Abstract
Many G protein-coupled receptors (GPCR) exert patterns of cell-specific signaling and function. Mounting evidence now supports the view that cytoplasmic adapter proteins contribute critically to this behavior. Adapter proteins recognize highly conserved motifs such as those for Src homology 3 (SH3), phosphotyrosine-binding (PTB), and postsynaptic density 95/discs-large/zona occludens (PDZ) docking sequences in candidate GPCRs. Here we review the behavior of the Na+/H+ exchange regulatory factor (NHERF) family of PDZ adapter proteins on GPCR signalling, trafficking, and function. Structural determinants of NHERF proteins that allow them to recognize targeted GPCRs are considered. NHERF1 and NHERF2 are capable also of modifying the assembled complex of accessory proteins such as β-arrestins, which have been implicated in regulating GPCR signaling. In addition, NHERF1 and NHERF2 modulate GPCR signaling by altering the G protein to which the receptor binds or affect other regulatory proteins that affect GTPase activity, protein kinase A, phospholipase C, or modify downstream signaling events. Small molecules targeting the site of NHERF1-GPCR interaction are being developed and may become important and selective drug candidates.
I. Introduction: Historical Background and Discovery
The discovery of the Na+/H+ exchange regulatory factor (NHERF1) family of scaffolding proteins has its origins in two distinct lines of investigation. The first has a longer history and began with studies directed at uncovering the structural basis for the targeting and asymmetric association of membrane-delimited proteins in polarized epithelial cells. This work led to the identification of ezrin, which was shown to link cell membranes with the cytoskeleton (Bretscher, 1983). These studies, in turn, led to a search for additional proteins that bind to ezrin and its related family members radixin, moesin, and merlin and that regulate cytoskeletal-plasma membrane interactions. Using immobilized N-terminal peptide fragments of ezrin and moesin, Reczek et al. (1997) identified a 50-kDa protein that they called ezrin-radixin-moesin-binding phosphoprotein 50.2 During the same time frame, Weinman et al. (1993) independently were searching for a phosphoprotein cofactor that was required for the inhibition of renal Na+/H+ exchanger isoform-3 (NHE3). Those studies uncovered a cytoplasmic protein that was a substrate for protein kinase A phosphorylation and was distinct from the Na+/H+ exchanger but regulated its activity. They named the protein NHERF for Na+/H+ exchanger regulatory factor (Morell et al., 1990; Weinman et al., 1995). It was appreciated that the two proteins were identical (Reczek et al., 1997).
The closely related gene product E3KARP, now called NHERF2 (Table 1), was discovered by Yun and Donowitz (1997, 1998) by using a yeast two-hybrid screen for cDNAs encoding proteins that interact with the cytoplasmic domain of NHE3, which was used as bait. The identified cDNA encoded a protein of 451 residues, which they named E3KARP (NHE3 kinase A regulatory protein). E3KARP and NHERF share 44% identity, mostly within the first 260 amino acids. Although they differ importantly in other ways, as detailed below, it is noteworthy that in tissues where they are coexpressed, they largely can support the same functions.
TABLE 1.
Gene names and synonyms for NHERF isoforms
All terminology is from http://www.uniprot.org (UniProt Consortium, 2011).
| Isoform | Gene | Synonyms |
|---|---|---|
| NHERF1 | SLC9A3R1 | Ezrin-radixin-moesin-binding phosphoprotein-50 (EBP50) |
| NHERF2 | SLC9A3R2 | NHE3 kinase A regulatory protein (E3KARP); tyrosine kinase activator protein-1 (TKA-1); SRY-interacting protein 1 (SIP-1) |
| NHERF3 | PDZK1 | CFTR-associated protein of 70 kDa (CAP70); Na/Pi cotransporter carboxyl-terminal-associated protein-1 (NaPi-Cap1); PDZ domain-containing protein-1 (PDZD1) |
| NHERF4 | PDZD3 | Intestinal and kidney-enriched PDZ protein (IKEPP); natrium-phosphate cotransporter IIa carboxy-terminal-associated protein-2 (NaPi-Cap2); PDZ domain-containing protein-2 (PDZK2) |
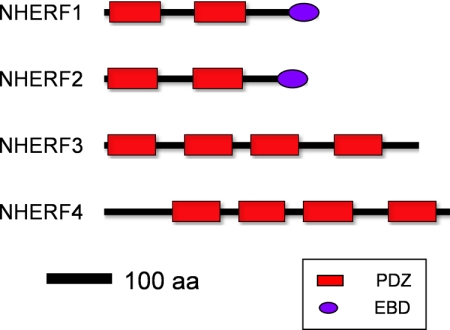
PDZK1, now called NHERF3 (Table 1), was also discovered using a yeast two-hybrid screen searching for proteins that were up-regulated in response to dietary phosphate restriction (Custer et al., 1997; Gisler et al., 2001). NHERF3 possesses four PDZ domains but lacks the carboxyl-terminal ezrin-binding domain found in NHERF1 and NHERF2 (Fig. 1). It is noteworthy that although NHERF3 plays an important role in phosphate homeostasis, its role is clearly different from that of NHERF1 (Kocher et al., 2003; Giral et al., 2011). Hence, they would not seem to have biologically redundant roles.
Fig. 1.
Schematic representation of human NHERF proteins discussed in this review. PDZ domains are indicated by the red rectangular boxes and EBDs by purple ellipses. The relative scale is shown on the bottom.
Finally, NHERF4, which closely resembles NHERF3 (Fig. 1) was identified in a search for proteins interacting with the type IIa sodium-phosphate cotransporter (Npt2a) and was initially named NaPi-Cap2 (Gisler et al., 2001) and independently GCC “interacting protein intestine and kidney enriched PDZ protein” (IKEPP) (Scott et al., 2002). It has since been designated PDZK2 and, now, NHERF4 (Table 1) (Thelin et al., 2005).
II. Structure and Structural Determinants of Na+/H+ Exchange Regulatory Factor Proteins
Mounting evidence highlights the role of cytoplasmic adapter proteins that are responsible for the cell-specific pattern of signaling of select GPCRs and contribute to the ligand bias displayed by these receptors. Most adapter proteins interact with their targets through well conserved modular protein-protein domains. Common modular motifs include SH2, SH3, WW, PTB, and PDZ domains (Scott and Pawson, 2009). Proteins containing PDZ domains, named for the first three proteins in which they were described (postsynaptic density 95/discs-large/zona occludens), are the most abundant protein modules that function as scaffolding agents to assemble multiprotein signaling complexes. Scaffolding proteins may harbor single or multiple PDZ domains and may include other protein-protein interaction modules as well. The Na+/H+ exchanger regulatory factors form a family of adaptor proteins consisting of four members.3 NHERF1 (SLC9A3R1) and NHERF2 (SLC9A3R2) possess two nonidentical, type 1 tandem PDZ domains and a carboxyl-terminal ezrin-binding domain (EBD), whereas NHERF3 (PDZK1) and NHERF4 (PDZD3) have four PDZ domains but lack an EBD (Fig. 1) (Seidler et al., 2009).
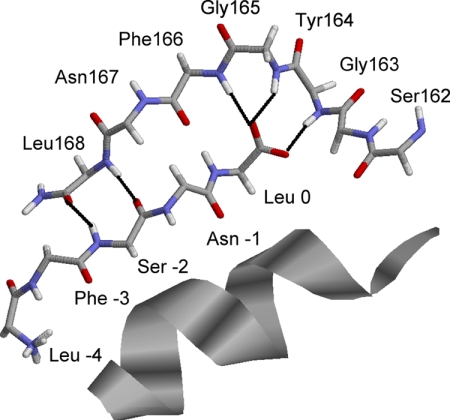
PDZ domains consist of an 80- to 90-amino-acid three-dimensional globular structure composed of six β-sheets and two α-helices (Karthikeyan et al., 2001b). The carboxylate-binding loop contains a core motif of GLGF (Ponting et al., 1997) or a related sequence (GYGF in the case of the NHERF proteins). Three classes of PDZ domains have been described on the basis of the binding motif of the ligand with which they interact. Class I PDZ proteins interact with ligands terminating in the sequence [S/T]-X-Φ, where X is promiscuous and Φ is a hydrophobic residue, generally Leu but also Ile, Val, or Met. By convention, numbering of the ligand sequence begins with 0 for the terminal position. Some class I PDZ proteins such as NHERF additionally favor [D/E] at -3. Class II PDZ modules prefer a ligand motif of the form X-Φ-X-Φ; and Class III PDZ proteins recognize [D/E/K/R]-X-Φ (Songyang et al., 1997; Stricker et al., 1997). PDZ ligands dock in a groove between the second β-sheet and the second α-helix. For Class I PDZ proteins, the terminal hydrophobic amino acid of the ligand occupies a hydrophobic cavity at the top of the groove (Fig. 2). The ligand preferences of class I and II PDZ domains result from the presence at the distal end of the second α-helix of a histidine in class I, which favors Ser or Thr at the -2 position of the ligand, and a hydrophobic pocket in class II that favors a hydrophobic amino acid at the corresponding location.
Fig. 2.
Position of the carboxyl-terminal PTHR (PTHR-CT ligand) in the binding groove of the PDZ2 domain (only the peptide backbone is shown). The α2-helix is displayed as a ribbon. The carboxylate binding loop (Gly-163, Tyr-164, Gly-165, and Phe-166) and β2 strand (Asn-167 and Leu-168) are shown as the polypeptide backbone chain (stick representation). The carbon atoms of the PTHR-CT ligand are yellow, the oxygen atoms are red, and the nitrogen atoms are blue. H-bond interactions are indicated by black dotted lines. The proximity of Ser-162 to this site is evident. Predicted electrostatic interactions between the Glu -3 side chain and Arg-180 (β3 strand) are depicted. (Graphic kindly prepared by Dr. T. Mamonova.)
Based on the particular amino acid sequence, a PDZ domain of either class may favor specific ligands on the basis of residues located at the -3, -4, and -5 positions (Songyang et al., 1997; Hock et al., 1998). The preferred carboxyl-terminal motifs for interaction with NHERF1 PDZ1 were first worked out independently by two different groups (Hall et al., 1998a; Wang et al., 1998a). Although different technical approaches were applied to define the preferred NHERF1 PDZ1-binding motif [unbiased screening of a peptide library (Wang et al., 1998a) versus targeted mutagenesis (Hall et al., 1998a)], both groups identified D-T-R-L as the sequence favored for binding by NHERF1 PDZ1. This sequence is found as the last four amino acids of CFTR. This led to the recognition of CFTR as a binding partner of NHERF family proteins (along with work by Short et al. (1998), who independently discovered the CFTR/NHERF1 interaction in a manner that did not involve working out the optimal motif for NHERF1 PDZ1 binding.
The PDZ domains of NHERF1 contain an arginine that can electrostatically interact with a glutamate or aspartate at the -3 position of the target binding partner (Fig. 2) (Karthikeyan et al., 2001a,b). Although it prefers targets with Glu/Asp at the -3 position, NHERF1 is still able to interact with targets that have a different amino acid at -3 (e.g., Npt2a, which terminates in A-T-R-L) (Shenolikar et al., 2004). Additional selectivity arises from upstream positions 5 to 7 that further define the specificity of interaction with the respective PDZ protein (Zhang et al., 2006). Residues as far as 18 amino acids upstream of the carboxyl terminus of NHERF1, for example, participate in establishing the recognition site (Mahon and Segre, 2004).
A. Na+/H+ Exchange Regulatory Factor Dimerization
NHERF1 and NHERF2 may homo- or heterodimerize. The precise means of multimerization is unclear. Two mechanisms of NHERF1 dimerization have been described. The first involves head-to-tail dimerization (Morales et al., 2007), whereas the second involves dimerization of PDZ domains (Fouassier et al., 2000; Lau and Hall, 2001; Shenolikar et al., 2001). Head-to-tail dimerization of NHERF1 is mediated by the interaction of the carboxyl terminus, which itself is a PDZ-binding sequence (F-S-N-L) with PDZ2 (Morales et al., 2007).
In some instances, NHERF dimerization is promoted by the presence of a carboxyl-terminal PDZ-recognition peptide, such as PDGFR-CT (Maudsley et al., 2000) or the β2-adrenergic receptor (β2-AR)-CT (Lau and Hall, 2001). Similar results occur with the parathyroid hormone receptor type I (PTH1R)-CT (B. Wang and P. Friedman, unpublished observations). Limited evidence for in vivo NHERF1 dimerization is available (Hammad et al., 2010), and it is uncertain whether dimerization occurs in vivo and its biologically meaning has been questioned (Garbett et al., 2010).
III. Tissue, Cell, and Subcellular Localization
Biochemical and cell biological studies suggest that where they coexist, NHERF proteins perform overlapping functions as regulators of transmembrane receptors, transporters, and other proteins localized at or near the plasma membrane (Voltz et al., 2001). Superficially, each of the NHERF family of PDZ proteins seems quite similar. Closer inspection, however, reveals many differences in cellular expression, subcellular localization, and biochemical properties. Despite the resemblance between their PDZ domains, NHERF proteins exhibit different affinities for PDZ-binding partners (Wang et al., 2010) that may determine unique localizations and functions for each family member. These differences probably affect in vivo function and will help explain the unique aspects of the NHERF proteins and the coexpression of such seemingly similar proteins within tissues such as kidney tubules and gastrointestinal tract (Thelin et al., 2005). Table 2 summarizes tissues exhibiting high levels of NHERF expression.
TABLE 2.
