dG)3 has been studied by 500 MHz 1H NMR in D2O (0.1 M NaCl) and in D2O/deuterated methanol mixtures. Two conformations, in slow equilibrium on the NMR time scale, were detected in methanolic solution. Two-dimensional nuclear Overhauser effect (NOE) experiments were used to assign the base and many of the sugar resonances as well as to determine structural features for both conformations. The results were consistent with the an equilibrium in solution between B-DNA and Z-DNA. The majority of the molecules have a B-DNA structure in low-salt D2O and a Z-DNA structure at high methanol concentrations. A cross-strand NOE between methyl groups on adjacent cytosines is observed for Z-DNA but not B-DNA. The B-DNA conformation predominates at low methanol concentrations and is stabilized by increasing temperature, while the Z-DNA conformation predominates at high methanol concentrations and low temperatures. 31P NMR spectra gave results consistent with those obtained by 1H NMR. Comparison of the 31P spectra with those obtained on poly(dG-m5dC) allow assignment of the lower field resonances to GpC in the Z conformation.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Nordheim A., Rich A. Formation of Z-DNA in negatively supercoiled plasmids is sensitive to small changes in salt concentration within the physiological range. EMBO J. 1983;2(5):649–655. doi: 10.1002/j.1460-2075.1983.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Cohen J. S., Behe M. B to Z transition of double-stranded poly[deoxyguanylyl(3'-5')-5-methyldeoxycytidine] in solution by phosphorus-31 and carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Apr 26;22(9):2136–2142. doi: 10.1021/bi00278a013. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Feigon J., Wright J. M., Denny W. A., Leupin W., Kearns D. R. Application of multiple-pulse H-NMR techniques to the study of two synthetic DNA decamers. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):207–217. doi: 10.1101/sqb.1983.047.01.026. [DOI] [PubMed] [Google Scholar]
- Fujii S., Wang A. H., van der Marel G., van Boom J. H., Rich A. Molecular structure of (m5 dC-dG)3: the role of the methyl group on 5-methyl cytosine in stabilizing Z-DNA. Nucleic Acids Res. 1982 Dec 11;10(23):7879–7892. doi: 10.1093/nar/10.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hartmann B., Thuong N. T., Pouyet J., Ptak M., Leng M. Spectroscopic studies of (m5dC-dG)3: thermal stability of B- and Z-forms. Nucleic Acids Res. 1983 Jul 11;11(13):4453–4466. doi: 10.1093/nar/11.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Valle R. P., Möller A., Nordheim A., Schur P. H., Rich A., Stollar B. D. Z-DNA-specific antibodies in human systemic lupus erythematosus. J Clin Invest. 1983 Feb;71(2):314–321. doi: 10.1172/JCI110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Morgenegg G., Celio M. R., Malfoy B., Leng M., Kuenzle C. C. Z-DNA immunoreactivity in rat tissues. Nature. 1983 Jun 9;303(5917):540–543. doi: 10.1038/303540a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Tran-Dinh S., Taboury J., Neumann J. M., Huynh-Dinh T., Genissel B., Gouyette C., Igolen J. B and Z double helical conformations of d-(m5C-G-C-G-m5C-G) in aqueous solution. FEBS Lett. 1983 Apr 18;154(2):407–410. doi: 10.1016/0014-5793(83)80192-6. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


