Abstract
Opioids are the most effective analgesic drugs for the management of moderate or severe pain, yet their clinical use is often limited because of the onset of adverse side effects. Drugs in this class produce most of their physiological effects through activation of the μ opioid receptor; however, an increasing number of studies demonstrate that different opioids, while presumably acting at this single receptor, can activate distinct downstream responses, a phenomenon termed functional selectivity. Functional selectivity of receptor-mediated events can manifest as a function of the drug used, the cellular or neuronal environment examined, or the signaling or behavioral measure recorded. This review summarizes both in vitro and in vivo work demonstrating functional selectivity at the μ opioid receptor in terms of G protein coupling, receptor phosphorylation, interactions with β-arrestins, receptor desensitization, internalization and signaling, and details on how these differences may relate to the progression of analgesic tolerance after their extended use.
I. Introduction
Opioid analgesics are the most efficacious drugs for the treatment of moderate to severe pain and represent the largest market share of prescription pain medications (Melnikova, 2010). Although opioids are effective pain relievers, they also produce a number of adverse side effects that can limit their clinical utility, including nausea and vomiting, constipation, and respiratory suppression. Moreover, long-term exposure to opioids is also associated with the development of analgesic tolerance, physical dependence, and addiction (Cherny et al., 2001; Harris, 2008). Given their numerous effects, a major goal in opioid research is to understand the molecular and cellular mechanisms that give rise to opioid-induced physiological and behavioral responses and adaptations to develop improved analgesics that can selectively provide pain relief while reducing the onset of these unwanted side effects.
The μ opioid receptor (μOR) belongs to the superfamily of G protein-coupled receptors (GPCRs) and has been shown to be the opioid receptor subtype that primarily mediates the physiological actions of clinically used opioids (Kieffer, 1999). At the cellular level, the μOR traditionally has been described to mediate opioids drug effects by coupling to heterotrimeric G proteins (Fig. 1A), particularly pertussis toxin-sensitive Gαi/o proteins, which act to inhibit adenylyl cyclases, modulate activity of certain ion channels, and signal through several second-messenger signal transduction cascades to promote signaling (for review, see Law, 2011).
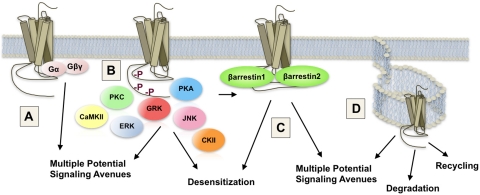
Fig. 1.
Schematic demonstrating key points in opioid receptor signaling and regulation that have been shown to be influenced by differential agonist occupation. A, heterotrimeric G proteins represent 16 individual gene products for Gα, 5 individual gene products for Gβ and 11 for Gγ proteins. Together, the diversity arising from heterotrimeric G protein subunit composition presents a gateway to potentially high diversification of agonist-directed coupling between μOR and G proteins. These interactions can determine access to secondary cascade activation. B, the μOR can be phosphorylated in response to agonist occupation by multiple kinases, each of which has multiple isoforms. Phosphorylation by a particular kinase may dictate secondary cascade interactions or subsequent receptor fate. CKII, casein kinase II. C, receptor interaction with scaffolding partners such as β-arrestins can be dependent or independent of receptor phosphorylation. Agonist occupancy may determine these interactions with potential binding partners. Such interactions can prevent (desensitization) or promote subsequent signaling. D, the μOR can be internalized in response to agonist occupancy. Endocytosis may involve clathrin- or caveolin-dependent processes and may result in the activation of subsequent signaling pathways, receptor recycling or degradation.
As with most GPCRs, the extent and duration of agonist-induced μOR signaling can be determined by several regulatory mechanisms including receptor desensitization, internalization, down-regulation, and resensitization. After agonist activation, the μOR can be rapidly phosphorylated by G protein-coupled receptor kinases (GRKs) or other second messenger-regulated kinases, including protein kinase C (PKC) (Fig. 1B). This may facilitate β-arrestin binding and the dampening of further coupling to G proteins, despite the continued presence of agonist (for review, see Ferguson, 2001; Ahn et al., 2003) (Fig. 1C). In addition to receptor desensitization, β-arrestins can act to facilitate receptor internalization, which can contribute to down-regulation or resensitization events (Ferguson et al., 1996) (Fig. 1D). More recently, it has been shown that GPCRs can also signal through β-arrestins independent of G proteins, both in cellular systems (Daaka et al., 1998; Luttrell et al., 1999; McDonald et al., 2000; Luttrell and Lefkowitz, 2002) and in vivo (Beaulieu et al., 2005, 2008; Schmid et al., 2008; Schmid and Bohn, 2010; Urs et al., 2010).
The conventional understanding of receptor pharmacology has been that responses elicited by GPCR activation are determined by the “intrinsic efficacy” of the ligand acting at the receptor. In this model, a full agonist maximally activates all signal transduction pathways to which the receptor is coupled and therefore has high intrinsic efficacy. Partial agonists, on the other hand, induce submaximal activation of these same pathways, and thus possess lower degrees of intrinsic efficacy, whereas inverse agonists reduce the basal activities of these pathways. Antagonists, which possess no intrinsic efficacy and do not shift any of the responses away from basal levels, occupy the receptor and block receptor responses induced by agonists. However, these definitions may not wholly conceptualize the full range of pharmacological profiles that are observed experimentally.
Over the past several decades, numerous studies have demonstrated that not all GPCR agonists activate the same intracellular signaling pathways, even though they may be acting at the same receptor (Kenakin, 2011). For example, an agonist may fully stimulate G protein coupling yet show very little efficacy in activating a mitogen-activated protein kinase pathway, whereas another agonist at the same receptor may fully activate mitogen-activated protein kinases yet weakly engage G protein coupling. The concept of “functional selectivity” (or “biased agonism” or “collateral efficacy”) has evolved to describe these differences in ligand-directed GPCR signaling, wherein efficacies and potencies are not conserved among diverse signaling cascades (Kenakin, 2005, 2007, 2009; Mailman, 2007; Urban et al., 2007; Bohn, 2009). It has been proposed that physical interactions between an agonist and a receptor impact upon the physical constraints of receptor conformation, which can result in a preferential or “biased” interaction with certain signaling components over others (Fig. 2) (Kenakin, 2007, 2009). Furthermore, the cellular environment, including the proteins expressed in close proximity with the receptor, can influence those interactions and thereby influence the degree of signaling induced by a particular ligand. In this way, the same ligand can induce differential signaling profiles when a receptor is expressed in different cell types (Bohn, 2009; Schmid and Bohn, 2009).
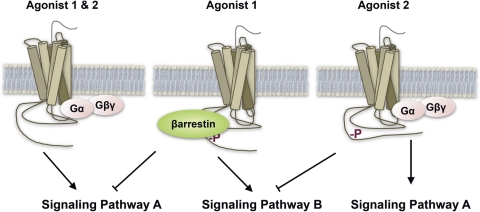
Fig. 2.
Schematic representation of functional selectivity at the level of GPCR and G protein or β-arrestin bias. In this diagram, agonists 1 and 2 both activate receptor G protein-coupling pathways, represented as “Signaling Pathway A.” However, agonist 1 leads to recruitment of β-arrestin but agonist 2 does not. Receptor-β-arrestin interactions can serve to disrupt receptor-G protein coupling and may also serve to facilitate GPCR signaling to alternate pathways such as Signaling Pathway B (Schmid and Bohn, 2010). By not recruiting β-arrestins, agonist 2 activates the receptor without engaging signaling pathway B while allowing signaling pathway A to proceed. Agonist 2 would be considered biased against β-arrestin signaling.
Functional selectivity has been demonstrated at the μOR, where it has been observed that different opioids can promote diverse cellular and physiological responses downstream of receptor activation that cannot be solely attributed to differences in the ligand's efficacy. In addition to being a function of the agonist, μOR regulation and signaling are proving to be contextual, whereby the cellular environment contributes to ligand-induced responses. This review summarizes work demonstrating agonist-determined differences in μOR responsiveness with respect to key signaling and regulatory events, including the activation of G protein and second-messenger signaling cascades, regulation by kinases and β-arrestins and subsequent receptor desensitization and internalization. Studies that demonstrate how these differences may relate to the progression of analgesic tolerance after extended use of opioid drugs in vivo are also reviewed. Although still at an early stage in development, these studies suggest that the development of functionally selective μOR agonists may allow for the fine-tuning of receptor signaling toward desired pathways and away from unwanted signaling pathways such as those mediating adverse side effects. The challenge remains in identifying those pathways in vivo.