Tissues highly enriched with NHERF family members
| NHERF | Tissue |
|---|---|
| NHERF 1 | Kidney (proximal tubules), gastrointestinal tract (small intestine, colon, and gastric parietal cells), airway epithelium, liver, mesothelium, breast, placenta, brain |
| NHERF 2 | Kidney (glomeruli, peritubular capillaries, collecting duct principal cells and weakly in proximal tubules), gastrointestinal tract (small intestine and colon), lung, colon cancer, brain |
| NHERF 3 | Kidney, gastrointestinal tract, liver |
| NHERF 4 | Kidney, gastrointestinal tract |
Expression studies show that mRNA for the four members of the NHERF family is differentially and widely expressed (Reczek et al., 1997; Yun et al., 1997; Kocher et al., 1998; Su et al., 2004). NHERF1 and NHERF2 seem to be the most broadly distributed and abundantly expressed (Thelin et al., 2005).
NHERF1 is enriched in tissues possessing extensive polarized epithelia. These include kidney, placenta, and liver (Reczek et al., 1997). NHERF1 is generally described as a brush-border protein in kidney proximal tubules and regions of the gastrointestinal tract (small intestine, colon); it is additionally found in the airway epithelium, gastric parietal cells, and brain (Weinman et al., 1995; Reczek et al., 1997; Yun et al., 1997; Mohler et al., 1999; Ingraffea et al., 2002; Scott et al., 2002; Kocher et al., 2003).
NHERF2 has a more restricted tissue distribution, with the highest expression found in lung. It is coexpressed with NHERF1 in kidney, albeit with a distinct distribution. Whereas NHERF1 is localized to proximal tubules, NHERF2 is expressed in glomeruli and in collecting ducts and not in proximal convoluted tubules. NHERF2 is also found throughout the renal vasculature, including the descending vasa rectae (Wade et al., 2001; Ingraffea et al., 2002).
Using antibodies specific to NHERF1 or NHERF2 for localization in murine tissues, Ingraffea et al. (2002) found that the cellular distribution of NHERF1 and NHERF2 is mutually exclusive. NHERF1 and ezrin are generally coexpressed in epithelial cells, such as intestinal epithelial cells, gastric parietal cells, tubular epithelial cells of the kidney proximal tubule, the terminal bronchiole of the lung, and in mesothelia. This correlation is not absolute, insofar as cells of the mucous epithelium of the stomach and in the glomerulus express ezrin but not detectable NHERF1, whereas the hepatic bile canaliculi express NHERF1 but not ezrin. NHERF2, conversely has no preference for coexpression with ezrin in polarized epithelia (Ingraffea et al., 2002). NHERF2 has also been localized in astrocytic processes and postsynaptic neuronal elements of the mouse brain (Paquet et al., 2006).
NHERF3 shares much of the tissue distribution with NHERF1 and NHERF2 including expression in the brush-border of epithelial cells forming the kidney proximal tubule and the small intestine (Kocher et al., 1998) and is present also in liver (Custer et al., 1997; Gisler et al., 2001).
NHERF4 is expressed at the apical cell surface of intestine and kidney epithelia (Gisler et al., 2001; Thelin et al., 2005). NHERF4 is distinct from the other family members in that it displays the most restricted tissue expression of all the NHERFs, being found only at significant levels in the gastrointestinal tract and kidney (Thelin et al., 2005).
Subcellular localization of NHERFs varies depending on the family member and the tissue (Table 3). In general, NHERFs are primarily localized at or near the actin cytoskeleton underlying the apical plasma membrane (Yun et al., 1998). NHERF1 and NHERF2 are predominantly found in brush-border membranes, although some NHERF1 is present in basolateral membranes (Weinman and Shenolikar, 1997; Wade et al., 2001). NHERF1 and -2 are substantially enriched at specialized cell surface structures associated with the ezrin-radixin-moesin family of proteins (Bretscher et al., 2002). NHERF1 and NHERF2 possess carboxyl-terminal ezrin-binding domains that indirectly tether these proteins to the actin cytoskeleton by additional protein interactions (Bretscher et al., 1997; Reczek et al., 1997; Short et al., 1998; Sun et al., 2000).
TABLE 3.
Subcellular distribution of NHERF proteins
| Location | |
|---|---|
| NHERF 1 | Brush-border and basolateral membranes (slight) |
| Actin-rich structures including membrane ruffles, microvilli, and filopodia | |
| Cell surface structures with ERM proteins | |
| Apical regions in the membrane | |
| NHERF 2 | Brush-border membranes |
| Cell surface structures with ERM proteins | |
| Apical and subapical regions and cytosol | |
| NHERF 3 | Brush-border membranes |
| Apical and subapical structures | |
| NHERF 4 | Subapical regions and cytosol |
NHERF2 is found in the brush-border membranes with NHERF1 but also at an adjacent site below this region, where NHERF2 but not NHERF1 colocalizes with clathrin. Electron microscopic studies showed that NHERF1 was exclusively detected in the brush-border microvilli. NHERF2, on the other hand, was largely restricted to an apical band of small vesicles and minimally associated with microvilli (Seidler et al., 2009). NHERF2 is commonly found in conjunction with moesin and radixin, especially in pulmonary alveoli (Ingraffea et al., 2002).
NHERF3 resides on or immediately under the plasma membrane (Gisler et al., 2003; Donowitz et al., 2005). In human colon cancer Caco-2 cells and renal proximal tubule cells, NHERF3 is predominantly found in apical brush-border membranes (Gisler et al., 2003; Donowitz et al., 2005). NHERF3 and NHERF1 are highly localized to the brush-border, whereas the targeting of NHERF2 varies somewhat between apical, at the base of this area, as well as more diffusely in the cytosol among different tissues (Sun et al., 2000; Wade et al., 2001; Ingraffea et al., 2002).
NHERF4 is found within vesicular structures of the subapical region of kidney proximal tubules but is largely absent from the brush-border membrane (Gisler et al., 2001). Although brush-border expression of NHERF4 is minimal or absent, it is distributed throughout the cytoplasm. Similar observations have been made in human Caco-2 colon cancer cells (Donowitz et al., 2005).
Despite the colocalization and interaction of some NHERF family members with GPCRs and transporters at apical membranes of polarized epithelial cells, the role of NHERFs in apical membrane targeting is controversial and unclear because many NHERF-binding partners retain their apical localization in the absence of NHERF (Nies et al., 2002; Benharouga et al., 2003; Ostedgaard et al., 2003; Milewski et al., 2005; Mahon, 2009), whereas others exhibit impaired apical localization (Shenolikar et al., 2002; Seidler et al., 2009). The overall thrust of the findings suggests that the main role of the NHERF family is transiently to assemble multiprotein complexes that modulate trafficking, transport, and signaling in polarized cells (Thelin et al., 2005).
IV. G Protein-Coupled Receptor Partners and Effects of Na+/H+ Exchange Regulatory Factor-1 on Trafficking and Signaling
Depending on the stringency of the PDZ-binding sequence, from a handful to a potentially large number of mammalian GPCRs have the ability to engage NHERF proteins. Table 4 summarizes selected GPCRs for which such interactions have been convincingly established and specifically linked to function. These interactions and their functional consequences are described below.
TABLE 4.
G protein-coupled receptors interacting with NHERF proteins
| GPCR | PDZ-Binding Motif | NHERF Target | Function | Reference |
|---|---|---|---|---|
| PTHR | E-T-V-M | PDZ1 NHERF1 | Gαs, Gαq, Gαi, PLCβ, PKA, PKC, MAP kinase signaling | Mahon et al., 2002; Sneddon et al., 2003; Mahon and Segre, 2004; Wang et al., 2007 |
| PDZ2 NHERF2 | Receptor trafficking & desensitization | Wheeler et al., 2008 | ||
| Npt2a regulation | Wang et al., 2008, 2009, 2010; Weinman et al., 2009, 2011 | |||
| β2-AR | D-S-L-L | PDZ1 NHERF1 | NHE3 & CFTR regulation | Hall et al., 1998; Naren et al., 2003; Taouil et al., 2003; Singh et al., 2009 |
| PDZ1,2 NHERF2 | ||||
| PDZ2 NHERF3 | ||||
| κOR | N-K-P-V | PDZ1 NHERF1 | Receptor recycling | Li et al., 2002 |
| NHE3 regulation | Huang et al., 2004; Liu-Chen, 2004 | |||
| mGluR5 | S-S-S-L | PDZ2 NHERF2 | Prolong calcium mobilization | Paquet et al., 2006 |
| Potentiate cell death | ||||
| P2Y1 | D-T-S-L | PDZ1 NHERF1 | PLCβ signaling | Hall et al., 1998 |
| AKAP activity (via ezrin) | Fam et al., 2005 | |||
| PDZ1, PDZ2 NHERF2 | CFTR regulation | Guerra et al., 2004 | ||
| Receptor dimerization | Choi et al., 2008 | |||
| LPA2 (LPA5) | D-S-T-L | PDZ2 NHERF2 | Gαq, Gαi, PLCβ, Akt, MAP kinase signaling | Oh et al., 2004 |
| Choi et al., 2010 | ||||
| CFTR regulation (LPA5: NHE3) | Li et al., 2005; Singh et al., 2009 | |||
| Antiapoptotic signaling | Yun et al., 2005; E et al., 2009; Lee et al., 2011 | |||
| Cell migration | Cha et al., 2010; Lin et al., 2010 | |||
| CCR5 | S-V-G-L | PDZ2 NHERF1 | Receptor internalization & dimerization | Hammad et al., 2010 |
| MAP kinase signaling | ||||
| CLRL | D-T-L-L | PDZ1 NHERF1 | Receptor internalization | Bomberger et al., 2005a,b |
| RAMP3 | ||||
| A2b | ? | NHERF2 | Membrane anchoring | Sitaraman et al., 2002 |
| TXA2 | Indirect | PDZ 1&2 NHERF1 | Gαq & PLCβ signaling | Rochdi et al., 2002; Rochdi and Parent, 2003 |
| Fzd (Fzd4) | X-S/T-X-V/L (ETVV) | PDZ2 NHERF2 | Wnt/β-catenin signaling Tumor suppressor in breast cancer | Wheeler et al., 2011 |
MAP, mitogen-activated protein; AKAP, A kinase anchoring protein; Akt, protein kinase B.
A. Parathyroid Hormone Receptor
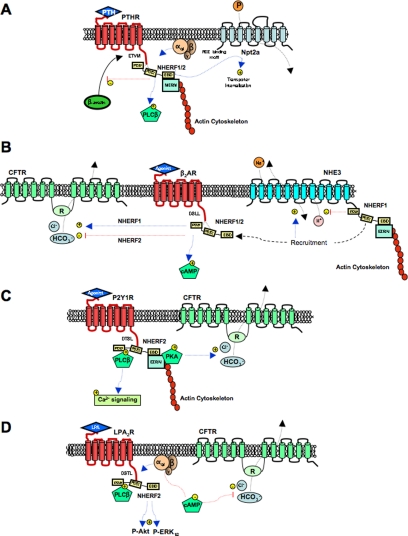
The PTH1R is a family B G protein-coupled receptor present primarily in bone and kidney. Interactions with its cognate ligands, parathyroid hormone (PTH) and parathyroid hormone-related protein, led to activation of Gαs and/or Gαq triggering activation of adenylyl cyclase-cAMP-protein kinase A- (PKA) and phospholipase C- (PLC) dependent pathways, respectively. A PDZ-binding motif (E-T-V-M) in the carboxyl-terminal tail of PTH1R allows the receptor to bind preferentially to the PDZ1 of NHERF1 or PDZ2 of NHERF2 (Fig. 3A) (Wang et al., 2010). NHERF1 and NHERF2 interactions with the PTH1R have been extensively studied, and the effects are summarized in Table 5. Mahon et al. (2002) reported that NHERF2 inhibited PTH-activated adenylyl cyclase by stimulating inhibitory Gαi and increasing PLCβ in PS120 cells transfected with the PTH1R. Thus, a signaling switch mechanism consisting of NHERF2-mediated assembling of PTH1R to PLCβ and inhibition of a Gαs-based adenylyl cyclase activity by Gαi was proposed.
Fig. 3.
Models of NHERF effects on GPCR signaling. Activation (+) or inhibition (−) of signaling pathways is shown with blue or red lines, respectively. A, E-T-V-M-binding domain at the carboxyl terminus of the PTHR binds to PDZ1 of NHERF1 or PDZ2 of NHERF2. In turn, through the EBD, NHERF1 associates with merlin, ezrin, radixin, or moesin (MERM) with the actin cytoskeleton. NHERF1 interactions with the PTHR increase the binding for Gαq [NHERF1 and -2] or Gαi [NHERF2] proteins (Wang et al., 2010). Upon PTH stimulation, NHERF1 increases PLCβ activity, internalizes the Npt2a Na-Pi cotransporter, and decreases β-arrestin recruitment to the receptor. B, upon agonist stimulation, the D-S-S-L-binding domain at the carboxyl terminus of the β2-AR) binds to PDZ1 of NHERF1 or PDZ1 or PDZ2 of NHERF2 (Lau and Hall, 2001). Interactions of the β2AR with NHERFs increase cAMP accumulation and also increase (NHERF1) or decrease (NHERF2) CFTR of anion extrusion (Naren et al., 2002; Taouil et al., 2003; Singh et al., 2009). Inhibitory effects of NHERF1 on NHE3 transporter activity decrease after NHERF1 releases the transporter when recruited to β2-AR. C, the D-T-S-L-binding domain at the carboxyl terminus of the P2Y1R binds PDZ2 of NHERF2 (Hall et al., 1998a; Fam et al., 2005), tethering the receptor indirectly to PLCβ and increasing calcium (Ca2+) signaling (Fam et al., 2005). NHERF2 associates with ezrin through the EBD and in turn ezrin bind PKA, which increases CFTR Cl− efflux (Guerra et al., 2004). D, the DSTL-binding sequence at the carboxyl terminus of the LPA2R binds to PDZ2 of NHERF2, thereby tethering the receptor indirectly to PLCβ and increasing Akt and ERK1/2 phosphorylation (P) and activity upon LPA stimulation (Oh et al., 2004; Yun et al., 2005). NHERF2 interactions with LPA2R also increase the binding for Gαq or Gαi (Li et al., 2005; Lee and Zheng, 2010). Recruitment of Gαi decreases adenylyl cyclase activity, reducing cAMP accumulation, and decreasing Cl− transport by the CFTR (Li et al., 2005; Singh et al., 2009).
TABLE 5.
NHERF effects on PTHR signaling
| Cell type | NHERF Isoform | Effect | Reference |
|---|---|---|---|
| PS120 | NHERF1/2 | Increased PLC activity | Mahon et al., 2002 |
| Increased Gαi and decreased adenylyl cyclase activity | |||
| ROS | NHERF1 | Increased PLC activity and cAMP and Ca2+ accumulation | Wheeler et al., 2008 |
| Renal tubule cells | NHERF1 | Increased PLC activity | Cunningham et al., 2005 |
| No changes in cAMP accumulation | Capuano et al., 2007 | ||
| CHO-N10-R3 | NHERF1 | No changes in cAMP accumulation | Wang et al., 2007 |
| Decreased ERK1/2 stimulation | Wang et al., 2008 | ||
| OK | NHERF1/2 | Increased PLC activity | Mahon and Segre, 2004 |
| Increased Ca2+ accumulation | |||
| HEK293 | NHERF1/2 | NHERF1: increased Gαq activity and Ca2+ accumulation | Wang et al., 2010 |
| NHERF2: increased Gαq and Gαi and decreases Gαs activity | |||
| Increased Ca2+ and decreases cAMP accumulation |
Despite the described effect of NHERF1 and NHERF2 to suppress cAMP signaling (Mahon et al., 2002; Mahon and Segre, 2004), NHERF1 increased PTH-stimulated cAMP accumulation in rat osteosarcoma (ROS) 17/2.8 cells (Wheeler et al., 2008). Moreover, in contrast to the observations in opossum kidney (OK) cells that Gαi-dependent activation of PLC by PTH induces Ca2+ signaling (Mahon and Segre, 2004), the induction of calcium responses by NHERF1 was not due to the activation of PLC in ROS cells (Wheeler et al., 2008). In the latter, upon activation of the PTH1R, Gs-dependent adenylyl cyclase produces high local concentrations of cAMP, which, in turn, activate PKA locally, inducing the phosphorylation of voltage-dependent Ca2+ channels, thus allowing Ca2+ entry (Wheeler et al., 2008).
Adding to the variability of these effects, no differences in PTH-stimulated cAMP formation were found in wild-type and NHERF1-null proximal tubule cells (Cunningham et al., 2004, 2005) or in Chinese hamster ovary CHO-N10-R3 cells in the presence or absence of NHERF1 (Wang et al., 2007).
Independent of the effects on adenylyl cyclase, both NHERF1 and NHERF2 increase PTH-stimulated PLC or intracellular calcium in PS120 cells, OK cells, and ROS 17/2.8 cells (Mahon et al., 2002; Mahon and Segre, 2004; Wheeler et al., 2008). Consistent with these observations, PTH activation of PLC is impaired in NHERF1-null mice (Capuano et al., 2007).
Recent findings demonstrate that NHERF interactions regulate PTH1R signaling at the level of G proteins. NHERF1 directs interactions with the PTH1R that enhance receptor-mediated stimulation of Gαq but has no effect on stimulation of Gαi or Gαs (Wang et al., 2010). In contrast, PTH1R association with NHERF2 augments receptor-mediated stimulation of both Gαq and Gαi but decreases stimulation of Gαs. These studies reveal that NHERF interactions with the PTH1R can directly modulate receptor coupling to G proteins. NHERF2 formed cellular complexes with both Gαq and Gαi, whereas NHERF1 interacted only with Gαq (Wang et al., 2010).
PTH1R also signals through mitogen-activated protein kinases and extracellular signal-related kinases 1 and 2 (ERK1/2). NHERF1 regulates ERK1/2 activation through a PTH1R-independent mechanism whereby decreasing the stimulatory effect of 14-3-3 binding to B-Raf inhibits ERK1/2 activation, whereas increasing the inhibitory influence of AKT negatively regulates ERK1/2 activation (Wang et al., 2008).
NHERF proteins also affect trafficking and desensitization of the PTH receptor. PTH1R undergoes β-arrestin/dynamin-mediated endocytosis in response to the biologically active forms of PTH, PTH(1–34), and PTH(1–84), but the synthetic antagonist PTH(7–34) and the naturally circulating PTH(7–84) peptide induce PTH1R endocytosis through an arrestin-independent mechanism (Sneddon et al., 2003). NHERF1 inhibits the arrestin-independent PTH1R endocytosis effects of the N-terminally truncated PTH(7–34) and PTH(7–840) (Sneddon et al., 2003; Sneddon et al., 2004).
In addition to the PDZ domains that interact with the PTH1R, NHERF1 and NHERF2 contain a carboxyl-terminal EBD (Fig. 1) that enables the tethering of ezrin, merlin, radixin, and moesin to the actin cytoskeleton (Reczek et al., 1997; Reczek and Bretscher, 1998; Yun et al., 1998; Voltz et al., 2001; Weinman et al., 2006a). These motifs were found to be essential to allow NHERF1 to bind to PTH1R and anchor it to the cytoskeleton, altering significantly the cellular distribution and diffusion of PTH1R and reducing the rate of ligand-induced PTH1R endocytosis (Wang et al., 2007; Wheeler et al., 2007, 2008). A dual mechanism to explain PTH1R endocytosis inhibition by NHERF1 was suggested; NHERF1 decreases the rate of arrestin binding to the receptor and blocks arrestin-independent endocytosis (Wang et al., 2007; Wheeler et al., 2007). Studies on the interactions of NHERF1 with arrestin and their effects on PTH1R are controversial. Several observations support models of NHERF1 interfering dynamically with the binding of arrestin to the PTH1R after ligand stimulation (Wang et al., 2007, 2009; Wheeler et al., 2007). It was suggested that NHERF1 sterically interferes with or displaces β-arrestin-2 binding to the PTH1R. The mechanism proposed may explain the decrease of PTH1R desensitization and endocytosis caused by arrestins (Wang et al., 2007, 2009; Wheeler et al., 2007). However, an alternative model has been suggested in which NHERF1 may serve as an adaptor, bringing β-arrestin-2 into proximity of the PTH1R. This model would facilitate β-arrestin-2 recruitment after receptor activation by stabilizing a ternary complex (Klenk et al., 2010). Such interactions may briefly occur when β-arrestins are recruited to NHERF1-bound PTH1R. In contrast to this static model of PTH1R-NHERF1-arrestin interaction proposed by Klenk et al. (2010), results from our group suggest dynamic interactions between NHERF1 and the PTH1R. Upon PTH stimulation, we observed rapid dissociation of NHERF1 from the PTH1R, with β-arrestin1/2 then recruited to the PTH1R, thereby initiating receptor internalization (Ardura et al., 2010).
NHERF1 not only regulates PTH1R signaling and trafficking but also modulates receptor effects on transporters, such as Npt2a. Mahon et al. (2003) showed that OK cells display PTH-mediated inhibition of inorganic phosphate (Pi) uptake via regulation of the Npt2a. However, a subclone of OK cells that expresses only modest levels of NHERF1 (OKH) was defective in PTH-mediated inhibition of Npt2a. Overexpression of NHERF1 in OKH cells rescued PTH-mediated inhibition of Npt2a. In vivo studies demonstrated increased urinary phosphate excretion in NHERF1-null mice compared with wild-type animals (Shenolikar et al., 2002). Npt2a protein was aggregated within the cytoplasm of renal proximal tubule cells of NHERF1-null mice. Mislocalization of Npt2a suggested that NHERF1 plays a role in the apical targeting and/or tethering of Npt2a in the mammalian kidney (Shenolikar et al., 2002; Cunningham et al., 2005, 2006). In addition, phosphate transport was inhibited by activation of PKA and protein kinase C, the two major signaling pathways of the PTH1R, in wild-type proximal tubule cells but not in NHERF1-null cells (Cunningham et al., 2006). Studies to test whether PTH-mediated phosphorylation of NHERF1 could affect Npt2a/NHERF1 complexes showed that murine NHERF1-null renal proximal tubule cells infected with an S77A-mutated NHERF1 exhibited increased phosphate transport compared with a phosphomimetic S77D mutation. Furthermore, these cells were resistant to the inhibitory effect of PTH on phosphate transport compared with cells infected with wild-type NHERF1 (Weinman et al., 2007). Together with the phosphorylation of NHERF1, the stimulation of PTH1R induced the dissociation of the Npt2a/NHERF1 complex, thereby increasing the membrane diffusion of Npt2a (Weinman et al., 2011). On the basis of these observations, Npt2a/NHERF1 complexes seem to be dissociated by PTH1R-dependent protein kinases releasing Npt2a, allowing its internalization and thus inhibiting phosphate transport at the apical surface of renal proximal tubule cells.
B. β2 Adrenergic Receptor
Three β-AR subtypes have been identified: β1-AR, β2-AR, and β3-AR. Ligand binding to these receptors leads to heterotrimeric G protein stimulation; all β-AR subtypes can activate the Gαs/adenylyl cyclase signaling pathway, but only β2-AR and β3-AR are able to activate Gαi (Xiao et al., 1995; Gauthier et al., 1996). Probing tissue extracts from various organs in blot overlay experiments, Hall et al. (1998b) showed that NHERF1 binds to the β2-AR tail, becoming the first GPCR described to interact with a NHERF family member. The residues responsible for β2-AR binding to NHERF1 were elucidated by mutagenesis and were located at the carboxyl terminus of the receptor. NHERF1-binding by the β2-AR is mediated by the last four amino acids (D-S-L-L) of the intracellular tail. The β2-AR binds preferentially to PDZ1 of NHERF1. In addition, the β2-AR carboxyl terminus binds strongly to NHERF2 PDZ1 and PDZ2 and to NHERF3 PDZ2 (He et al., 2006).
β2-ARs are characterized by their rapid and efficient recycling to the plasma membrane after agonist stimulation in human embryonic kidney 293 cells (Cao et al., 1999). Truncation of the distal portion of the cytoplasmic tail that includes the NHERF-binding motif caused pronounced, ligand-induced degradation. Candidate proteins involved in this sorting operation and that bind to the cytoplasmic tail of the wild-type β2-ARs were searched (Cao et al., 1999). NHERF1, with its EBD, can assemble a network of protein connectivity linking integral membrane proteins to actin (Bretscher et al., 2000). NHERF1 was suggested as the β2-AR sorting protein based on indirect actin connectivity as the source of β2-AR recycling (Cao et al., 1999). However, neither NHERF1 nor NHERF2 are known to localize to endosomes, and although actin connectivity is sufficient to promote recycling of engineered receptors in the absence of a natural PDZ motif, this connectivity does not fully recapitulate the natural characteristics of β2-AR recycling (Lauffer et al., 2009). In fact, simultaneous knockdown of NHERF1 and -2 did not have a major effect on β2-AR recycling. Other PDZ proteins that might function in endosome-to-plasma membrane trafficking of β2-AR were considered and, sorting nexin 27 (SNX27) arose as the PDZ-bearing protein with a key role on β2-AR directed recycling (Lauffer et al., 2010).
Agonist-induced association of NHERF1 with the β2-AR proved to modulate cell pH by regulating NHE3 by a mechanism distinct from G-protein activation (Hall et al., 1998b). Preliminary studies showed that phosphorylation of both NHE3 and NHERF mediated by PKA lead to NHERF1 binding to and subsequent inhibition of NHE3 (Weinman and Shenolikar, 1993; Kurashima et al., 1997). Based on these findings and on the β2-AR–NHERF1 interaction, direct agonist-promoted association of the β2-AR with NHERF1 was postulated to displace NHERF1 from NHE3, thereby preventing NHE3 inhibition that would normally be expected to follow adenylyl cyclase activation by β2-AR (Fig. 3B) (Hall et al., 1998b).
In addition to NHE3, regulation of the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel in vivo is believed to be accomplished by activation of surface receptors that couple to adenyl cyclase and raise cellular cAMP (Chan et al., 1997). This was demonstrated for human airway mucosa, where β2-AR stimulation with isoproterenol activated CFTR-dependent Cl- transport in vivo (Walker et al., 1997). Moreover, interactions of NHERF1, as well as NHERF2 and NHERF3, with CFTR had been previously proposed (Hall et al., 1998a; Short et al., 1998; Wang et al., 1998b; Guggino and Stanton, 2006; Lamprecht and Seidler, 2006). These studies suggest a model where CFTR, β2-AR, and NHERF1 exist as a complex at the apical surface of epithelial cells (Fig. 3B). In this setting, agonist activation of the receptor increases local concentrations of cAMP and subsequent compartmentalization and specific signaling of CFTR (Naren et al., 2003; Taouil et al., 2003). An in vivo study in mice lacking one or more of the NHERF proteins linked β2-AR signaling to CFTR-mediated duodenal HCO3− secretion (Singh et al., 2009). Total abrogation of the HCO3− secretory response to the β2-AR agonist clenbuterol was observed in duodenum of NHERF1-null mice. However, deletion of NHERF2 resulted in increased HCO3− secretion after forskolin stimulation, suggesting that NHERF2 exerts an inhibitory effect on intestinal CFTR function. In contrast to NHERF1 or NHERF2 ablation, the secretory response to forskolin in NHERF3-null mice was normal (Singh et al., 2009). Thus, the absence of each of the three CFTR-interacting NHERF proteins resulted in distinct alterations in the regulation of CFTR-dependent murine duodenal HCO3− secretion by the β2-AR.