II. Biased Agonism with Respect to G Protein Coupling
Opioid receptors mediate many of their cellular and physiological affects by coupling to different inhibitory type Gα subunits, including Gαi and Gαo (Fig. 1A); however, the specific Gαi subunits to which the μOR couples are dictated by the agonist and the cellular system. Massotte et al. (2002) compared the coupling efficiencies for several agonists with the μOR and Gαi1 or Gαi2 fusion proteins in HEK-293 cells. They reported that [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) fully activates Gαi1 and only partially activates Gαi2, whereas morphine behaves as a partial agonist at both Gαi1 and Gαi2 and can stimulate both Gα subunits equally. Buprenorphine, although more potent than either morphine or DAMGO, also only acts as a partial agonist at both subunits. Likewise, through the reconstitution of membranes prepared from μOR expressing HEK-293 cells (in which the endogenous G proteins were denatured) with specific purified G proteins, Saidak et al. (2006) demonstrated that DAMGO and the endogenous opioid ligands (endomorphin-1 and -2 and β-endorphin) maximally activate both Gαi1 and GαoA, whereas the synthetic and natural product opioid ligands (fentanyl, methadone, morphine, and buprenorphine) were only partial agonists for either subunit. In contrast, DAMGO, [d-Ala2,d-Leu5]-enkephalin, and morphine stimulate α-azidoanilido [32P]GTP incorporation into different Gα subunits expressed in CHO cells with varying efficacies (Gαi3 > Gαo2 > Gαi2 = Gαunknown subunit); however, no differences in potencies were apparent among agonists (Chakrabarti et al., 1995). In the above studies, the stimulation of particular Gα subunits did not correlate to activation of particular downstream signaling pathways as Gαi1 and Gαi2 were equally effective at inhibiting cAMP in HEK-293 cells (Massotte et al., 2002).
Researchers in the Garzón laboratory (Sánchez-Blázquez et al., 1995, 1999, 2001) have presented studies that begin to address the significance of different Gα activation profiles for distinct opioid agonists by individually silencing specific G proteins through intracerebroventricular injection of antisense oligodeoxynucleotides and then determining supraspinal antinociceptive responses in the tail-flick assay for various agonists in mice. Morphine and DAMGO were shown to predominantly use Gαi2 and Gαz (and Gαq for DAMGO) in their induction of spinal antinociception, whereas methadone, heroin, and buprenorphine use different combinations of a wider range of Gα subunits (including Gαi2, Gαz, Gαi3, Gαi1, Gαo1, Gα11, Gαo2, and Gαq) to mediate their antinociceptive responses (Sánchez-Blázquez et al., 1995, 1999, 2001). Together, these studies exemplify the extent of influence the agonist has on early points in receptor-transmitted signals.
To add further to the complexity of signaling, the μOR can also switch between coupling to inhibitory and stimulatory G proteins, which was first shown by Crain and Shen (1995, 1996). In mouse dorsal root ganglion (DRG) preparations, ultra low doses of the inverse agonist naloxone stimulate μOR/Gαs-mediated signaling. It is noteworthy that short- versus long-term administration of the same agonist induces differential activation of various Gα subunits. In vehicle-treated rats, short-term morphine treatment activates Gαo in the striatum, and both Gαo and Gαi in the spinal cord and periaqueductal gray. After long-term treatment with morphine (twice-daily injections of 10 mg/kg morphine for 7 days), there is a switch from the inhibitory Gαi/o coupling to the stimulatory Gαs coupling in all three brain regions. Moreover, the switch in the Gα subunit coupling is associated with a stimulation of adenylyl cyclases (Wang et al., 2005; Wang and Burns, 2006). These studies not only provide evidence for a switch in Gα coupling but also illustrate how morphine-mediated μOR coupling can differ depending upon the tissue in which it is expressed.
III. Biased Agonism with Respect to Activation of Second Messengers
Activation of the μOR can lead to the stimulation of a range of downstream effectors, including the activation of G protein-coupled inwardly rectifying potassium channels (e.g., Kir3) and the inhibition of voltage-gated calcium channels (Cav), as well as the activation of several second messenger systems, including protein kinase A (PKA), PKC, calcium/calmodulin kinase II (CaMKII), phospholipase C, extracellular regulated kinase 1/2 (ERK1/2), and c-jun N-terminal kinase (JNK) (for review, see Williams et al., 2001; Law, 2011). In addition to agonist-mediated differences in G protein coupling, ligands have also been shown to lead to distinct induction of some of these downstream signaling cascades. For example, Quillan et al. (2002) demonstrated that different opioid agonists differentially induce multiphasic increases in cytosolic free Ca2+ levels in μOR-expressing HEK-293 cells. They reported that the activation of the μOR causes two waves of Ca2+ signaling in cells: a first peak that is dependent upon extracellular Ca2+, and a second peak that results from Ca2+ release from intracellular stores. Morphine activates both phases of Ca2+ signaling with equal potency, whereas etorphine is much less potent at stimulating the first peak compared with the second. Looking at a different effector, Koch et al. (2003) showed that DAMGO activates recombinant phospholipase D2 in HEK-293 cells, as assessed by a transphosphatidylation assay, whereas morphine does not.
Differences in signaling induced by distinct opioid agonists may be influenced by alterations in receptor localization within the plasma membrane. Zheng et al. (2008a) and Ge et al. (2009) demonstrated that in the basal state, the μOR is located within lipid raft domains in the plasma membrane of both HEK-293 cells and in the mouse hippocampus. Treatment with etorphine, but not morphine, induces translocation of the μOR from lipid raft domains to nonraft domains through the initiation of differential interactions with GRIN1, a proposed μOR-associated protein that tethers the μOR in lipid raft domains (Zheng et al., 2008a; Ge et al., 2009). Etorphine treatment reduces the interaction between the μOR and GRIN1, allowing the μOR to migrate from raft domains. Morphine, however, has no effect on μOR-GRIN1 associations; therefore, the morphine-bound μOR remains localized to the raft domains (Zheng et al., 2008a; Ge et al., 2009). Overall, the in vivo consequences of such diverse agonist-directed signaling events have yet to be fully realized; however, further evaluation of signaling in relevant tissues and neuronal populations may lead to a means to exploit these differences for therapeutic development.
IV. Biased Agonism with Respect to μOR Phosphorylation
After exposure to an agonist, intracellular serine, threonine, and tyrosine residues of the μOR are phosphorylated; however, the extent to which the μOR is phosphorylated as well as the sites phosphorylated may be determined by the nature of the agonist bound to the receptor (Fig. 3). For example, 32P labeling of μOR immunoprecipitated from CHO cells revealed that agonists such as methadone, etorphine, and DAMGO induce robust μOR phosphorylation, whereas morphine and buprenorphine barely increase receptor phosphorylation above basal levels (Yu et al., 1997). Likewise, immunoprecipitation of an hemagglutinin-tagged (HA) μOR from HEK-293 cells showed that morphine and heroin weakly stimulate μOR phosphorylation, as determined by 32P incorporation, whereas etorphine, methadone, and fentanyl induce robust phosphorylation of the receptor. These findings have been confirmed and expanded for multiple agonists and in various cell types (Zhang et al., 1998; Koch et al., 2001; Schulz et al., 2004; Johnson et al., 2006; Groer et al., 2007; Clayton et al., 2010). A similar pattern of phosphorylation has also been demonstrated in vivo, wherein both morphine and DAMGO cause an increase in the phosphorylation of μOR immunoprecipitated from the rat thalamus and striatum; however, the increase in the level of phosphorylation for morphine was much less than that for DAMGO (Deng et al., 2000).
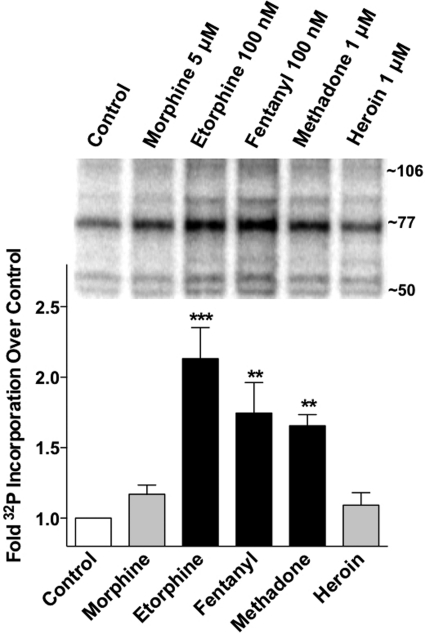
Fig. 3.
μOR phosphorylation in response to diverse opioid ligands. Radiolabeling of phosphorylated receptors was performed according to the methods described in detail by Oakley et al. (1999). HEK-293 cells expressing HA-μOR 1 were incubated in phosphate-free media in the presence of [32P]orthophosphate (100 μCi/ml) for 1 h at 37°C. Opioid agonists were included at the doses indicated for 15 min at 37°C. Cells were lysed, and equivalent levels of receptor per sample (as calculated by simultaneous radioligand binding assays) were immunoprecipitated with an anti-HA antibody and protein A Sepharose beads. Immunoprecipitates were resolved on 10% SDS-polyacrylamide gels, and the resulting gel was subjected to autoradiographic detection. Densitometric analyses normalized to control cells (untreated, collected at same time) are shown in the graph, and a representative autoradiograph is shown. Drug treatment versus control: ***, p < 0.001; **, p < 0.01, one-way analysis of variance, Dunnett's multiple comparison test, n = 5 to 6.
Differences between morphine- and DAMGO-induced μOR phosphorylation vary depending upon which splice variant of the receptor is activated as well. Although more than 26 splice variants of the mouse μOR have been identified, three with variations in the C terminus have been studied for their phosphorylation profiles: MOR-1C, MOR-1D, and MOR-1E (Pan et al., 1999). Koch et al. (2001) demonstrated that, when expressed in HEK-293 cells, the MOR-1C variant is phosphorylated in a manner similar to that observed for the wild-type (WT) μOR, in which DAMGO phosphorylates the receptor to a greater extent than morphine. In contrast, the agonist-specific differences disappear between the MOR-1D and MOR-1E variants. Abbadie et al. (2000a,b,c) show that these splice variants have markedly different expression profiles in the central nervous system; therefore, these differences could have functional consequences in vivo.
Site-directed mutagenesis studies suggest that at least 12 serine and threonine residues in the C terminus, as well as tyrosine residues in the intracellular cytoplasmic loops of the μOR, are susceptible to phosphorylation in response to receptor activation (Wolf et al., 1999; Deng et al., 2000; El Kouhen et al., 2001; Wang et al., 2002), and the specific pattern of residue phosphorylation depends upon the agonist used. For example, agonist activation can lead to μOR phosphorylation at Ser375, and in cell culture studies, mutation of Ser375 attenuates both morphine- and DAMGO-induced μOR phosphorylation. Although both agonists still stimulate phosphorylation of the Ser375 mutant, morphine only weakly phosphorylates the receptor compared with DAMGO (Deng et al., 2000; El Kouhen et al., 2001; Schulz et al., 2004). These studies suggest that in HEK-293 cells, morphine may predominantly cause phosphorylation at this residue, whereas DAMGO may stimulate phosphorylation at multiple residues.
A. Biased Phosphorylation of the μ Opioid Receptor by Intracellular Kinases
The differential degrees of μOR phosphorylation induced by various opioid agonists have been correlated to an agonist's ability to promote interactions between the receptor and different intracellular kinases (Fig. 1B). Several kinases, including GRKs, PKA, PKC, CaMKII, and ERK1/2 have been shown to phosphorylate the μOR in response to agonist activation (Koch et al., 1997; Chakrabarti et al., 1998; Polakiewicz et al., 1998; Wang et al., 1999; Wang, 2000; Johnson et al., 2006). Of these, GRKs and PKC have been shown to phosphorylate the μOR in a biased manner. Receptor mutation studies have shown that PKC phosphorylates the μOR specifically at Ser363 in the C terminus (Feng et al., 2011). Separation of HEK-293 cell homogenates on a sucrose gradient reveals that treatment with morphine for 5 min induces PKC translocation from the cytosolic fraction to the μOR containing membrane fraction, whereas PKC remained in the cytosolic fraction after the 5-min DAMGO treatment (Chu et al., 2010). Moreover, pretreatment of HEK-293 cells with the PKC inhibitor 3-[1-[3-(dimethylaminopropyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione monohydrochloride (GF109203X) significantly attenuates morphine-induced phosphorylation of the μOR, although morphine is still able to stimulate phosphorylation over vehicle levels. In contrast, GF109203X pretreatment has no effect on DAMGO-induced phosphorylation of the receptor (Johnson et al., 2006), suggesting that other kinases are involved in mediating DAMGO-induced phosphorylation of the μOR. However, Kramer and Simon (1999) reported that DAMGO does recruit PKC to the membrane of SHSY5Y neuroblastoma cells after longer incubation with the agonist (2–6 h); therefore, PKC may in fact phosphorylate the DAMGO-bound μOR in certain cell types or under certain treatment conditions.
The μOR can also be phosphorylated by GRKs in an agonist-specific manner. Seven GRK isoforms have been identified; however, only four (GRK2, -3, -5, and -6) are thought to regulate the μOR in vivo, because two are exclusively found in the eye (GRK1 and GRK7), and GRK4 is predominantly expressed in the Purkinje cells of the cerebellum, the testes, and the kidneys (for review, see Premont and Gainetdinov, 2007). Several studies demonstrate that the expression of various GRKs can affect μOR phosphorylation in vitro (Zhang et al., 1998; Deng et al., 2000; Schulz et al., 2004). Zhang et al. (1998) demonstrated that overexpression of GRK2 in HEK-293 cells can significantly enhance morphine-induced μOR phosphorylation to the same levels as those observed with etorphine. Furthermore, GRK-mediated phosphorylation has been shown to specifically affect Ser375, in that GRK2 overexpression enhances μOR phosphorylation at this residue for both DAMGO and morphine in HEK-293 cells (Schulz et al., 2004). However, they show that morphine-mediated phosphorylation is abrogated, whereas DAMGO-induced phosphorylation is decreased only when Ser375 is mutated in HEK-293 cells. This suggests that GRK-mediated phosphorylation of the μOR could have a greater effect on receptor regulation for some agonists than for others. Because GRK phosphorylation of GPCRs has been shown to facilitate subsequent interactions with β-arrestins, the differences in agonist-mediated phosphorylation of the μOR may affect β-arrestin recruitment as well.
V. Biased Agonism with Respect to Recruitment of β-Arrestin2
The extent of β-arrestin2 recruitment is also determined by the agonist bound to the receptor. Fluorescently tagged β-arrestins and μORs were initially used to demonstrate differences in agonist-directed β-arrestin recruitment profiles by live cell confocal microscopy. In these cell systems, some agonists, such as morphine and heroin, proved to be weak recruiters of β-arrestin2, whereas other agonists, such as DAMGO and etorphine, were able to robustly translocate β-arrestins to the μOR (Zhang et al., 1998; Whistler et al., 1999; Bohn et al., 2004; Groer et al., 2007). Although the earliest reports in the HEK-293 cells suggested that the morphine-bound MOR cannot interact with β-arrestins (β-arrestin2 in Zhang et al., 1998; undisclosed β-arrestin in Whistler and von Zastrow, 1998), more recent studies have shown that morphine can indeed lead to β-arrestin2 recruitment in other cell lines or under certain conditions.
Studies employing assays such as PathHunter (DiscoveRx, Fremont, CA), which uses enzyme fragment complementation to quantitate β-arrestin2 interactions with the μOR, as well as fluorescence resonance energy transfer (FRET) and bioluminescence resonance energy transfer, have recently allowed for alternate means to measure β-arrestin2 recruitment to the μOR. These approaches have facilitated comparison of the ability of different opioid agonists to recruit β-arrestin2 to the receptor, and several recent reports have assessed recruitment profiles for up to approximately 20 compounds each using these methods. These reports have confirmed the confocal studies, in which Met-enkephalin > etorphine > DAMGO > fentanyl induce robust interactions between the μOR and β-arrestin2, whereas morphine = oxycodone > buprenorphine are less robust recruiters in these optimized cell lines (McPherson et al., 2010; Molinari et al., 2010). In addition, the ability of these μOR agonists to induce β-arrestin2 interactions with the μOR does not directly correlate with their ability to activate G proteins. G protein coupling and β-arrestin binding to numerous GPCRs have been reported to occur within the intracellular loops and the C terminus tail of the receptor, respectively, suggesting that one event may not necessarily preclude the other (Gurevich and Gurevich, 2006). Molinari et al. (2010) used bioluminescence resonance energy transfer to compare the ability of opioid ligands to promote μOR coupling to either G proteins or β-arrestin2 and found no direct correlation between the ability of an agonist to induce μOR-mediated G protein activation and β-arrestin2 binding. Agonists such as morphine and oxymorphone were full agonists with respect to G protein coupling but only partial agonists for β-arrestin2. In contrast, the McPherson et al. (2010) study that assessed G protein coupling alongside the PathHunter and FRET β-arrestin2-recruitment assays observed some correlation between an agonist's efficacy in initiating G protein coupling and β-arrestin2 binding for a panel of opioid agonists. The difference in results between the two studies may be due to the context of the assays used; McPherson et al. (2010) assessed G protein coupling by standard GTPγS assays and β-arrestin2 recruitment by FRET in intact HEK-293 cells, whereas Molinari et al. (2010) were able to eliminate competition from endogenous β-arrestins by using membranes isolated from HEK-293 cells. In another study using FRET to assess Gαi and β-arrestin2 interactions with μOR, Frölich et al. (2011) showed that three metabolites of morphine (normorphine, 6-acetylmorphine, and morphine-6-glucuronide) preferentially lead to recruitment of β-arrestin2 over Gαi; this differs from morphine, which is more efficacious at promoting interactions with G proteins over β-arrestins.
Studies with the novel agonist herkinorin and its derivatives further exemplify functional selectivity at the μOR. Herkinorin is fully efficacious at inducing the activation of G protein coupling, the phosphorylation of the downstream kinases ERK1/2, and the inhibition of cAMP in μOR-expressing HEK-293 or CHO cells. However, unlike opioids such as DAMGO and fentanyl, which are also fully efficacious with regard to these same responses, herkinorin does not recruit β-arrestins to the μOR (Harding et al., 2005; Groer et al., 2007; Xu et al., 2007). Certain chemical derivatives of herkinorin have also been described as full agonists at the μOR with respect to the activation ERK1/2 yet are biased against interactions with β-arrestin2. It is noteworthy that one herkinorin derivative, herkamide, has been shown to recruit β-arrestin2 to μOR expressed in HEK-293 cells (Tidgewell et al., 2008), demonstrating that minor changes in the structure of the agonist can have dramatic effects on μOR regulation. Collectively, these findings suggest that ligand bias between signaling pathways can be independent of “intrinsic efficacy,” wherein an agonist can be highly efficacious in stimulating one pathway (G protein coupling) and lack efficacy in another (β-arrestin recruitment).