C. Opioid Receptors
Opioid receptors (OR) belong to GPCR family A and are divided in different subclasses, δ, κ, μ, and nociceptin, each with distinct and characteristic splice variants. Opioid receptors are well known for their roles in pain modulation/analgesia and reward (Waldhoer et al., 2004; van Rijn et al., 2010).
The κOR was the first opioid receptor shown to interact with NHERF proteins (Li et al., 2002). κOR immunoprecipitated with NHERF1 PDZ1 but not PDZ2. It is noteworthy that the carboxyl-terminal sequence of the κOR, N-K-P-V, was established as the region interacting with NHERF1 although it is not a canonical PDZ ligand of the form [D/E]-[S/T]-X-Φ. Thus, NHERF proteins may have a broader binding specificity permitting interaction with a subset of different GPCRs that do not possess canonical PDZ-binding motifs. Binding of NHERF1 to the carboxyl-tail of the κOR is much weaker than its binding to the carboxyl-tail of the β2-adrenergic receptor (Li et al., 2002). However, upon agonist stimulation, coimmunoprecipitation of NHERF1 with κOR significantly increased indicating that the agonist-occupied receptor has greater binding affinity for NHERF1 (Li et al., 2002).
NHERF1 binding to the κOR carboxyl terminus blocked agonist-induced down-regulation of the receptor; this effect was due to an increase in the recycling rate of internalized receptors and not to decreased κOR endocytosis. NHERF1/κOR association seems to serve as a signal for internalized κOR to be sorted to the recycling pathway for receptor resensitization (Li et al., 2002; Liu-Chen, 2004).
In addition, it was shown that the κOR enhanced Na+/H+ exchange in OK cells, stimulating NHE3 activity (Huang et al., 2004). However, this stimulatory effect was pertussis toxin-resistant, indicating that κOR regulated the activity of NHE3 by a mechanism independent of Gi/Go protein activation, the main G protein pathway triggered by κOR (Law et al., 2000). OK cells that expressed low amounts of NHERF1 showed no κOR activation of NHE3 but increasing NHERF levels in these cells by transient transfection restored the stimulatory effect of κOR on NHE3 activity. On the basis of these results it was hypothesized that binding of the κOR to NHERF1 facilitates oligomerization of NHERF1, leading to Na+/H+ exchange acceleration by NHE3 stimulation (Huang et al., 2004).
A key role for NHERF1 on δOR translocation to the membrane of neural synapses recently has been identified (Bie et al., 2010). NHERF1 normally exhibits low affinity and interaction with the carboxyl terminus of the δOR (Heydorn et al., 2004; Huang et al., 2004). However, in brainstem from rats treated with the opioid receptor agonist morphin, the amount of NHERF1 coimmunoprecipitated with δOR significantly increased (Bie et al., 2010). In many types of central neurons, the δOR is normally sorted to the lysosomal pathway for degradation or is constitutively retained in intracellular compartments, resulting in a low level of functional δOR at the plasma membrane (Tsao and von Zastrow, 2000; Wang and Pickel, 2001; Ma et al., 2006). Nevertheless, δOR is driven to the surface membrane of central synaptic terminals and becomes functional by a mechanism that depends on NHERF1. According to the model proposed, up-regulation by morphin of NHERF1 expression induced by the activation of the neurotrophic tyrosine kinase receptor/PLCγ/phosphatidylinositol 3-kinase/Ca2+/calmodulin-dependent protein kinase II pathway increases the interaction between NHERF1 and phosphorylated δOR, leading to exocytotic translocation of intracellular δOR to surface membrane. Thus, NHERF1 may redirect the sorting of intracellularly targeted δOR to the plasma membrane (Bie et al., 2010).
The key role of NHERF proteins in the efficient recycling of δOR was recently established by generating an engineered receptor ligated to an interaction module that binds directly to actin filaments and mimics the ability of NHERF proteins to bind receptors to the actin cytoskeleton. The domains mediating direct actin connectivity promoted receptor recycling with similar high efficiency to PDZ protein-binding determinants such as NHERF1 but without its capabilities to regulate receptor trafficking (Lauffer et al., 2009).
D. Purinergic P2Y1 Receptors
Extracellular nucleotides are a class of signaling molecules that mediate different biological effects by binding to and activating their respective cell surface purinergic (P2Y) GPCRs. They can be divided in adenine nucleotide-preferring receptors that respond mainly to ADP and ATP (P2Y1R, P2Y11R, P2Y12R, P2Y13R) and the uracil nucleotide-preferring receptors (P2Y2R, P2Y4R, P2YR6) (Abbracchio and Burnstock, 1994; Guerra et al., 2004).
Among the P2Y receptors, the P2Y1R contains a canonical NHERF1-binding motif (D-T-S-L), whereas the P2Y2R ends in a motif that is less ideal because of the lack of a hydroxyl-bearing amino acid at the -2 position (D-I-R-L). Consistent with this scheme, the P2Y1R tail exhibits robust binding of NHERF1 and NHERF2, whereas the P2Y2R tail exhibits poor NHERF binding (Hall et al., 1998a; Fam et al., 2005).
P2Y1R and NHERF2 interaction in C6 glioma cells facilitates tethering of the receptor to key downstream effectors such as PLCβ, thereby prolonging P2Y1R-mediated Ca2+ signaling (Fig. 3C) (Fam et al., 2005).
Moreover, P2Y1R stimulation of Cl− efflux induced by the agonist 2-methylthio-ADP is inhibited by truncation of the PDZ1 domain of NHERF2 (Guerra et al., 2004). P2Y1R stimulates CFTR by a mechanism mainly involving the PKA-dependent signal transduction pathway, which can be amplified in a PKC-dependent manner. These data suggest that NHERF may play an important role in facilitating activation of CFTR at the cell membrane. According to this view, ezrin, by virtue of its AKAP (A-kinase anchor protein) activity, promotes CFTR phosphorylation by forming a CFTR-NHERF2-ezrin macromolecular complex, thereby anchoring the catalytic subunit of PKA in the vicinity of CFTR (Fig. 3C) (Guerra et al., 2004).
NHERF2 affects not only P2Y1R signaling but also its dimerization (Choi et al., 2008). Excision of the carboxyl-terminal DTSL motif of the receptor blocked constitutive dimerization. This effect of NHERF2, it was proposed, suggests that P2Y1R clustering on a scaffold favors the presence of constitutive homodimerization (Choi et al., 2008).
E. Lysophosphatidic Acid Receptors
Lysophosphatidic acid (LPA) mediates its effects predominantly through the GPCRs LPA1R, LPA2R, and LPA3R. LPA2R, but neither LPA1R nor LPA3R, specifically interacts with the PDZ2 of NHERF2, thereby potentiating LPA-induced PLCβ and ERK activation in COS-7 cells, HeLa cells, and Rat-1 cells (Oh et al., 2004; Choi et al., 2010). PDZ2 of NHERF2 indirectly links the LPA2R to PLC-β3 to form a ternary complex (Oh et al., 2004). At the apical surface of intestinal epithelial cells, another macromolecular complex consisting of LPA2, NHERF2 and the anion transporter CFTR was reported (Fig. 3D) (Li et al., 2005; Singh et al., 2009). In the model proposed, upon LPA2R stimulation, Gi inhibits adenylyl cyclase, resulting in decreased cAMP levels. This decreased local or compartmentalized accumulation of cAMP results in the reduced activation of CFTR (Li et al., 2005). Furthermore, in vivo studies with NHERF2-null mice show increased duodenal HCO3− secretion (Singh et al., 2009). Conversely, LPA inhibited forskolin-stimulated HCO3− secretion in wild-type mice but not in NHERF2-null animals. These observations suggest that NHERF2 modulates duodenal HCO3− secretion by coupling the LPA2R to CFTR.
In colon cancer cells, LPA2R signaling is largely mediated by its ability to interact with NHERF2 (Yun et al., 2005). Silencing NHERF2 significantly attenuated activation of Akt and Erk1/2. LPA2 activity is dynamically regulated by NHERF2 and by another PDZ- protein, MAGI3. NHERF2 competes with MAGI3 for binding to LPA2R and PLCβ3. NHERF2 preferentially promotes interaction between LPA2 and Gαq, whereas MAGI3 increases the interaction of LPA2R with Gα12, favoring migration and invasion of colon cancer cells or inhibiting these processes, respectively (Lee et al., 2011).
LPA2R also elicits prosurvival responses to prevent and rescue cells from apoptosis. This effect depends on the formation of a ternary complex consisting of LPA2R, NHERF2, and the zinc finger protein TRIP6 (E et al., 2009). This complex provides a ligand-dependent signal amplification mechanism required for LPA2R-mediated activation of antiapoptotic signaling.
Signaling through LPA5R, LPA is a potent stimulant of NHE3 and fluid absorption by the intestine. Stimulation of NHE3 by LPA5R requires coexpression and interaction with NHERF2 in colonic epithelial cells (Lin et al., 2010). LPA stimulates NHE3 and fluid absorption, in part, by increasing NHE3 abundance at the brush-border membrane of intestinal epithelial cells. Finally, it was recently reported that upon LPA stimulation NHE3 acutely increased its mobility by a NHERF2-dependent mechanism that allows NHE3 to transit dynamically over the microvilli (Cha et al., 2010).
F. Effects on Other G-Protein-Coupled Receptors
1. Thromboxane A2 Receptor
Thromboxane A2 receptors (TXA2Rs), part of the GPCR superfamily, signal through Gαq/11 (Kinsella, 2001). Studying the role of NHERF1 on TXA2R signaling through the Gαq pathway, Rochdi et al. (2002) reported that agonist stimulation of the TXA2R increased the interaction between NHERF1 and Gαq, implying that NHERF1 preferentially interacts with activated Gαq. Moreover, NHERF1 inhibited Gαq signaling by preventing the interaction between Gαq and the TXA2R and also between activated Gαq and PLCβ1. Both NHERF PDZ domains are involved in this interaction. Further studies revealed that constitutively active Gαq induces TXA2R internalization through a PLCβ- and protein kinase C-independent pathway that was blocked by NHERF1 (Rochdi and Parent, 2003).
2. Adenosine 2b Receptor
Adenosine is a ubiquitous extracellular signaling molecule that is released during inflammation and interacts with four different adenosine GPCR subtypes A1, A2a, A2b, and A3 (Palmer and Stiles, 1995). Upon agonist stimulation the A2bR is recruited to the membrane of intestine cells, where it interacts directly with NHERF2 and ezrin (Sitaraman et al., 2002). A2bR does not contain a consensus PDZ-binding motif in the classic carboxyl terminus location, but this sequence is present in the third intracellular loop. The authors speculate that the interaction between the A2bR and NHERF2 may not only anchor the receptor to the surface of the membrane but also function to stabilize it in a signaling complex at the plasma membrane.
3. CCR5 Chemokine Receptor
Chemokine receptors are a specialized subset of the GPCR superfamily that mediate immune responses and, like some GPCRs, are able to dimerize (Agrawal et al., 2007; Sohy et al., 2009). The CCR5 chemokine receptor carboxyl-terminal tail contains a PDZ recognition motif constituted by S-V-G-L that binds to PDZ2 of NHERF1 (Heydorn et al., 2004). Studies of homo- and heterodimerization of CCR5 and CXCR4 (a chemokine receptor lacking a PDZ binding motif) showed that that NHERF1 binds only to the CCR5 homodimer and not to other combinations (CXCR4 homodimer or CXCR4-CCR5 heterodimer) (Hammad et al., 2010). These findings underscore the selectivity of receptor signaling complexes for NHERF family members. NHERF1 enhanced ERK activation and increased the internalization of CCR5 (Hammad et al., 2010).
4. Adrenomedullin Receptor
The calcitonin receptor-like receptor (CRLR) is a GPCR that requires association with receptor activity-modifying proteins (RAMPs) for plasma membrane expression and determination of ligand specificity and receptor phenotype. Coexpression of RAMP1 with CRLR yields a functional calcitonin gene-related peptide receptor, whereas coexpression of RAMP2 or RAMP3 with CRLR produces a receptor responsive to adrenomedullin (McLatchie et al., 1998; Bühlmann et al., 1999). Similar to the C terminus of the β2-AR, RAMP3 possesses a carboxyl terminus type-I PDZ motif (-D-T-L-L). The CRLR itself, as well as RAMP1 and RAMP2, does not contain a PDZ motif (Bomberger et al., 2005a). The CRLR-RAMP3 complex interacts with NHERF1 via the PDZ domain of NHERF1 (Bomberger et al., 2005b). Furthermore, NHERF1 altered the trafficking pattern of the receptor complex, inhibiting CRLR internalization by tethering the receptor complex to the actin cytoskeleton through interactions between RAMP3 and NHERF1.
5. Metabotropic Glutamate Receptors
Metabotropic glutamate receptors are members of GPCR family C. Three mGluR subtypes based on sequence, signaling, and pharmacology are now defined. mGluRs engage several PDZ proteins including PICK1, shank, tamalin, syntenin, and glutamate receptor-interacting protein in an isotype-specific manner. Group I mGluR5, but not mGluR1, binds NHERF2, even though both possess identical S-S-S-L carboxyl-terminal PDZ recognition ligands (Paquet et al., 2006). NHERF2 potentiates Gq-coupled Ca2+ signaling by mGluR5a, but not mGluR1a. No trafficking function of this interaction has been reported.
6. Frizzled Receptors
The Frizzled group of GPCRs (Fzd) transduces signals from the Wnt/β-catenin pathway (Wawrzak et al., 2009). Eight of the 10 human Frizzled receptors terminate in a canonical PDZ ligand (X-S/T-X-V/L): E-T-T-V (Fzd1, Fzd2), E-T-V-V (Fzd4), L-S-H-V (Fzd5), E-T-A-V (Fzd7), L-S-Q-V (Fzd8), P-T-H-L (Fzd9), and P-T-C-V (Fzd10), which makes these receptors potential candidates to bind NHERF family members. Frizzleds and their downstream effectors, β-catenin and cyclin D1, have been implicated in malignant transformation and acquisition of an invasive tumor phenotype in breast cancer (Shtutman et al., 1999). A recent report showed that NHERF1 expression caused Fzd4 to aggregate along actin fibers and impaired Wnt-induced β-catenin activation by Fzd2, -4, and -7 (Wheeler et al., 2011). Moreover, loss of NHERF1 in mammary tissue revealed enhanced Wnt/β-catenin signaling by increasing the recruitment and activation of disheveled (Dvl) (Wheeler et al., 2011). Dvl is otherwise recruited by Frizzled and prevents the constitutive destruction of cytosolic β-catenin complex. Thus, in the model proposed binding of NHERF1 to Fzd reduces Fzd-Dvl precoupling, impairing Wnt signaling and decreasing epithelial-to-mesenchymal transition, malignancy, and tumor metastasis (Wheeler et al., 2011).
V. Hormonal Regulation of Na+/H+ Exchange Regulatory Factor-1 Expression
Estrogens are presently the only known regulators of NHERF1 expression. Studies using a luciferase reporter system, in which promoter region fragments of mouse NHERF1 ranging from −2500 to −161 base pairs upstream of the transcription start site were ligated into firefly luciferase, failed to disclose a stimulatory action of estradiol on MCF7 breast cancer cells, which are highly estrogen-responsive (Weinman et al., 1999). In contrast, estradiol directly stimulated NHERF RNA levels in MCF7 cells by 6-fold in a time- and concentration-dependent manner (Ediger et al., 1999). Other steroid hormones were without effect, and antiestrogens blocked the stimulatory action of estradiol. Further analysis revealed that although NHERF1 does not possess a canonical estrogen response element, it contains numerous ERE half-sites (TGACC and GGTCA) (Ediger et al., 2002). Subcloning of the 3.5 kilobases of the 5′-flanking region, which contains all 13 ERE half-sites, in a CAT reporter and cotransfection with the estrogen receptor α in MDA-MB-231 breast cancer cells displayed a 29-fold stimulation in response to estradiol. Subsequent experiments established that the estrogen receptor binds to the half-palindromic ERE sequences. These observations are consistent with the finding that endometrial NHERF expression varies directly with estrogen levels (Stemmer-Rachamimov et al., 2001). Likewise, estradiol treatment substantially increases NHERF1 expression in MCF7 cells (G. Romero and P. Friedman, unpublished observations). The reason for the discrepancy between the mouse and human NHERF1 promoters is unclear. The mouse studies employed a 5′ sequence sufficiently long to contain putative ERE half-sites (Weinman et al., 1999). Moreover, the limited published sequence of the mouse promoter indicates the presence of several of these half-sites (GenBank accession no. NM_012030). Further inspection of the human genomic NHERF1 sequence reveals the presence of half-palindromic vitamin D response elements. Given the importance of NHERF1 on mineral ion economy, it might be productive to explore the vitamin D responsiveness of NHERF1 expression.
VI. Biological Actions of Na+/H+ Exchange Regulatory Factors
NHERF proteins apparently interact in vitro with a disarmingly large array of cell membrane-delimited proteins, including GPCRs. The range of their biological actions may be more accurately represented by the phenotype of NHERF-null mice and by the symptoms of patients harboring polymorphisms or mutations of NHERF1. Mice genetically deficient in NHERF1 were generated by homologous recombination of exon 1 (Shenolikar et al., 2002) or exons 1 to 4 (Morales et al., 2004). Both strains of NHERF1-null mice exhibit a spectrum of mineral ion disorders that includes prominent hypophosphatemia. The animals were noted to have lower bone mineral density and mineral content and high rates of fractures (Shenolikar et al., 2002). The diminished bone mineralization was interpreted to arise from renal phosphate and calcium wasting. Polymorphisms (L110V, R153Q) or mutation (E225K) was found only in patients with a low threshold of renal phosphate excretion (tubular maximum for phosphate corrected for glomerular filtration rate) (Karim et al., 2008). These patients presented with renal phosphate wasting (similar to the NHERF1-null mice), recurrent renal stones, and a history of bone fracture or low bone density. Again, the skeletal findings were thought to be a secondary consequence of the mineral ion disorder. Together, these findings point to an important role for NHERF1 in the regulation of mineral ion homeostasis.
NHERF2-null mice were generated by a retroviral gene-trapping procedure that resulted in protein abolition from kidney, lung, stomach, heart, brain, and colon (Broere et al., 2007). No evident morphological, behavioral, mineral-ion, or uric acid wasting phenotype was evident. (Broere et al., 2007; Cunningham et al., 2008). Recent results indicate morphological changes of the intestine of NHERF2-null mice with shorter villi, deeper crypts, and decreased epithelial cell number (Donowitz et al., 2011; Murtazina et al., 2011). NHERF2-null mice display augmented forskolin-stimulated intestinal HCO3− secretion and are refractory to the inhibitory action of lysophosphatidic acid on forskolin-stimulated HCO3− secretion (Singh et al., 2009). Thus, NHERF2 exhibits specificity for regulating LPA signaling, where actions on CFTR are mediated by the LPA2 receptor (Li et al., 2005), whereas actions on NHE3 involve the LPA5 receptor (Lin et al., 2010). Select aspects of acute regulation of intestinal NHE3 is disrupted in NHERF2-null animals (Murtazina et al., 2011). A proteomic analysis of NHERF2-null mice indicates significant, albeit slight, up-or down-regulation of proteins associated with ion transport, signaling, and cytoskeleton (Donowitz et al., 2011).
NHERF3-null mice were generated by homologous recombination (Kocher et al., 2003). The mice developed normally, lacked gross phenotypic abnormalities, and exhibited normal mineral-ion homeostasis (Kocher et al., 2003). Like NHERF2-null mice, the absence of NHERF3 was associated with modest reduction of forskolin-induced Na and HCO3− transport (Hillesheim et al., 2007). These results contrast with others showing reduced basal but normal forskolin-induced HCO3− secretion (Singh et al., 2009). More strikingly, NHERF3-null mice develop lipoprotein abnormalities and atherosclerosis (Kocher et al., 2008; Kocher et al., 2010). NHERF3 may also uniquely be associated with the intestinal organic anion-transporting polypeptide (Sugiura et al., 2010). It is noteworthy that at this juncture it would seem that only NHERF1 and NHERF2 abnormalities are associated with disordered GPCR function.
VII. Post-Translational Modifications
A. Phosphorylation
NHERF1 contains 31 Ser and 9 Thr residues, which make up roughly 12% of the molecule. This suggests that there is ample opportunity for regulated post-translational phosphorylation. It is noteworthy that although NHERF2 harbors a nearly equal number of Ser/Thr residues, it is not a phosphoprotein (Lamprecht et al., 1998). NHERF1 is devoid of canonical PKA (K/R-K/R-X-S/T) or PKC (S/T-X-R/K) phosphorylation motifs. Nonetheless, both PKA and PKC phosphorylate NHERF1. NHERF1 is phosphorylated at Ser-77 (Voltz et al., 2007) and Thr-95 (Weinman et al., 2010b), and in a serine cluster at 287, 289, and 290, which is required for biological activity, and is located between PDZ2 and the ezrin-binding domain (Weinman et al., 1998). Two Ser residues in particular exert pivotal actions on NHERF1 activity: Ser-289 is constitutively phosphorylated in rabbit (Ser-290 of human sequence) and its mutation abrogates NHERF1 oligomerization (Lau and Hall, 2001); Ser-77, located in PDZ1, is phosphorylated by PTH and is critical in supporting NHERF1 action on Na+/H+ exchange and Na-Pi cotransport (Weinman et al., 2007, 2010a).
The mechanism and sites of NHERF1 phosphorylation are somewhat enigmatic. Neither 8-bromo-cAMP (Lamprecht et al., 1998) nor purified PKA phosphorylates full-length NHERF1 or recombinant PDZ1 (Weinman et al., 2007), respectively. However, cAMP induced phosphorylation in vivo. Furthermore, mutation of Ser-287, Ser-289, and Ser-290 abolishes NHERF1 phosphorylation but does not prevent 8-bromo-cAMP mediated inhibition of NHE3 (Zizak et al., 1999). Thus, PKA-mediated phosphorylation either occurs through a noncanonical site or is mediated downstream by another kinase first activated by PTH (see below). In contrast, PKC directly phosphorylates PDZ1 (Weinman et al., 2007) and full-length NHERF1 (Thr-71, Ser-77, Ser-277, Ser-287, Ser-288, Ser-299, Ser-337, and Ser-338; Ser-339, Ser-340- in human NHERF1) (Fouassier et al., 2005). The latter two sites were identified as the primary residues phosphorylated by PKC.
Likewise, PKC mediates phosphorylation of Ser-162, although this site is may be shielded from the kinase by the engaged carboxyl-terminal EB domain (Raghuram et al., 2003; Li et al., 2007). Ser-162 is present in human and cynomolgus monkey NHERF1 (Fig. 4) but is otherwise not conserved across species, and its physiological relevance has been questioned (Voltz et al., 2007). Nonetheless, Ser-162 phosphorylation is required for microvillar clustering and function (Raghuram et al., 2003; Garbett et al., 2010). Moreover, Ser-162 is closely located to two other critical domains of PDZ2. Ser-162 is immediately adjacent to the core-binding motif of PDZ2 163GYGF166. Thus, it is not unreasonable to theorize that Ser-162 phosphorylation affects binding of PDZ ligands that prefer PDZ2. Ser-162 is also close to Arg-153 in the PDZ binding loop, which may be critically altered in some inherited disorders (see section VIII). These findings may be especially relevant for PTH1R, which activates both PKA and PKC (Friedman et al., 1996) and underscores the limitations of reliance upon predicted consensus sites for analyzing NHERF1 phosphorylation.
Fig. 4.
Alignment of NHERF1 by species. The consensus sequence and percentage conservation are shown at the bottom.
GRK6a terminates in a three-residue class 1 PDZ ligand, T-R-L, that mediates its specific interaction with NHERF1 (Hall et al., 1999). GRK6a mediates the constitutive phosphorylation at Ser-289 (Hall et al., 1999; Fouassier et al., 2005). This site is a consensus GRK6 phosphorylation motif (R-X-X-S/T). Moreover, the interaction of GRK6a is required for phosphorylation. GRK6a constructs harboring mutations of the PDZ ligand, or GRK6 isoforms lacking the carboxyl-terminal PDZ ligand fail to phosphorylate NHERF1. It is noteworthy that GRK6a itself harbors several canonical PKA phosphorylation sites, leading to the possibility that PKA phosphorylates GRK6a, which in turn phosphorylates NHERF1.
VIII. Na+/H+ Exchange Regulatory Factor Mutations and Polymorphisms
A. Phosphate Wasting
All four NHERF proteins are capable of binding the Npt2a renal phosphate transporter in vitro, though only NHERF1 seems to be critically involved in native tissues or whole organisms (Gisler et al., 2001; Capuano et al., 2005; Cunningham et al., 2008). NHERF1-null mice exhibit pronounced renal phosphate excretion accompanied by hypophosphatemia (Shenolikar et al., 2002; Morales et al., 2004). Likewise, patients harboring polymorphisms or mutations of NHERF1 exhibit similar symptoms (Karim et al., 2008). The limited ability of the kidneys to absorb phosphate stems from reduced tethering of the Npt2a phosphate transporter at apical brush-border membranes of the proximal nephron (Biber et al., 2009; Cunningham et al., 2010). In OKH cells, a strain expressing only low levels of NHERF1, PTH stimulates cAMP formation but does not inhibit NaPi cotransport. Transfection with NHERF1 restores the normal inhibitory action of PTH (Mahon et al., 2003). OK cells transfected with the mutant NHERF1 variant L110V, R153Q, or E225K displayed impaired phosphate uptake (Karim et al., 2008). These loss-of-function mutations had no effect on basal phosphate transport but potentiated PTH-induced cAMP accumulation and thereby inhibition of phosphate transport. The structural basis whereby mutations in the linker region between PDZ1 and PDZ2, or mutations in PDZ2 but remote from the core-binding motif, affect NHERF1 function is unclear, especially since Npt2a binds PDZ1 (Gisler et al., 2001; Gisler et al., 2008).
B. Bone Disease
Mature mice lacking NHERF1 and adult humans harboring NHERF1 mutations display osteopenia (Weinman et al., 2006b; Karim et al., 2008). Initial assessments concluded that the bone defects were secondary to renal phosphate wasting because of high urinary phosphate excretion, and that NHERF1 was not detected in osteoblasts (Shenolikar et al., 2002; Karim et al., 2008). Mice lacking the renal Npt2a Na-Pi cotransporter; however, exhibit equivalent or greater urinary phosphate loss and lower serum phosphate that is accompanied by a complex, age-dependent bone phenotype, but not by osteomalacia or a mineralization defect (Beck et al., 1998). Resolution of growth plate abnormalities in Npt2a-null mice is associated with transient increases of serum 1,25(OH)2-vitamin D (Miedlich et al., 2010). In contrast to Npt2a-null mice, where the bone phenotype spontaneously resolves with age, the osteopenia and osteomalacia associated with NHERF1-null mice or patients with NHERF1 mutations, the skeletal changes persist. NHERF1-null mice and humans with NHERF1 variants also exhibit elevated 1,25(OH)2-vitamin D levels (Shenolikar et al., 2002; Weinman et al., 2006b; Karim et al., 2008). Hence, the bone phenotype associated with NHERF1 cannot be attributed to compromised vitamin D status per se, although altered vitamin D receptor levels cannot be excluded. NHERF1 is well expressed in mouse mineralizing osteoblasts and on human osteoblasts (H. Blair and P. Friedman, unpublished observations). Together, these findings are consistent with the view that the bone phenotype likely arises from a combination of intrinsic and extrinsic factors including hypophosphatemia, some element of the vitamin D axis, and the absence of NHERF1, or its mutations, in bone.