A number of studies have demonstrated that the agonist-directed differences in β-arrestin2 recruitment are a function of the agonist's ability to phosphorylate the μOR (Fig. 1C). For instance, morphine and heroin, which weakly phosphorylate the μOR, also weakly recruit β-arrestin2 to the receptor in HEK-293, whereas DAMGO and etorphine lead to robust μOR phosphorylation and β-arrestin2 recruitment (Whistler and von Zastrow, 1998; Zhang et al., 1998; Bohn et al., 2004; Groer et al., 2007). Furthermore, overexpression of GRK2 enhances both morphine-induced μOR phosphorylation and β-arrestin2-GFP recruitment (Zhang et al., 1998; Bohn et al., 2004; Groer et al., 2007). In fact, overexpression of any of the ubiquitously expressed GRKs in HEK-293 cells is sufficient to enhance morphine-induced β-arrestin2-GFP translocation to the receptor (Fig. 4) (Raehal et al., 2009). McPherson et al. (2010) also assessed μOR phosphorylation at Ser375 in their analysis of β-arrestin2 recruitment profiles for approximately 20 agonists and found a strong correlation between an agonist's ability to phosphorylate the μOR at this residue and its ability to induce the recruitment β-arrestin2 to the receptor. This correlation was also observed with the agonist herkinorin, which does not induce β-arrestin2 interactions with the μOR and induces minimal receptor phosphorylation at Ser375 in HEK-293 cells (Groer et al., 2007).
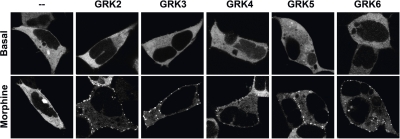
Fig. 4.
GRK overexpression augments morphine-induced β-arrestin2-GFP translocation. HEK-293 cells were transiently transfected with HA-μOR1 (5 μg), βarr2-green fluorescent protein (GFP) (2 μg), and GRKs (2 μg) as described in detail by Groer et al. (2007). After a 30-min serum-free period, cells were treated with either DAMGO (1 μM) or morphine (10 μM). Images were taken between 10 and 15 min after treatment. Receptor expression was confirmed via immunolabeling HA-μOR on the membrane of live cells (not shown). Without coexpression of a GRK, morphine-induced translocation is barely detectable in the HEK-293 cells. When any GRK (GRK2, -3, -4, -5, or -6A) is coexpressed, morphine-induced βarr2-GFP translocation becomes readily apparent by confocal microscopy analysis (puncta formation, lower panels).
A. Biased Agonism with Respect to β-Arrestin1 versus β-Arrestin2 Interactions
In addition to determining the extent of β-arrestin2 interactions with the μOR, different ligands can affect the recruitment of β-arrestin1 as well. β-Arrestin1 typically displays lower affinity than β-arrestin2 for some GPCRs, including the μOR (Oakley et al., 2000). It is noteworthy that although etorphine induces translocation of both β-arrestin1 and β-arrestin2 to the μOR, morphine induces translocation of only β-arrestin2 (Bohn et al., 2004). In these studies, β-arrestin1/2 double-knockout mouse embryonic fibroblasts (βarr1/2-KO MEFs) were used to evaluate agonist-induced translocation of fluorescently tagged β-arrestin1 or β-arrestin2. The benefit of the βarr1/2-KO MEFs is that there are no endogenous β-arrestins that could potentially compete with the tagged β-arrestins; this in turn can enhance the ability to visualize β-arrestin interactions with the receptor even when they occur at relatively low levels. Under these conditions, morphine recruits β-arrestin2 to the μOR in the absence of GRK overexpression but is unable to promote β-arrestin1 translocation (Bohn et al., 2004). These studies further demonstrate how μOR regulation differs depending upon the agonist acting at the receptor. Moreover, growing evidence indicates that although β-arrestin1 and β-arrestin2 share a great deal of sequence homology, they have different functional roles in regulating GPCRs (Gurevich and Gurevich, 2006; Violin and Lefkowitz, 2007); therefore, selective interactions between the μOR and one isoform over the other could have implications on receptor responsiveness.
VI. Biased Agonism with Respect to Receptor Desensitization
Sustained stimulation of the μOR leads to diminished receptor responsiveness over time, such that the receptor can no longer respond to agonist stimulation, a process referred to as desensitization. A number of in vitro studies have demonstrated that opioids cause varying degrees of μOR desensitization but that this is highly dependent upon temporal factors, the signaling pathway assessed, and the cell type studied. For example, HEK-293 cells pretreated with DAMGO for either 5 or 30 min are no longer able to stimulate GTP exchange efficiently or inhibit adenylyl cyclase, whereas cells pretreated with morphine retain their ability to stimulate G protein coupling and inhibit adenylyl cyclases (Whistler and von Zastrow, 1998). Likewise, a 1-h pretreatment with DAMGO led to a reduction in the DAMGO-induced conductance of Kir3 channels in AtT20 cells, whereas a 1-h pretreatment with morphine did not affect the ability of DAMGO to induce Kir3-mediated conductance (Celver et al., 2004). Conversely, pretreatment with morphine for 30 min induces desensitization of μOR-mediated intracellular calcium release in HEK-293 cells more rapidly and to a greater extent than DAMGO (Chu et al., 2008). Although in AtT20 mouse pituitary tumor cells and in sensory neurons isolated from the trigeminal ganglia of mice, a 3- or 10-min pretreatment with DAMGO and morphine induces rapid desensitization of μOR-mediated inhibition of Cav to a similar extent (Borgland et al., 2003; Johnson et al., 2006).
Differences in agonist-mediated desensitization of μOR responsiveness have also been observed in locus ceruleus brain slice preparations. In these neurons, methadone and DAMGO cause rapid desensitization of Met-enkephalin-induced Kir3 currents after short-term exposure (10 min) in whole-cell patch-clamp recordings, whereas morphine, morphine-6-glucuronide, and oxycodone lead to far less desensitization in these same preparations (Alvarez et al., 2002; Virk and Williams, 2008). Moreover, in locus ceruleus neurons from transgenic mice expressing an epitope-tagged FLAG-μOR, Met-enkephalin, etorphine, methadone, morphine, and oxymorphone all induce significant desensitization of Met-enkephalin-induced hyperpolarizations, whereas oxycodone does not (Arttamangkul et al., 2008). A recent study by Quillinan et al. (2011) shows that long-term treatment of rats with morphine decreases recovery of short-term Met-enkephalin-induced Kir3 channel desensitization in locus ceruleus slice preparations and also inhibits receptor recycling, effects that were not observed in rats or in β-arrestin2 knockout (βarr2-KO) mice receiving long-term treatment with methadone. These observations suggest that agonists may affect not only the rate of desensitization but also the ability of the receptor to resensitize to allow for further stimulation, which will ultimately contribute to the overall extent of desensitization observed after long-term treatment.
Different brain regions also display diverse μOR desensitization profiles in response to long-term morphine treatment. For instance, differences in [35S]GTPγS binding in rat brain slices have been reported wherein long-term morphine treatment produces uncoupling of the μOR from G proteins in specific nuclei in the brainstem (dorsal raphe nucleus, locus ceruleus, and parabrachial nucleus), whereas no changes in coupling were observed in other brain regions, including the striatum, thalamus, and hypothalamus (Sim et al., 1996). In contrast to long-term morphine exposure, long-term exposure to heroin does cause a reduction in μOR coupling to G proteins in the medial and mediodorsal thalamus, as well as the dorsal raphe nucleus, locus ceruleus, rostral nucleus accumbens, lateral parabrachial nucleus, and periaqueductal gray (Sim-Selley et al., 2000). Long-term morphine treatment has also been shown to induce desensitization of the μOR in the thalamus and periaqueductal gray, but not in caudate putamen or nucleus accumbens, as measured by the inhibition of adenylyl cyclase (Noble and Cox, 1996), further suggesting that the receptor can be differentially regulated in different anatomical brain regions. Overall, these studies demonstrate that μOR desensitization is not only agonist-dependent but also neuron- or region-dependent. Furthermore, a recent study by Sim-Selley et al. (2007), showed the degree of μOR desensitization increases in some brain regions and becomes significant in others when the dose of long-term morphine administration was increased. Although this may be somewhat expected, this study drives home the effect of dose and duration of exposure on the long-term adaptive events that occur after prolonged drug usage across different neuronal populations.