C. Breast Cancer
As noted earlier, NHERF1 possesses an estrogen response element (Ediger et al., 1999, 2002). Thus, high correlation between NHERF1 and estrogen receptor expression comes as no surprise (Stemmer-Rachamimov et al., 2001; Cardone et al., 2007; Song et al., 2007; Wheeler et al., 2011). But whether this is a positive or negative correlation is extremely controversial and contradictory. NHERF1 has been proposed to function as a tumor suppressor by some (Schindelmann et al., 2002; Dai et al., 2004; Kreimann et al., 2007; Zheng et al., 2010; Wheeler et al., 2011) but has been associated with invasiveness, metastatic progression, and poor prognosis by others (Cardone et al., 2007; Song et al., 2007; Mangia et al., 2009). Knockdown of NHERF1 increases cellular proliferation and migration of various breast cancer cell lines (Pan et al., 2006; Pan et al., 2008). Furthermore, when introduced in a mouse xenograft model, NHERF1-null cells were more aggressive and produced greater numbers of metastases (Pan et al., 2006). NHERF1 mutations occur in 3% of the human breast tumors, whereas loss of heterozygosity at the NHERF1 locus (17q25.1) occurs in more than 50% of primary beast tumors (Dai et al., 2004). Both are associated with a poor prognosis and early death (Dai et al., 2004). Recent results show that loss of NHERF1 heterozygosity in breast cancer cell lines enhances canonical Wnt signaling and Wnt-dependent cell proliferation (Wheeler et al., 2011). Furthermore, the mammary glands of NHERF1-knockout mice exhibit increased mammary duct density accompanied by increased proliferation and β-catenin activity. Finally, NHERF1 and nuclear β-catenin in human breast carcinomas are negatively correlated (Wheeler et al., 2011). How can the various and conflicting results be reconciled? Georgescu (2008) advanced an attractive comprehensive explanation for these contradictory results. She proposed that NHERF1, when localized at the cell membrane, acts as a tumor suppressor, whereas when it is localized to the cytoplasm, it behaves as an oncogenic protein.
The mechanism by which NHERF1 modulates its tumor suppressor or oncogenic activity involves an array of ezrin-like molecules or proteins harboring PDZ ligands with which NHERF1 interacts. These proteins include phosphatase and tensin homolog (PTEN) (Takahashi et al., 2006; Morales et al., 2007), NF2 (Voltz et al., 2001; Dai et al., 2004; Hennigan et al., 2010), β-catenin (Shibata et al., 2003; Kreimann et al., 2007; Morales et al., 2007; Theisen et al., 2007; Wheeler et al., 2011), PDGFR (Maudsley et al., 2000; Demoulin et al., 2003; Takahashi et al., 2006; Theisen et al., 2007), epidermal growth factor receptor (Lazar et al., 2004; Curto et al., 2007). The tumor suppressor PTEN has a carboxyl-terminal PDZ-binding sequence (T-K-V) and coimmunoprecipitates with NHERF1 (Takahashi et al., 2006). More importantly, NHERF1 assembles a ternary complex consisting of PTEN-NHERF1-PDGFR. Through this association, PTEN suppresses PDGFR signaling. In cells devoid of NHERF1, PDGF increases phosphatidylinositol-3-kinase signaling, whereas in cells expressing NHERF1, downstream signaling of AKT controls cell proliferation and survival. Consistent with these observations, NHERF1 is overexpressed in glioblastomas but is now primarily expressed in the cytoplasms rather than associated with the plasma membrane (Molina et al., 2010).
NF2 (merlin) is one of the FERM proteins that engage the EBD of NHERF1, thereby interacting with the cytoskeleton. NF2/merlin regulates the Hippo/SWH (Sav/Wts/Hpo) signaling pathway, which plays a pivotal role in tumor suppression by restricting proliferation and promoting apoptosis (Li et al., 2010b). Paradoxically, whereas NF2/merlin acts as a tumor suppressor, ezrin exerts oncogenic actions (Hunter, 2004; Khanna et al., 2004; Li et al., 2010a).
IX. Conclusions and Future Directions
The interaction between NHERF proteins and their binding partners is a subject of expanding investigation (http://www.gopubmed.com)4 and consequently a rapidly changing arena. It is now clear that many of the heretofore inexplicable or apparently contradictory reported findings between GPCR signaling, trafficking, and function in different cells or in response to distinct ligands now can be attributed to the participation of NHERF proteins and their ability to confer ligand- and cell-specific actions on GPCRs, thereby adding to the remarkable diversity of actions a single receptor can display. Much of this work examined stable interactions of PDZ proteins with GPCRs. However, for the most part, these are low-affinity and transient interactions. To understand better the dynamic mechanisms by which PDZ proteins assemble multiprotein complexes, we need now to apply techniques that permit analyzing these short-lived interactions. Such approaches, including time-resolved fluorescence resonance energy transfer, fluorescence recovery after photobleaching, bimolecular fluorescence complementation, and total internal reflection fluorescence, take advantage of high-quantum-yield fluorescence proteins that permit analyzing protein-protein interactions in time and space. Moreover, the examination of the interactions of PDZ proteins with GPCRs has largely relied upon heterologous cell models and extensive overexpression. Under these circumstances it is not surprising that many putative interactions can be detected that do not fit with described phenotypes from animal models or humans harboring spontaneous or engineered mutations in the PDZ protein. Hence, it will be critical for future work to concentrate on native cells and tissues that express constitutive levels of the GPCR and PDZ protein partner if we are to understand their true biological actions.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK054171, DK069998] (to P.A.F.). We thank our present and former colleagues Dr. Bin Wang, Dr. Tatyana Mamonova, Dr. Guillermo Romero, Dr. Alessandro Bisello, Dr. David Wheeler, Dr. Bruce Sneddon, and Yanmei Yang for their critical contributions; collaborators Drs. Randy Hall and Abdul Abou-Samra, and especially Dr. Edward Weinman for encouragement, support and generously supplying reagents.
Authorship Contributions
Participated in research design: Ardura and Friedman.
Wrote or contributed to the writing of the manuscript: Ardura and Friedman.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.110.004176.
Gene designations and synonyms for human NHERF proteins are summarized in Table 1.
In all cases, we refer to human NHERF proteins and genes unless otherwise noted.
Enter “NHERF” as the search term. Then, click on “statistics” to see the number of publications by year, demographics, and networking associated with NHERF.
- AR
- adrenergic receptor
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CRLR
- calcitonin receptor-like receptor
- CT
- carboxyl terminus
- E3KARP
- NHE3 kinase A regulatory protein
- EBD
- ezrin-binding domain
- ERE
- estrogen response element
- ERK
- extracellular signal-related kinase
- GPCR
- G protein-coupled receptor
- GRK
- G protein-coupled receptor kinase
- LPA
- lysophosphatidic acid
- LPA2R
- lysophosphatidic acid receptor 2
- NF2
- neurofibromatosis 2
- NHE3
- Na+/H+ exchanger isoform-3
- NHERF
- Na+/H+ exchange regulatory factor
- Npt2a
- type IIa sodium-phosphate cotransporter
- OK
- opossum kidney
- OKH
- subclone of OK cells that expresses only modest levels of NHERF1
- OR
- opioid receptor
- PDGFR
- platelet-derived growth factor receptor
- PDZ
- postsynaptic density 95/discs-large/zona occludens
- Pi
- inorganic phosphate
- PICK1
- protein interacting with C kinase 1
- PKA
- protein kinase A
- PLC
- phospholipase C
- PTEN
- phosphatase and tensin homolog
- PTH
- parathyroid hormone
- PTH1R
- parathyroid hormone receptor 1
- RAMP
- receptor activity-modifying protein
- ROS
- rat osteosarcoma
- TXA2R
- thromboxane A2 receptor.
References
- Abbracchio MP, Burnstock G. (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64:445–475 [DOI] [PubMed] [Google Scholar]
- Agrawal L, Jin Q, Altenburg J, Meyer L, Tubiana R, Theodorou I, Alkhatib G. (2007) CCR5Δ32 protein expression and stability are critical for resistance to human immunodeficiency virus type 1 in vivo. J Virol 81:8041–8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardura JA, Vilardaga JP, Friedman PA. (2011) Protein-exchange dynamics at GPCR micro-domains: a case study with the parathyroid hormone receptor (Abstract). Biophys J 98 (3 Suppl 1):432a [Google Scholar]
- Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95:5372–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benharouga M, Sharma M, So J, Haardt M, Drzymala L, Popov M, Schwapach B, Grinstein S, Du K, Lukacs GL. (2003) The role of the C terminus and Na+/H+ exchanger regulatory factor in the functional expression of cystic fibrosis transmembrane conductance regulator in nonpolarized cells and epithelia. J Biol Chem 278:22079–22089 [DOI] [PubMed] [Google Scholar]
- Biber J, Hernando N, Forster I, Murer H. (2009) Regulation of phosphate transport in proximal tubules. Pflugers Arch 458:39–52 [DOI] [PubMed] [Google Scholar]
- Bie B, Zhang Z, Cai YQ, Zhu W, Zhang Y, Dai J, Lowenstein CJ, Weinman EJ, Pan ZZ. (2010) Nerve growth factor-regulated emergence of functional delta-opioid receptors. J Neurosci 30:5617–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS. (2005a) Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem 280:9297–9307 [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N. (2005b) Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem 280:23926–23935 [DOI] [PubMed] [Google Scholar]
- Bretscher A. (1983) Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol 97:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. (2000) ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 16:113–143 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3:586–599 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Reczek D, Berryman M. (1997) Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci 110:3011–3018 [DOI] [PubMed] [Google Scholar]
- Broere N, Hillesheim J, Tuo B, Jorna H, Houtsmuller AB, Shenolikar S, Weinman EJ, Donowitz M, Seidler U, de Jonge HR, et al. (2007) Cystic fibrosis transmembrane conductance regulator activation is reduced in the small intestine of Na+/H+ exchanger 3 regulatory factor 1 (NHERF-1)- but not NHERF-2-deficient mice. J Biol Chem 282:37575–37584 [DOI] [PubMed] [Google Scholar]
- Bühlmann N, Leuthäuser K, Muff R, Fischer JA, Born W. (1999) A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology 140:2883–2890 [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401:286–290 [DOI] [PubMed] [Google Scholar]
- Capuano P, Bacic D, Roos M, Gisler SM, Stange G, Biber J, Kaissling B, Weinman EJ, Shenolikar S, Wagner CA, et al. (2007) Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol 292:C927–C934 [DOI] [PubMed] [Google Scholar]
- Capuano P, Bacic D, Stange G, Hernando N, Kaissling B, Pal R, Kocher O, Biber J, Wagner CA, Murer H. (2005) Expression and regulation of the renal Na/phosphate cotransporter NaPi-IIa in a mouse model deficient for the PDZ protein PDZK1. Pflugers Arch 449:392–402 [DOI] [PubMed] [Google Scholar]
- Cardone RA, Bellizzi A, Busco G, Weinman EJ, Dell'Aquila ME, Casavola V, Azzariti A, Mangia A, Paradiso A, Reshkin SJ. (2007) The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol Biol Cell 18:1768–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B, Zhu XC, Chen W, Jones M, Ryoo S, Zachos NC, Chen TE, Lin R, Sarker R, Kenworthy AK, et al. (2010) NHE3 mobility in brush borders increases upon NHERF2-dependent stimulation by lyophosphatidic acid. J Cell Sci 123:2434–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HC, Fong SK, So SC, Chung YW, Wong PY. (1997) Stimulation of anion secretion by β-adrenoceptors in the mouse endometrial epithelium. J Physiol (Lond) 501:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Lim S, Oh YS, Kim EK, Kim SH, Kim YH, Heo K, Kim J, Kim JK, Yang YR, et al. (2010) Subtype-specific role of phospholipase C-β in bradykinin and LPA signaling through differential binding of different PDZ scaffold proteins. Cell Signal 22:1153–1161 [DOI] [PubMed] [Google Scholar]
- Choi RC, Simon J, Tsim KW, Barnard EA. (2008) Constitutive and agonist-induced dimerizations of the P2Y1 receptor: relationship to internalization and scaffolding. J Biol Chem 283:11050–11063 [DOI] [PubMed] [Google Scholar]
- Cunningham R, Biswas R, Steplock D, Shenolikar S, Weinman E. (2010) Role of NHERF and scaffolding proteins in proximal tubule transport. Urol Res 38:257–262 [DOI] [PubMed] [Google Scholar]
- Cunningham R, Esmaili A, Brown E, Biswas RS, Murtazina R, Donowitz M, Dijkman HB, van der Vlag J, Hogema BM, De Jonge HR, et al. (2008) Urine electrolyte, mineral, and protein excretion in NHERF-2 and NHERF-1 null mice. Am J Physiol Renal Physiol 294:F1001–F1007 [DOI] [PubMed] [Google Scholar]
- Cunningham R, Steplock D, Wang F, Huang H, Xiaofei E, Shenolikar S, Weinman EJ. (2004) Defective PTH regulation of NHE3 activity and phosphate adaptation in cultured NHERF-1−/− renal proximal tubule cells. J Biol Chem 279:37815–37821 [DOI] [PubMed] [Google Scholar]
- Cunningham R, Steplock D, Xiaofei E, Biswas RS, Wang F, Shenolikar S, Weinman EJ. (2006) Adenoviral expression of NHERF-1 in NHERF-1 null mouse renal proximal tubule cells restores Npt2a regulation by low phosphate media and parathyroid hormone. Am J Physiol Renal Physiol 291:F896–F901 [DOI] [PubMed] [Google Scholar]
- Cunningham R, Xiaofei E, Steplock D, Shenolikar S, Weinman EJ. (2005) Defective PTH regulation of sodium-dependent phosphate transport in NHERF-1−/− renal proximal tubule cells and wild-type cells adapted to low phosphate media. Am J Physiol Renal Physiol 289:F933–F938 [DOI] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. (2007) Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol 177:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer M, Spindler B, Verrey F, Murer H, Biber J. (1997) Identification of a new gene product (diphor-1) regulated by dietary phosphate. Am J Physiol 273:F801–F806 [DOI] [PubMed] [Google Scholar]
- Dai JL, Wang L, Sahin AA, Broemeling LD, Schutte M, Pan Y. (2004) NHERF (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene 23:8681–8687 [DOI] [PubMed] [Google Scholar]