These neuronal-specific differences in the desensitization of the μOR may underlie some of the differences in the physiological effects of opioids as well. Long-term administration of morphine leads to desensitization of the μOR in brain regions associated with the development of antinociceptive tolerance (which is discussed in section IX), such as the periaqueductal gray and brainstem (Noble and Cox, 1996; Sim et al., 1996; Bohn et al., 1999, 2000). In contrast, a lack of desensitization is observed after long-term exposure to agonist in regions such as the striatum (Sim et al., 1996). This lack of desensitization may be related to the enhanced locomotor responses observed after continued opioids administration (a condition referred to as “reverse tolerance” or “sensitization”) (Di Chiara and Imperato, 1988; Robinson and Berridge, 2000). These distinctions in neuronal-specific regulation of the μOR emphasize the need to study neuronal and cellular mechanisms in relevant neuronal populations if these regulatory events are to be associated with physiological conditions. In addition to studying cellular responses within appropriate neuronal environments, it also is important to evaluate responses in temporally relevant periods, as well as at physiologically consistent dosing.
A. Effect of Biased Agonism on Desensitization Mechanisms
Agonists not only dictate the extent of desensitization but also seem to determine the mechanism used to desensitize receptors. Receptor phosphorylation has been shown to be involved in mediating μOR desensitization for some agonists. For instance, μOR mutants with alanines instead of Thr394 and Thr383 show greatly attenuated desensitization of the inhibition of cAMP after treatment with DAMGO in CHO cells (Pak et al., 1997; Deng et al., 2000). Moreover, mutation of Thr180 in the second intracellular loop of the μOR abrogates DAMGO-induced desensitization of Kir3 channels in Xenopus laevis oocytes (Celver et al., 2001). Given that mutation of Ser375 reduces morphine-stimulated μOR phosphorylation to a greater extent than that induced by DAMGO, it is unsurprising that mutation of Ser375 completely attenuates morphine-mediated desensitization of the inhibition of adenylyl cyclase or stimulation of ERK1/2 and only slightly decreases DAMGO-mediated desensitization in HEK-293 cells (Schulz et al., 2004). These studies indicate that those kinases involved in the agonist-induced phosphorylation of the μOR may play a key role in determining desensitization.
Several studies suggest that DAMGO-induced μOR phosphorylation is mediated primarily by GRKs, whereas morphine induces μOR phosphorylation by both GRKs and PKC. The differential interactions with these kinases have been shown to play prominent roles in the agonist-directed desensitization of the μOR, the DAMGO-coupled μOR being predominantly desensitized via a GRK/β-arrestin2-mediated mechanism, and morphine-mediated desensitization also occurs via a PKC-dependent mechanism. For example, modulation of GRK expression or activity levels affects both DAMGO and morphine-mediated desensitization of the μOR, as is evidenced by two studies showing that pharmacological inhibition of GRK2 reduces DAMGO-induced desensitization of Kir3 channel activation in mouse locus ceruleus neurons (Hull et al., 2010), and pretreatment with the GRK inhibitors β-adrenergic receptor kinase 1-inhibitor or 3-[(8S)-8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl[-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione hydrochloride (Ro32-0432) significantly attenuates DAMGO-induced desensitization of Cav in mouse locus ceruleus neurons (Hull et al., 2010). Furthermore, overexpression of GRK2 in HEK-293 cells reduces μOR-mediated inhibition of adenylyl cyclase activity by morphine, suggesting that GRK2 enhanced the desensitization of the μOR (Zhang et al., 1998).
On the other hand, activation of PKC selectively affects the morphine activated μOR. For instance, in mature rat locus ceruleus neurons, activation of PKC induces robust and rapid desensitization of the μOR in response to short-term morphine treatment, as measured by whole-cell patch-clamp recordings (Bailey et al., 2004). Furthermore, PKC inhibitors reverse morphine-induced desensitization after 3 days of morphine treatment in vivo or after 6 to 9 h of morphine treatment of locus ceruleus slice preparations in vitro (Bailey et al., 2009a). Inhibition of PKC by a peptide or the pharmacological inhibitor staurosporine significantly attenuates morphine- but not DAMGO-induced desensitization of Kir3 channel currents in HEK-293 cells expressing rat μOR1 and Kir3 channels (Johnson et al., 2006). In mature rat locus ceruleus neuronal preparations, pharmacological inhibition of PKCα also attenuates morphine- but not DAMGO-induced desensitization as measured by whole-cell patch-clamp electrophysiology (Bailey et al., 2009b; Hull et al., 2010). Moreover, locus ceruleus neuronal preparations isolated from PKCα-KO mice have attenuated desensitization of whole-cell currents after morphine administration, whereas DAMGO-mediated desensitization remains intact (Bailey et al., 2009b). Finally, inhibition of PKC by either RNA interference or PKC subtype-specific pharmacological inhibitors reduces morphine- but not DAMGO-induced μOR desensitization (as monitored by intracellular Ca2+ release) in HEK-293 cells (Chu et al., 2010). These studies emphasize how the agonist will determine both the extent and mechanism of desensitization.
Considerable evidence suggests that the mechanism for desensitization differs depending upon the cell type in which the receptor is expressed. For instance, some studies indicate that in certain cell types, the morphine-bound receptor may be selectively desensitized in a GRK-independent manner. In X. laevis oocytes, overexpression of GRK3 or GRK5 and β-arrestin2 enhances DAMGO- and fentanyl-induced desensitization of μOR-mediated Kir3 currents but has no effect on morphine-induced currents (Kovoor et al., 1998; Celver et al., 2001). Moreover, in βarr1/2-KO MEF cells, DAMGO- but not morphine-induced desensitization of μOR-stimulated intracellular calcium release was attenuated (Chu et al., 2008). Furthermore, dominant-negative mutants of GRK2 have no effect on morphine-induced desensitization of Kir3 channel activation in locus ceruleus brain slice preparations but almost completely abrogate DAMGO-induced desensitization (Johnson et al., 2006; Bailey et al., 2009b). Likewise, although the studies presented above suggest that DAMGO-mediated desensitization of the μOR occurs primarily via a GRK-dependent mechanism, Narita et al. (2001) suggest that it may induce desensitization through a PKC-dependent mechanism in the spinal cord, wherein spinal cord membranes from PKCγ-KO mice show enhanced μOR coupling to G proteins compared with membranes from WT mice, after treatment with DAMGO, morphine, and endomorphin-1 and -2.
Positive correlations have also been made between the ability of an agonist to induce μOR desensitization in some signaling pathways and its ability to induce receptor phosphorylation and β-arrestin2 recruitment. For example, highly efficacious agonists, including etorphine, Met-enkephalin, methadone, fentanyl, and DAMGO, promote robust μOR phosphorylation and β-arrestin recruitment and lead to substantial desensitization of the receptor, whereas agonists such as morphine, oxycodone, or oxymorphone, which produce less μOR phosphorylation and β-arrestin interactions, also lead to less robust desensitization as measured by [35S]GTP coupling, inhibition of adenylyl cyclases and the induction of potassium currents in a number of different cellular systems (Whistler and von Zastrow, 1998; Zhang et al., 1998; Dang and Williams, 2005; Arttamangkul et al., 2008).
VII. Biased Agonism with Respect to Receptor Internalization
μOR responsiveness is also regulated through the internalization of the receptor, another event that is determined by agonist action at the receptor (Fig. 1D). Using confocal microscopy and HEK-293 cells transfected with an epitope-tagged μOR, Arden et al. (1995) first demonstrated agonist-specific μOR trafficking profiles, wherein DAMGO, but not morphine, promoted robust internalization of the receptor. Numerous studies in HEK-293 and AtT20 cells have confirmed these findings, showing that DAMGO, etorphine, methadone, fentanyl, and enkephalin all induce robust internalization of the μOR, whereas morphine is less effective (Keith et al., 1996; Whistler and von Zastrow, 1998; Zhang et al., 1998; Celver et al., 2004; Schulz et al., 2004; Groer et al., 2007). DAMGO, etorphine, methadone, fentanyl, oxycodone, and morphine have been shown to be full agonists with respect to stimulating [35S]GTPγS binding in HEK-293 cells, whereas oxycodone and morphine only weakly promote receptor internalization compared with the other agonists (McPherson et al., 2010). Moreover, herkinorin fully activates G protein coupling and ERK1/2 phosphorylation compared with DAMGO but does not promote any measurable receptor internalization (Harding et al., 2005; Groer et al., 2007). Therefore, ligands such as morphine and herkinorin are biased toward the activation of these signaling cascades but against receptor internalization.
Agonist-dependent differences in μOR internalization processes are conserved in some neuronal cell types as well as determined by immunohistochemical studies. Sternini et al. (1996) demonstrated that etorphine induces internalization of μORs expressed in guinea pig ileum, whereas morphine does not cause detectable internalization of the receptor. Immunolabeling of the μOR in the rat locus ceruleus shows similar findings, etorphine inducing a substantial increase in the intracellular localization of the μOR, whereas morphine does not induce a change in the membrane distribution of the μOR (Van Bockstaele and Commons, 2001). Furthermore, DAMGO, but not morphine, induces robust μOR internalization in cortical neuronal cultures prepared from embryonic rats (Schulz et al., 2004). Agonist-induced internalization was also measured in locus ceruleus neuronal preparations from transgenic mice expressing an epitope-tagged FLAG-μOR wherein Met-enkephalin, etorphine, and methadone were shown to induce significant μOR internalization, whereas morphine, oxymorphone, and oxycodone did not (Arttamangkul et al., 2008). With the exception of the FLAG-tagged receptor studies above, all of the neuronal studies have used antibodies to the C terminus of the receptor and therefore have depended upon the availability of this epitope. If the C terminus of the receptor is involved in scaffolding intracellular proteins, the epitope may be occluded. Additional studies using antibodies directed to other regions of the receptor or tagged receptors may be critical in determining whether receptors are truly internalized after treatment with agonist (Scherrer et al., 2009).
There is a strong correlation between an agonist's ability to internalize the μOR and its abilities to promote receptor phosphorylation and β-arrestin recruitment. In cell culture systems, agonists that promote robust receptor phosphorylation and β-arrestin2 recruitment, such as etorphine and DAMGO, also promote robust μOR internalization as measured by confocal microscopy, whereas the morphine-bound receptor induces less internalization, receptor phosphorylation and β-arrestin2 recruitment overall (Whistler and von Zastrow, 1998; Bohn et al., 2004). Similar to its effects on facilitating phosphorylation and β-arrestin2 recruitment, overexpression of GRK2 can also augment morphine-induced internalization of the μOR (Zhang et al., 1998; Whistler et al., 1999; Groer et al., 2007). Furthermore, mutation of Ser375 inhibits DAMGO-induced internalization of the μOR in HEK-293 cells (Schulz et al., 2004). It is noteworthy that overexpression of GRK2 did not allow for herkinorin-induced μOR internalization (Groer et al., 2007; Tidgewell et al., 2008).
There have also been attempts to correlate an agonist's ability to promote the desensitization of the μOR and its ability to internalize the receptor. Such correlations have been observed under certain conditions, as demonstrated by the comparison of the agonist-induced desensitization of μOR-mediated inhibition Cav current and receptor internalization profiles in AtT20 cells, which reveals that the agonists studied have a similar rank order of efficacies for promoting both events, with DAMGO > methadone > morphine > pentazocine (Borgland et al., 2003). However, this pattern is not conserved for all agonists, or in all cell types, as demonstrated in a study by Arttamangkul et al. (2008). They observed that although Met-enkephalin, etorphine, and methadone result in the robust desensitization of Met-enkephalin-induced hyperpolarization and internalization of the receptor in locus ceruleus neurons expressing FLAG-tagged μORs, oxycodone is unable to promote either event, whereas the agonists morphine and oxymorphine both induce receptor desensitization without detectable internalization. Although desensitization and internalization may be interrelated, particularly because receptor sequestration into intracellular vesicles can disrupt further coupling between the receptor and certain downstream effectors, internalization has been shown to facilitate receptor signaling to additional signaling cascades for other GPCRs (Luttrell et al., 1997; Daaka et al., 1998; Lin et al., 1998; Yuen et al., 2008). Therefore, internalization and desensitization cannot reliably be considered as synchronous events.
As observed for other endpoints discussed, internalization profiles also vary depending upon the cell type in which the receptor is expressed. This is nicely demonstrated by studies from Haberstock-Debic et al. (2003, 2005). Using immunocytochemistry, they report that morphine induces rapid μOR trafficking in striatal primary neurons but not in nucleus accumbens neurons. It is noteworthy, however, that a more detailed assessment of μOR trafficking within the neurons of the nucleus accumbens revealed a “compartment-selective” internalization of the μOR, wherein morphine does not induce receptor endocytosis in the neuronal somas but does induce receptor internalization in the dendrites (Haberstock-Debic et al., 2003). These results further suggest that the interactions between the receptor and intracellular regulatory proteins may differ between cell types and even between compartments within the same cell, resulting in different agonist-induced responses and that evaluations made in cell culture systems cannot always be reliably translated to predict receptor responses in neuronal populations.
VIII. Biased Agonism with Respect to β-Arrestin-Mediated Signaling
In addition to promoting μOR desensitization and internalization, β-arrestins can serve to facilitate GPCR signaling cascades independent of G protein coupling. There is evidence that μOR-mediated activation of the ERK1/2 pathway can occur through the traditional G protein-mediated pathway or via a β-arrestin-dependent pathway in vitro. Using βarr1/2-KO MEFs, Zheng et al. (2008b, 2011) demonstrated that morphine and methadone mediate ERK1/2 activation through a PKC-dependent pathway, whereas etorphine-, fentanyl-, and DAMGO-mediated phosphorylation of ERK1/2 is β-arrestin2-dependent. It is noteworthy that this group has shown that the inhibition of μOR phosphorylation by mutating Ser363, Thr370, and Ser375 to alanines causes etorphine, fentanyl, and DAMGO to use PKC in the β-arrestin2-dependent activation of ERK1/2 (Zheng et al., 2011). Additional studies from the Law laboratory (Zheng et al., 2008b, 2010a,b) have suggested that these differential mechanisms for ERK1/2 activation have functional consequences, because PKC-activated ERK1/2 remains cytosolic and acts on 90RSK (a cytosolic ERK1/2 substrate) to activate cAMP response element-binding protein, whereas β-arrestin-mediated ERK1/2 phosphorylation results in ERK1/2 translocation into the nucleus (Zheng et al., 2008b). The translocation of ERK1/2 to the nucleus corresponds with an up-regulation of β-arrestin2 and GRK2 (Zheng et al., 2008b) as well as altered expression of certain microRNAs (Zheng et al., 2010a,b). Finally, Rozenfeld and Devi (2007) demonstrated that DAMGO induces ERK1/2 phosphorylation through a β-arrestin2-dependent mechanism downstream of the μOR-δOR heterodimer in CHO cells. However, when the μOR monomer is activated by DAMGO, PKC mediates ERK1/2 phosphorylation. These data suggest that activation of μOR homo- and heterodimers leads to differential signaling and regulation of the receptor.
The biased mechanisms that different opioids agonists use to mediate the phosphorylation of ERK1/2 further reveal the differential roles that β-arrestins play in mediating μOR responsiveness. In some instances, β-arrestins seem to facilitate μOR-mediated signaling, whereas in others they seem to dampen signaling cascades. For example, Zheng et al. (2008b) demonstrated in HEK-293 cells that overexpression of β-arrestin2 potentiates etorphine-mediated ERK1/2 phosphorylation, suggesting that β-arrestin2 is a facilitator of ERK1/2 signaling for etorphine. In contrast, overexpressing β-arrestin2 decreased the amount of ERK1/2 phosphorylation observed after morphine treatment, suggesting that β-arrestin2 is acting to desensitize this μOR-mediated response when this agonist is bound to the receptor (Zheng et al., 2008b). However, it is important to recognize that ERK1/2 activation as an indicator of μOR responsiveness must be carefully compared among agonists because the net stimulation of ERK1/2 is a factor of the phosphorylation and dephosphorylation states and kinetics, such that different doses and time points can produce effects that are not wholly dependent on receptor potencies and efficacies.
In addition to cell culture studies, facilitation of μOR signaling by β-arrestins has also been demonstrated in neuronal culture preparations by Walwyn et al. (2007), wherein DAMGO and morphine were shown to be less efficacious in inhibiting Cav in DRG neuronal cultures from βarr2-KO mice compared with WT mice. Walwyn et al. (2007) demonstrate that the activation of c-Src is necessary for the μOR-mediated inhibition of Cav by DAMGO and that DAMGO is unable to fully activate c-Src in βarr2-KO neurons. Furthermore, they show that the localization of c-Src is disrupted in the absence of β-arrestin2. β-Arrestin2-mediated signaling has also been observed downstream of μOR activation in mouse primary striatal neurons (Macey et al., 2006). Short-term treatment with fentanyl, but not morphine, stimulates ERK1/2 phosphorylation in neurons from WT but not μOR-KO mice. Moreover, knockdown of β-arrestin2 by siRNA attenuates fentanyl-induced ERK1/2 activation. It is noteworthy that the authors show that overexpression of a constitutively active β-arrestin2 (R170E mutant) leads to ERK1/2 activation after morphine treatment, suggesting that morphine's inability to activate ERK1/2 in striatal neurons is an artifact of its weak β-arrestin2 recruitment profile (Macey et al., 2006).