- Demoulin JB, Seo JK, Ekman S, Grapengiesser E, Hellman U, Rönnstrand L, Heldin CH. (2003) Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF. Biochem J 376:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. (2005) NHERF family and NHE3 regulation. J Physiol (Lond) 567:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M, Singh S, Singh P, Chakraborty M, Chen Y, Murtazina R, Gucek M, Cole RN, Zachos NC, Salahuddin FF, et al. (2011) Alternations in the proteome of the NHERF2 knockout mouse jejunal brush border membrane vesicles. Physiol Genomics 43:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- E S, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, et al. (2009) Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem 284:14558–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ediger TR, Kraus WL, Weinman EJ, Katzenellenbogen BS. (1999) Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology 140:2976–2982 [DOI] [PubMed] [Google Scholar]
- Ediger TR, Park SE, Katzenellenbogen BS. (2002) Estrogen receptor inducibility of the human Na+/H+ exchanger regulatory factor/ezrin-radixin-moesin binding protein 50 (NHE-RF/EBP50) gene involving multiple half-estrogen response elements. Mol Endocrinol 16:1828–1839 [DOI] [PubMed] [Google Scholar]
- Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, Smith Y, Yun CC, Hall RA. (2005) P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA 102:8042–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouassier L, Nichols MT, Gidey E, McWilliams RR, Robin H, Finnigan C, Howell KE, Housset C, Doctor RB. (2005) Protein kinase C regulates the phosphorylation and oligomerization of ERM binding phosphoprotein 50. Exp Cell Res 306:264–273 [DOI] [PubMed] [Google Scholar]
- Fouassier L, Yun CC, Fitz JG, Doctor RB. (2000) Evidence for ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J Biol Chem 275:25039–25045 [DOI] [PubMed] [Google Scholar]
- Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA. (1996) Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving PKA and PKC. Endocrinology 137:13–20 [DOI] [PubMed] [Google Scholar]
- Garbett D, LaLonde DP, Bretscher A. (2010) The scaffolding protein EBP50 regulates microvillar assembly in a phosphorylation-dependent manner. J Cell Biol 191:397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. (1996) Functional β3-adrenoceptor in the human heart. J Clin Invest 98:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Morales FC, Molina JR, Hayashi Y. (2008) Roles of NHERF1/EBP50 in cancer. Curr Mol Med 8:459–468 [DOI] [PubMed] [Google Scholar]
- Giral H, Lanzano L, Caldas Y, Blaine J, Verlander JW, Lei T, Gratton E, Levi M. (2011) Role of PDZK1 protein in apical membrane expression of renal sodium-coupled phosphate transporters. J Biol Chem 286:15032–15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisler SM, Kittanakom S, Fuster D, Wong V, Bertic M, Radanovic T, Hall RA, Murer H, Biber J, Markovich D, et al. (2008) Monitoring protein-protein interactions between the mammalian integral membrane transporters and PDZ-interacting partners using a modified split-ubiquitin membrane yeast two-hybrid system. Mol Cell Proteomics 7:1362–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, et al. (2003) PDZK1: I. A major scaffolder in brush borders of proximal tubular cells. Kidney Int 64:1733–1745 [DOI] [PubMed] [Google Scholar]
- Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H. (2001) Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem 276:9206–9213 [DOI] [PubMed] [Google Scholar]
- Guerra L, Favia M, Fanelli T, Calamita G, Svetlo M, Bagorda A, Jacobson KA, Reshkin SJ, Casavola V. (2004) Stimulation of Xenopus P2Y1 receptor activates CFTR in A6 cells. Pflugers Arch 449:66–75 [DOI] [PubMed] [Google Scholar]
- Guggino WB, Stanton BA. (2006) New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7:426–436 [DOI] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. (1998a) A C-terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA 95:8496–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, et al. (1998b) The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392:626–630 [DOI] [PubMed] [Google Scholar]
- Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, Lefkowitz RJ. (1999) G protein-coupled receptor kinase 6A phosphorylates the Na+/H+ exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem 274:24328–24334 [DOI] [PubMed] [Google Scholar]
- Hammad MM, Kuang YQ, Yan R, Allen H, Dupré DJ. (2010) Na+/H+ exchanger regulatory factor-1 is involved in chemokine receptor homodimer CCR5 internalization and signal transduction but does not affect CXCR4 homodimer or CXCR4-CCR5 heterodimer. J Biol Chem 285:34653–34664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bellini M, Inuzuka H, Xu J, Xiong Y, Yang X, Castleberry AM, Hall RA. (2006) Proteomic analysis of β1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem 281:2820–2827 [DOI] [PubMed] [Google Scholar]
- Hennigan RF, Foster LA, Chaiken MF, Mani T, Gomes MM, Herr AB, Ip W. (2010) Fluorescence resonance energy transfer analysis of merlin conformational changes. Mol Cell Biol 30:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Søndergaard BP, Ersbøll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. (2004) A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J Biol Chem 279:54291–54303 [DOI] [PubMed] [Google Scholar]
- Hillesheim J, Riederer B, Tuo B, Chen M, Manns M, Biber J, Yun C, Kocher O, Seidler U. (2007) Down regulation of small intestinal ion transport in PDZK1- (CAP70/NHERF3) deficient mice. Pflugers Arch 454:575–586 [DOI] [PubMed] [Google Scholar]
- Hock B, Böhme B, Karn T, Yamamoto T, Kaibuchi K, Holtrich U, Holland S, Pawson T, Rübsamen-Waigmann H, Strebhardt K. (1998) PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci USA 95:9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Steplock D, Weinman EJ, Hall RA, Ding Z, Li J, Wang Y, Liu-Chen LY. (2004) κ Opioid receptor interacts with Na+/H+-exchanger regulatory factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50) to stimulate Na+/H+ exchange independent of Gi/Go proteins. J Biol Chem 279:25002–25009 [DOI] [PubMed] [Google Scholar]
- Hunter KW. (2004) Ezrin, a key component in tumor metastasis. Trends Mol Med 10:201–204 [DOI] [PubMed] [Google Scholar]
- Ingraffea J, Reczek D, Bretscher A. (2002) Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur J Cell Biol 81:61–68 [DOI] [PubMed] [Google Scholar]
- Karim Z, Gérard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, et al. (2008) NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359:1128–1135 [DOI] [PubMed] [Google Scholar]
- Karthikeyan S, Leung T, Ladias JA. (2001a) Structural basis of the Na+/H+ exchanger regulatory factor PDZ1 interaction with the carboxyl-terminal region of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 276:19683–19686 [DOI] [PubMed] [Google Scholar]
- Karthikeyan S, Leung T, Birrane G, Webster G, Ladias JA. (2001b) Crystal structure of the PDZ1 domain of human Na+/H+ exchanger regulatory factor provides insights into the mechanism of carboxyl-terminal leucine recognition by class I PDZ domains. J Mol Biol 308:963–973 [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nature Med 10:182–186 [DOI] [PubMed] [Google Scholar]
- Kinsella BT. (2001) Thromboxane A2 signalling in humans: a ‘Tail’ of two receptors. Biochem Soc Trans 29:641–654 [DOI] [PubMed] [Google Scholar]
- Klenk C, Vetter T, Zürn A, Vilardaga JP, Friedman PA, Wang B, Lohse MJ. (2010) Formation of a ternary complex among NHERF1, β-arrestin, and parathyroid hormone receptor. J Biol Chem 285:30355–30362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O, Birrane G, Tsukamoto K, Fenske S, Yesilaltay A, Pal R, Daniels K, Ladias JA, Krieger M. (2010) In vitro and in vivo analysis of the binding of the C terminus of the HDL receptor scavenger receptor class B, type I (SR-BI), to the PDZ1 domain of its adaptor protein PDZK1. J Biol Chem 285:34999–35010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O, Comella N, Tognazzi K, Brown LF. (1998) Identification and partial characterization of PDZK1: a novel protein containing PDZ interaction domains. Lab Invest 78:117–125 [PubMed] [Google Scholar]
- Kocher O, Pal R, Roberts M, Cirovic C, Gilchrist A. (2003) Targeted disruption of the PDZK1 gene by homologous recombination. Mol Cell Biol 23:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O, Yesilaltay A, Shen CH, Zhang S, Daniels K, Pal R, Chen J, Krieger M. (2008) Influence of PDZK1 on lipoprotein metabolism and atherosclerosis. Biochim Biophys Acta 1782:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimann EL, Morales FC, de Orbeta-Cruz J, Takahashi Y, Adams H, Liu TJ, McCrea PD, Georgescu MM. (2007) Cortical stabilization of β-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene 26:5290–5299 [DOI] [PubMed] [Google Scholar]
- Kurashima K, Yu FH, Cabado AG, Szabó EZ, Grinstein S, Orlowski J. (1997) Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. phosphorylation-dependent and -independent mechanisms. J Biol Chem 272:28672–28679 [DOI] [PubMed] [Google Scholar]
- Lamprecht G, Seidler U. (2006) The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol 291:G766–G777 [DOI] [PubMed] [Google Scholar]
- Lamprecht G, Weinman EJ, Yun CH. (1998) The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273:29972–29978 [DOI] [PubMed] [Google Scholar]
- Lau AG, Hall RA. (2001) Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl-termini and by phosphorylation. Biochemistry 40:8572–8580 [DOI] [PubMed] [Google Scholar]
- Lauffer BE, Chen S, Melero C, Kortemme T, von Zastrow M, Vargas GA. (2009) Engineered protein connectivity to actin mimics PDZ-dependent recycling of G protein-coupled receptors but not its regulation by Hrs. J Biol Chem 284:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. (2010) SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol 190:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40:389–430 [DOI] [PubMed] [Google Scholar]
- Lazar CS, Cresson CM, Lauffenburger DA, Gill GN. (2004) The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol Biol Cell 15:5470–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC. (2011) MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology 140:924–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Zheng JJ. (2010) PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, et al. (2005) Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Poulikakos PI, Dai Z, Testa JR, Callaway DJ, Bu Z. (2007) Protein kinase C phosphorylation disrupts Na+/H+ exchanger regulatory factor 1 autoinhibition and promotes cystic fibrosis transmembrane conductance regulator macromolecular assembly. J Biol Chem 282:27086–27099 [DOI] [PubMed] [Google Scholar]
- Li J, Tu Y, Wen J, Yao F, Wei W, Sun S. (2010a) Role for ezrin in breast cancer cell chemotaxis to CCL5. Oncol Rep 24:965–971 [DOI] [PubMed] [Google Scholar]
- Li JG, Chen C, Liu-Chen LY. (2002) Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem 277:27545–27552 [DOI] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, et al. (2010b) Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 140:477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, et al. (2010) Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology 138:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chen LY. (2004) Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci 75:511–536 [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. (2006) Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol 69:1137–1145 [DOI] [PubMed] [Google Scholar]
- Mahon MJ. (2009) The parathyroid hormone 1 receptor directly binds to the FERM domain of ezrin, an interaction that supports apical receptor localization and signaling in LLC-PK1 cells. Mol Endocrinol 23:1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon MJ, Cole JA, Lederer ED, Segre GV. (2003) Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 17:2355–2364 [DOI] [PubMed] [Google Scholar]
- Mahon MJ, Donowitz M, Yun CC, Segre GV. (2002) Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417:858–861 [DOI] [PubMed] [Google Scholar]
- Mahon MJ, Segre GV. (2004) Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cβ, and actin increases intracellular calcium in opossum kidney cells. J Biol Chem 279:23550–23558 [DOI] [PubMed] [Google Scholar]
- Mangia A, Chiriatti A, Bellizzi A, Malfettone A, Stea B, Zito FA, Reshkin SJ, Simone G, Paradiso A. (2009) Biological role of NHERF1 protein expression in breast cancer. Histopathology 55:600–608 [DOI] [PubMed] [Google Scholar]
- Maudsley S, Zamah AM, Rahman N, Blitzer JT, Luttrell LM, Lefkowitz RJ, Hall RA. (2000) Platelet-derived growth factor receptor association with Na+/H+ exchanger regulatory factor potentiates receptor activity. Mol Cell Biol 20:8352–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin- receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- Miedlich SU, Zhu ED, Sabbagh Y, Demay MB. (2010) The receptor-dependent actions of 1,25-dihydroxyvitamin D are required for normal growth plate maturation in NPt2a knockout mice. Endocrinology 151:4607–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewski MI, Lopez A, Jurkowska M, Larusch J, Cutting GR. (2005) PDZ-binding motifs are unable to ensure correct polarized protein distribution in the absence of additional localization signals. FEBS Lett 579:483–487 [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. (1999) Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol 147:879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JR, Morales FC, Hayashi Y, Aldape KD, Georgescu MM. (2010) Loss of PTEN binding adapter protein NHERF1 from plasma membrane in glioblastoma contributes to PTEN inactivation. Cancer Res 70:6697–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. (2004) Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA 101:17705–17710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FC, Takahashi Y, Momin S, Adams H, Chen X, Georgescu MM. (2007) NHERF1/EBP50 head-to-tail intramolecular interaction masks association with PDZ domain ligands. Mol Cell Biol 27:2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell G, Steplock D, Shenolikar S, Weinman EJ. (1990) Identification of a putative Na+-H+ exchanger regulatory cofactor in rabbit renal BBM. Am J Physiol 259:F867–F871 [DOI] [PubMed] [Google Scholar]
- Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. (2011) NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol 301:C126–C136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, et al. (2003) A macromolecular complex of β2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA 100:342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies AT, König J, Cui Y, Brom M, Spring H, Keppler D. (2002) Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2). Eur J Biochem 269:1866–1876 [DOI] [PubMed] [Google Scholar]