Although these studies were performed in cultured neurons and DRGs, a physiological role for μOR signaling via a β-arrestin-dependent pathway has yet to be directly demonstrated in live animals. However, a number of behavioral responses to morphine are decreased in the βarr2-KO mice, suggesting that for these responses, β-arrestin2 may be playing a facilitatory role. For example, short-term morphine-induced respiratory suppression and constipation are attenuated in βarr2-KO mice treated with morphine (Raehal and Bohn, 2005; Raehal and Bohn, 2011). Furthermore, naloxone-precipitated withdrawal is also attenuated in βarr2-KO mice after long-term morphine treatment, suggesting that the onset of physical dependence or the manifestation of withdrawal symptoms is diminished in the absence of β-arrestin2 (Raehal and Bohn, 2011). On the contrary, fentanyl, methadone, and oxycodone do not show differences among genotypes in their ability to induce physical dependence, which further exemplifies agonist-mediated differences with regard to β-arrestin2 function (Raehal and Bohn, 2011). Similar to the in vitro studies, the in vivo studies also suggest that β-arrestin2 can serve as both a facilitator of μOR signaling (as its deletion disrupts morphine-induced constipation, respiratory suppression, and dependence) and a desensitizing agent (as its deletion enhances morphine-induced antinociception and disrupts antinociceptive tolerance). Because each of these behavioral responses originates from and is controlled by diverse neuronal regulatory centers, it is attractive to hypothesize that such diversity in regulation is dependent on the cellular environment, such that differences in the intracellular proteins expressed in proximity with the μOR between the various neuronal populations can determine the ultimate function that the β-arrestin protein serves (Bohn, 2009).
The biochemical and electrophysiological studies presented thus far in this review demonstrate that μOR agonists have different abilities to induce receptor signaling and regulatory events. These differences cannot be explained merely by differences in an agonist's “intrinsic efficacy,” because ligands can fully activate some pathways, such as the stimulation of G protein coupling or ERK activation, yet have no agonist activity in other pathways, such as β-arrestin recruitment, in the same cell line (Table 1). The observed patterns in agonist-directed regulation of the μOR do suggest that some events downstream of μOR activation may be interrelated, in that the degree of μOR phosphorylation induced by agonists seems to correlate with both the degree of β-arrestin recruitment and the extent of receptor internalization. Moreover, as detailed above, many of these instances of ligand bias have also been observed in both neuronal tissues and in more physiologically relevant cell lines, such as primary striatal neuronal cultures and isolated DRGs. Therefore, agonist-directed signaling and regulation at the μOR may affect μOR-mediated responsiveness in vivo, and the second half of this review will focus on how functional selectivity at the μOR may specifically affect the development of antinociceptive tolerance.
TABLE 1.
Summary of biased agonism among several different μOR agonists with regard to different cellular responses
Relative efficacy ratios were extracted from quantitative studies in HEK-293 cells.
| DAMGO | Morphine | Methadone | Fentanyl | Etorphine | Herkinorin | |
|---|---|---|---|---|---|---|
| [35S]GTPγS binding | +++a,b | +++a,c | +++a | +++a,c | +++a,c | +++b |
| ERK1/2 phosphorylation | +++d | +++d | N.D. | N.D. | N.D. | +++d |
| μOR phosphorylation | +++a,d,e | +a,d,e,f | +++a | +++a | +++a,f | +e |
| β-Arrestin2 recruitment | +++a | +a,c | +++a | ++a,c | +++a,c | –e |
| μOR internalization | +++a,e | +a | +++a | ++a | +++a | –e |
N.D., not determined.
IX. Biased Agonism and Antinociceptive Tolerance
Long-term exposure to opioids can lead to the development of analgesic tolerance, which can be problematic in the clinical setting because drug levels must be escalated to manage pain. Despite numerous studies, the cellular mechanisms that contribute to the development of tolerance are not completely understood. Although the overall expression of tolerance involves complex adaptive processes, considerable evidence suggests that μOR regulation affects the degree of tolerance induced by opioids.
Similar to the in vitro studies described above in which agonists induce differential signaling and regulation of the μOR, different agonists also produce different degrees of analgesic tolerance in humans and rodents. For example, in rats receiving a constant infusion of different opioid agonists for 7 days, the rank order for the degree of tolerance that developed in the hot plate test was morphine > [d-Ala2,d-Leu5]-enkephalin ≫ sufentanil = DAMGO (Stevens and Yaksh, 1989). Likewise, long-term infusion with doses of morphine, fentanyl, and methadone that produce equiefficacious antinociceptive responses in normal mice when given in the short-term induce differing degrees of tolerance with morphine (2.9-fold) > fentanyl (2.4-fold) > methadone (1.8-fold) (Raehal and Bohn, 2011).
It has been proposed that the ability of an opioid agonist to promote μOR desensitization and internalization in vitro is inversely related to the degree of tolerance that develops after long-term treatment in vivo. For example, treatment with morphine promotes very little μOR desensitization and internalization in cell culture and produces a greater degree of tolerance in rodents than other highly efficacious agonists such as etorphine (Zhang et al., 1998; Whistler and von Zastrow, 1998; Kohout et al., 2001; Bohn et al., 2004; Koch et al., 2005; Groer et al., 2007). Furthermore, long-term treatment with buprenorphine produces even greater tolerance in the tail-flick, hot plate, or electrical tail stimulation tests in rats than did treatment with equivalent doses of etonitazene (Walker and Young, 2001; Grecksch et al., 2006). This is consistent with an earlier study in which etonitazene, but not buprenorphine, promotes μOR endocytosis in HEK-293 cells (Koch et al., 2005). Moreover, several studies from the Yoburn laboratory (Duttaroy and Yoburn, 1995; Pawar et al., 2007; Kumar et al., 2008; Sirohi et al., 2008; Madia et al., 2009) have shown that agonists that promote robust μOR desensitization and internalization, such as etorphine, methadone, and fentanyl, also produce less antinociceptive tolerance to the radiant-heat tail-flick test in rodents than agonists such as morphine, oxycodone, hydromorphone, and hydrocodone, which are less effective at promoting these regulatory events when continuously infused at equi-efficacious doses. Although there is apparent inverse correlation between μOR desensitization and internalization in vitro and the development of antinociceptive tolerance, the molecular mechanisms underlying the physiological responses or accounting for the differences between the agonists are not defined. We will review those studies that have assessed mechanisms for tolerance development to multiple agonists in vivo in section IX.A.
The “relative activity/versus endocytosis” hypothesis is based on the idea that receptor desensitization and internalization serve to promote the recycling and resensitization of receptors (Whistler et al., 1999; Schulz et al., 2004; He and Whistler, 2005; Koch et al., 2005). In this manner, the sustained activation of receptors caused by agonists that weakly induce μOR desensitization and internalization results in cellular adaptations, such as down-regulation of the receptor and the development of tolerance, whereas agonists that robustly induce desensitization and internalization avoid these cellular adaptations and prevent tolerance development (Whistler et al., 1999). Although weak internalizers such as morphine do induce greater degrees of tolerance than agonists such as fentanyl and methadone, the robust internalizers still produce marked tolerance in humans (Kreek, 1973; Collett, 1998) and in mice (Sirohi et al., 2008; Madia et al., 2009; Raehal and Bohn, 2011). Therefore, internalization of the receptor per se is not sufficient for the prevention of tolerance. Moreover, long-term treatment with etorphine and fentanyl, but not morphine or oxycodone, actually leads to robust μOR down-regulation in mouse brain and spinal cord as opposed to facilitating resensitization (Stafford et al., 2001; Patel et al., 2002; Yoburn et al., 2004; Pawar et al., 2007; Sirohi et al., 2008). Although the relative activity/versus endocytosis hypothesis supports an attractive idea that internalization may facilitate resensitization, it does not take into account that dephosphorylation of a receptor does not have to occur in a vesicle and therefore, resensitization may occur independent of internalization (Koch et al., 2004). Furthermore, ligands that activate the receptor and facilitate the recruitment of an appropriate phosphatase may optimize μOR longevity and decrease the development of antinociceptive tolerance.
A. Biased Agonism and G Protein-Coupled Receptor Kinases in Antinociception and Antinociceptive Tolerance
Given the agonist-directed role that GRKs play in mediating receptor phosphorylation and the subsequent regulation of μOR responsiveness, these kinases may be involved in modulating μOR responsiveness in vivo. Genetically modified mice lacking individual GRKs and pharmacological GRK inhibitors have been employed to explore the effect of GRK regulation of μOR signaling on the development of antinociception and antinociceptive tolerance to opioids in vivo. Terman et al. (2004) showed that although mice lacking GRK3 display a similar degree of antinociception compared with WT controls in response to short-term fentanyl and morphine treatment in the hot plate test, they develop significantly less antinociceptive tolerance compared with WT controls after long-term infusion with fentanyl. In contrast, WT and GRK3-KO mice develop tolerance to morphine to a similar degree (Table 2) (Terman et al., 2004). These findings are consistent with data demonstrating that GRK3 mediates fentanyl- but not morphine-induced desensitization of the μOR in oocytes (Kovoor et al., 1998). Using a JNK inhibitor and GRK3-KO mice, Melief et al. (2010) showed that morphine, morphine-6-glucuronide, and buprenorphine require JNK activation, but not GRK3, to produce acute tolerance in the tail-flick test. In contrast, fentanyl, methadone, and oxycodone require GRK3 but not JNK activation to produce acute tolerance in the same assay. A recent study by Hull et al. (2010) also showed that intracerebroventricular administration of the GRK2 inhibitor β-adrenergic receptor kinase 1 or the nonspecific GRK2/3/5 inhibitor Ro 32-0432 after a rapid 8-h tolerance-induction treatment paradigm reverses the degree of antinociceptive tolerance that develops in rats in response to intracerebroventricular DAMGO, but not subcutaneous morphine, fentanyl, or meperidine in the tail-flick test, further demonstrating that there are specific differences in GRK regulation of μOR-mediated responses with respect to different opioid agonists.