- Oh YS, Jo NW, Choi JW, Kim HS, Seo SW, Kang KO, Hwang JI, Heo K, Kim SH, Kim YH, et al. (2004) NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-β3 activation. Mol Cell Biol 24:5069–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Randak C, Rokhlina T, Karp P, Vermeer D, Ashbourne Excoffon KJ, Welsh MJ. (2003) Effects of C-terminal deletions on cystic fibrosis transmembrane conductance regulator function in cystic fibrosis airway epithelia. Proc Natl Acad Sci USA 100:1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TM, Stiles GL. (1995) Adenosine receptors. Neuropharmacology 34:683–694 [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang L, Dai JL. (2006) Suppression of breast cancer cell growth by Na+/H+ exchanger regulatory factor 1 (NHERF1). Breast Cancer Res 8:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Weinman EJ, Dai JL. (2008) Na+/H+ exchanger regulatory factor 1 inhibits platelet-derived growth factor signaling in breast cancer cells. Breast Cancer Res 10:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, Asay MJ, Fam SR, Inuzuka H, Castleberry AM, Oller H, Smith Y, Yun CC, Traynelis SF, Hall RA. (2006) The PDZ scaffold NHERF-2 interacts with mGluR5 and regulates receptor activity. J Biol Chem 281:29949–29961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Phillips C, Davies KE, Blake DJ. (1997) PDZ domains: targeting signalling molecules to sub-membranous sites. Bioessays 19:469–479 [DOI] [PubMed] [Google Scholar]
- Raghuram V, Hormuth H, Foskett JK. (2003) A kinase-regulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc Natl Acad Sci USA 100:9620–9625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Berryman M, Bretscher A. (1997) Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol 139:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Bretscher A. (1998) The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J Biol Chem 273:18452–18458 [DOI] [PubMed] [Google Scholar]
- Rochdi MD, Parent JL. (2003) Gαq-coupled receptor internalization specifically induced by Gαq signaling. Regulation by EBP50. J Biol Chem 278:17827–17837 [DOI] [PubMed] [Google Scholar]
- Rochdi MD, Watier V, La Madeleine C, Nakata H, Kozasa T, Parent JL. (2002) Regulation of GTP-binding protein αq (Gαq) signaling by the ezrin-radixin-moesin-binding phosphoprotein-50 (EBP50). J Biol Chem 277:40751–40759 [DOI] [PubMed] [Google Scholar]
- Schindelmann S, Windisch J, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. (2002) Expression profiling of mammary carcinoma cell lines: correlation of in vitro invasiveness with expression of CD24. Tumour Biol 23:139–145 [DOI] [PubMed] [Google Scholar]
- Scott JD, Pawson T. (2009) Cell signaling in space and time: where proteins come together and when they're apart. Science 326:1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RO, Thelin WR, Milgram SL. (2002) A novel PDZ protein regulates the activity of guanylyl cyclase C, the heat-stable enterotoxin receptor. J Biol Chem 277:22934–22941 [DOI] [PubMed] [Google Scholar]
- Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B. (2009) The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann NY Acad Sci 1165:249–260 [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Minkoff CM, Steplock DA, Evangelista C, Liu M, Weinman EJ. (2001) N-terminal PDZ domain is required for NHERF dimerization. FEBS Lett 489:233–236 [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Voltz JW, Cunningham R, Weinman EJ. (2004) Regulation of ion transport by the NHERF family of PDZ proteins. Physiology (Bethesda) 19:362–369 [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. (2002) Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA 99:11470–11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S. (2003) EBP50, a β-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 38:178–186 [DOI] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. (1998) An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem 273:19797–19801 [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. (1999) The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96:5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Riederer B, Krabbenhöft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, et al. (2009) Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119:540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL. (2002) The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem 277:33188–33195 [DOI] [PubMed] [Google Scholar]
- Sneddon WB, Magyar CE, Willick GE, Syme CA, Galbiati F, Bisello A, Friedman PA. (2004) Ligand-selective dissociation of activation and internalization of the parathyroid hormone (PTH) receptor: conditional efficacy of PTH peptide fragments. Endocrinology 145:2815–2823 [DOI] [PubMed] [Google Scholar]
- Sneddon WB, Syme CA, Bisello A, Magyar CE, Rochdi MD, Parent JL, Weinman EJ, Abou-Samra AB, Friedman PA. (2003) Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50). J Biol Chem 278:43787–43796 [DOI] [PubMed] [Google Scholar]
- Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, Parmentier M, Springael JY. (2009) Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem 284:31270–31279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Bai J, Yang W, Gabrielson EW, Chan DW, Zhang Z. (2007) Expression and clinicopathological significance of oestrogen-responsive ezrin-radixin-moesin-binding phosphoprotein 50 in breast cancer. Histopathology 51:40–53 [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275:73–77 [DOI] [PubMed] [Google Scholar]
- Stemmer-Rachamimov AO, Wiederhold T, Nielsen GP, James M, Pinney-Michalowski D, Roy JE, Cohen WA, Ramesh V, Louis DN. (2001) NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol 158:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NL, Christopherson KS, Yi BA, Schatz PJ, Raab RW, Dawes G, Bassett DE, Jr, Bredt DS, Li M. (1997) PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequences. Nat Biotechnol 15:336–342 [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101:6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Otake T, Shimizu T, Wakayama T, Silver DL, Utsumi R, Nishimura T, Iseki S, Nakamichi N, Kubo Y, et al. (2010) PDZK1 regulates organic anion transporting polypeptide Oatp1a in mouse small intestine. Drug Metab Pharmacokinet 25:588–598 [DOI] [PubMed] [Google Scholar]
- Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. (2000) E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem 275:29539–29546 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Morales FC, Kreimann EL, Georgescu MM. (2006) PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J 25:910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taouil K, Hinnrasky J, Hologne C, Corlieu P, Klossek JM, Puchelle E. (2003) Stimulation of βs-adrenergic receptor increases cystic fibrosis transmembrane conductance regulator expression in human airway epithelial cells through a cAMP/protein kinase A-independent pathway. J Biol Chem 278:17320–17327 [DOI] [PubMed] [Google Scholar]
- Theisen CS, Wahl JK, 3rd, Johnson KR, Wheelock MJ. (2007) NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell 18:1220–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin WR, Hodson CA, Milgram SL. (2005) Beyond the brush border: NHERF4 blazes new NHERF turf. J Physiol (Lond) 567:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PI, von Zastrow M. (2000) Type-specific sorting of G protein-coupled receptors after endocytosis. J Biol Chem 275:11130–11140 [DOI] [PubMed] [Google Scholar]
- UniProt Consortium (2011) Ongoing and future developments at the Universal Protein Resource. Nucleic Acids Res 39:D214–D219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. (2010) Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol 10:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. (2007) Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J Biol Chem 282:33879–33887 [DOI] [PubMed] [Google Scholar]
- Voltz JW, Weinman EJ, Shenolikar S. (2001) Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 20:6309–6314 [DOI] [PubMed] [Google Scholar]
- Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. (2001) Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol 280:C192–C198 [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. (2004) Opioid receptors. Annu Rev Biochem 73:953–990 [DOI] [PubMed] [Google Scholar]
- Walker LC, Venglarik CJ, Aubin G, Weatherly MR, McCarty NA, Lesnick B, Ruiz F, Clancy JP, Sorscher EJ. (1997) Relationship between airway ion transport and a mild pulmonary disease mutation in CFTR. Am J Respir Crit Care Med 155:1684–1689 [DOI] [PubMed] [Google Scholar]
- Wang B, Ardura JA, Romero G, Yang Y, Hall RA, Friedman PA. (2010) Na/H exchanger regulatory factors control PTH receptor signaling by differential activation of Gα protein subunits. J Biol Chem 285:26976–26986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. (2007) NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem 282:36214–36222 [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. (2001) Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. J Neurosci 21:3242–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yang Y, Abou-Samra AB, Friedman PA. (2009) NHERF1 regulates parathyroid hormone receptor desensitization: interference with β-arrestin binding. Mol Pharmacol 75:1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yang Y, Friedman PA. (2008) Na/H exchange regulator factor 1, a novel AKT-associating protein, regulates extracellular signal-related signaling through a B-Raf-mediated pathway. Mol Biol Cell 19:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. (1998a) Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR). FEBS Lett 427:103–108 [DOI] [PubMed] [Google Scholar]
- Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. (1998b) Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR). FEBS Lett 427:103–108 [DOI] [PubMed] [Google Scholar]
- Wawrzak D, Luyten A, Lambaerts K, Zimmermann P. (2009) Frizzled-PDZ scaffold interactions in the control of Wnt signaling. Adv Enzyme Regul 49:98–106 [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Shenolikar S. (1993) Regulation of the renal brush border membrane Na+-H+ exchanger. Annu Rev Physiol 55:289–304 [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Shenolikar S. (1997) The Na-H exchanger regulatory factor. Exp Nephrol 5:449–452 [PubMed] [Google Scholar]
- Weinman EJ, Biswas R, Steplock D, Douglass TS, Cunningham R, Shenolikar S. (2010a) Sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) transduces signals that mediate dopamine inhibition of sodium-phosphate co-transport in mouse kidney. J Biol Chem 285:13454–13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Biswas RS, Peng G, Peng Q, Shen L, Turner CL, E X, Steplock D, Shenolikar S, Cunningham R. (2007) Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117:3412–3420 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. (2006a) The association of NHERF adaptor proteins with G protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol 68:491–505 [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, Shenolikar S, Cunningham R. (2006b) Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 Null mice. Am J Physiol Renal Physiol 290:F838–F843 [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Cha B, Kovbasnjuk O, Frost NA, Cunningham R, Shenolikar S, Blanpied TA, Donowitz M. (2009) PTH transiently increases the percent mobile fraction of Npt2a in OK cells as determined by FRAP. Am J Physiol Renal Physiol 297:F1560–F1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Shenolikar S. (1993) cAMP-mediated inhibition of the renal brush border membrane Na+-H+ exchanger requires a dissociable phosphoprotein cofactor. J Clin Invest 92:1781–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Shenolikar S, Blanpied TA. (2011) Dynamics of PTH-induced disassembly of Npt2a/NHERF-1 complexes in living OK cells. Am J Physiol Renal Physiol 300:F231–F235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Tate K, Hall RA, Spurney RF, Shenolikar S. (1998) Structure-function of recombinant Na/H exchanger regulatory factor (NHE-RF). J Clin Invest 101:2199–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Wang Y, Shenolikar S. (1995) Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na+/H+ exchanger. J Clin Invest 95:2143–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Zhang X, Akhter S, Shenolikar S. (1999) Molecular cloning of the cDNA and promoter sequences for the mouse sodium-hydrogen exchanger regulatory factor. Biochim Biophys Acta 1447:71–76 [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Zhang Y, Biswas R, Bloch RJ, Shenolikar S. (2010b) Cooperativity between the phosphorylation of Thr95 and Ser77 of NHERF-1 in the hormonal regulation of renal phosphate transport. J Biol Chem 285:25134–25138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Garrido JL, Bisello A, Kim YK, Friedman PA, Romero G. (2008) Regulation of PTH1R dynamics, traffic and signaling by the Na+/H+ exchanger regulatory factor-1 (NHERF1) in rat osteosarcoma ROS 17/28 cells. Mol Endocrinol 22:1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Sneddon WB, Wang B, Friedman PA, Romero G. (2007) NHERF-1 and the cytoskeleton regulate the traffic and membrane dynamics of G protein-coupled receptors. J Biol Chem 282:25076–25087 [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Barrick SR, Grubisha MJ, Brufsky AM, Friedman PA, Romero G. (2011) Direct interaction between NHERF1 and Frizzled regulates β-catenin signaling. Oncogene 30:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP, Ji X, Lakatta EG. (1995) Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol 47:322–329 [PubMed] [Google Scholar]
- Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. (2005) LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol 289:C2–C11 [DOI] [PubMed] [Google Scholar]
- Yun CH, Lamprecht G, Forster DV, Sidor A. (1998) NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem 273:25856–25863 [DOI] [PubMed] [Google Scholar]
- Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. (1997) cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94:3010–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh S, Appleton BA, Held HA, Kausalya PJ, Phua DC, Wong WL, Lasky LA, Wiesmann C, Hunziker W, et al. (2006) Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J Biol Chem 281:22299–22311 [DOI] [PubMed] [Google Scholar]
- Zheng JF, Sun LC, Liu H, Huang Y, Li Y, He J. (2010) EBP50 exerts tumor suppressor activity by promoting cell apoptosis and retarding extracellular signal-regulated kinase activity. Amino Acids 38:1261–1268 [DOI] [PubMed] [Google Scholar]
- Zizak M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun CH, Weinman EJ. (1999) cAMP-induced phosphorylation and inhibition of Na+/H+ exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor. J Biol Chem 274:24753–24758 [DOI] [PubMed] [Google Scholar]