TABLE 2.
Summary of hot plate antinociception and antinociceptive tolerance responses in mice deficient in individual GRKs in response to short-term morphine and fentanyl treatment
| GRK2-HT | GRK3-KO | GRK4-KO | GRK5-KO | GRK6-KO | |
|---|---|---|---|---|---|
| Hot plate antinociception | |||||
| Morphine | =a | =b | =a | =a | =a,c |
| Fentanyl | N.D. | =b | N.D. | N.D. | N.D. |
| Hot plate antinociceptive tolerance | |||||
| Morphine | =d | =b | =d | =d | =c |
| Fentanyl | N.D. | ↓b | N.D. | N.D. | N.D. |
=, equivalent to wild type (WT) response at 10 mg/kg s.c.; ↓, significant decrease in response; N.D., not determined; HT, heterozygote.
K. Raehal and L. Bohn, unpublished observations.
Mice lacking GRK4, -5, or -6, as well as mice heterozygous for GRK2 (because the GRK2-KO is lethal to embryonic mice) have also been evaluated for their antinociceptive responsiveness to morphine. Each individual KO line displays short-term antinociceptive responses to morphine similar to those of their WT counterparts. Moreover, each of these knockout lines develops antinociceptive tolerance to morphine upon long-term drug administration to the same degree as WT mice (Table 2) (Raehal et al., 2009). These studies suggest that the contribution of GRKs to the development of tolerance to morphine in vivo is minimal. Alternatively, the lack of differences in the individual GRK-KO mice may be confounded by the ability of the kinases to substitute for each other in vivo. This is supported by the cell culture study presented in Fig. 4, demonstrating that overexpression of any of the nonvisual GRKs is sufficient to enhance morphine-mediated β-arrestin2 translocation in HEK-293 cells. Furthermore, GRK2, -3, -5, and -6 are widely distributed throughout the central nervous system, whereas GRK4 is expressed only in Purkinje cells of the cerebellum, testes, and kidney (Premont et al., 1996; Erdtmann-Vourliotis et al., 2001; Sanada et al., 2006). In rats, short-term treatment with a moderate dose of morphine induces an increase in GRK2 and GRK5 mRNA expression levels in the cerebral cortex, hippocampus, and lateral thalamic nuclei and a decrease in GRK5 expression levels in the periaqueductal gray (Fan et al., 2002). In contrast, administration of morphine for 9 days results in a significant down-regulation of GRK2 expression in cerebral cortex, hippocampus, thalamus, and locus ceruleus, whereas GRK5 mRNA expression levels remained unchanged in these regions (Fan et al., 2002). These findings indicate that GRK regulation of the morphine-activated μOR is complex, can differ among brain regions, and is contingent upon the duration of treatment. Studies in which multiple GRKs are knocked out or simultaneously inhibited may reveal more specific roles for GRK involvement in the regulation of morphine-mediated antinociception.
B. Biased Agonism and β-Arrestins in Antinociception and Antinociceptive Tolerance
Agonist-directed interactions between the μOR and β-arrestins also influence opioid-induced antinociception and antinociceptive tolerance in vivo. For example, βarr2-KO mice display enhanced and prolonged antinociception after short-term treatment with morphine and heroin in the hot plate test, a model of thermal antinociception (Table 3) (Bohn et al., 1999, 2000, 2002, 2004; Raehal and Bohn, 2011). Antinociceptive tolerance is also significantly attenuated in βarr2-KO mice in response to long-term morphine treatment in this same assay (Bohn et al., 2000, 2002; Raehal and Bohn, 2011). Likewise, Li et al. (2009) showed that siRNA inhibition of β-arrestin2 in periaqueductal gray matter in mice enhances morphine-induced antinociception and delays the development of antinociceptive tolerance in the hot plate test. Knocking down β-arrestin2 in the rat spinal cord by intrathecal delivery of antisense oligonucleotides also reduces the development of tolerance to morphine in the tail-flick test (Przewlocka et al., 2002). These enhanced and prolonged antinociceptive responses to morphine in the absence of β-arrestin2 are consistent with the described role of β-arrestin2 as a desensitizing agent. Likewise, in brain regions involved in regulating pain, such as the periaqueductal gray and brainstem, agonist-stimulated [35S]GTPγS coupling remains intact in βarr2-KO but not WT mice after long-term treatment with morphine (Bohn et al., 2000). In contrast to the βarr2-KO mice, βarr1-KO mice show similar short-term antinociceptive responses to morphine in the hot plate test compared with their WT littermates (Table 3). Likewise, siRNA knockdown of β-arrestin1 in the periaqueductal gray also had no affect on morphine-induced antinociceptive profiles or on the development of tolerance to the hot plate test (Li et al., 2009). These findings are not unexpected, because morphine does not seem to promote recruitment of β-arrestin1 to the receptor in vitro (Bohn et al., 2004). Collectively, these studies demonstrate that morphine is biased toward β-arrestin2 regulation of the μOR.
TABLE 3.
Summary of hot plate antinociceptive responses in mice lacking β-arrestin1 or β-arrestin2 in response to different opioid agonists
| Morphine | Methadone | Fentanyl | Etorphine | Oxycodone | Heroin | |
|---|---|---|---|---|---|---|
| Hot plate antinociception | ||||||
| βarr1-KO | =a | N.D. | N.D. | N.D. | N.D. | N.D. |
| βarr2-KO | ↑a,,–d | =a,d | =a,d | =a | =d | ↑a |
| Hot plate antinociceptive tolerance | ||||||
| βarr2-KO | ↓b,d | =d | =d | N.D. | =d | N.D. |
=, equivalent to wild type (WT) response at 10 mg/kg s.c.; ↑, significant increase in response; ↓, significant decrease in response; N.D., not determined.
Initially, it was considered paradoxical that the βarr2-KO displayed such dramatic differences in their response profiles to morphine when it had been previously shown, in HEK-293 cells, that morphine did not recruit β-arrestins to the MOR (Whistler and von Zastrow, 1998; Zhang et al., 1998). However, Zhang et al. (1998) showed that overexpression of GRK2 could promote MOR–β-arrestin2 interactions in this cell line. Other studies demonstrated that morphine could recruit β-arrestin2 to MOR in other cell lines (reviewed in section X). Considering the differences between the scaffolding partners expressed in HEK-293 cells versus neurons, as well as the particular nuances of any overexpression system, the environment in the HEK-293 cell lines may simply not be favorable to observe the morphine-driven interaction. Moreover, the fact that multiple physiological responses are altered in the β-arrestin2-KO mice in response to morphine further argues that there is a interaction between these two proteins when morphine occupies the receptor (Raehal and Bohn, 2005).
A number of studies have shown that diverse kinases and other regulatory proteins are also involved in the development of morphine tolerance. In mice, spinal tolerance induced by repeated systemic treatment with meperidine, morphine, or fentanyl over an 8-h period was effectively reversed with intracerebroventricular administration of the PKC inhibitor 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (Gö6976) (Hull et al., 2010). In fact, the role for PKC in morphine tolerance becomes even more evident in the absence of β-arrestin2, because the morphine-tolerant βarr2-KO mice can be completely resensitized to morphine after a single systemic injection of the nonselective PKC inhibitor chelerythrine (Bohn et al., 2002). Moreover, peripheral tolerance to intraplantar morphine is reversed by pretreatment with the PKC inhibitors calphostin C, Gö6976, and 2,2′,3,3′,4,4′-hexahydroxy-1,1′-biphenyl-6,6′-dimethanol dimethyl ether (Inoue and Ueda, 2000; Ueda et al., 2001). Therefore, morphine may selectively engage PKC regulation of the μOR in the spinal cord to produce spinal antinociceptive tolerance.
In contrast to studies with morphine, in response to short-term treatment with etorphine, methadone, and fentanyl, βarr2-KO mice display degrees of antinociception similar to those of their WT counterparts (Bohn et al., 2004; Raehal and Bohn, 2011), and long-term administration of these agonists produces antinociceptive tolerance to an equal extent in both genotypes (Raehal and Bohn, 2011). The differential antinociceptive responses after short- and long-term administration of morphine and these other opioid agonists in the βarr2-KO mice are consistent with in vitro studies showing that morphine and heroin selectively induce μOR interactions with β-arrestin2, whereas etorphine, methadone, and fentanyl recruit both β-arrestins to the receptor (Bohn et al., 2004). Therefore, it is likely that β-arrestin1 is able to functionally substitute for β-arrestin2 in the knockout mice when etorphine, methadone, and fentanyl, but not morphine, are bound to the μOR.
C. Multiple Regulators of the μ Opioid Receptor and Morphine Tolerance
In addition to the ligand, the contribution of the receptor's immediate cellular environment can determine how the μOR is regulated and the extent of antinociception and tolerance produced in vivo. This concept is not entirely new, in that others have shown differential desensitization profiles for μOR across brain regions as well as spinal cord, as detailed in the first half of this review (Sim et al., 1996; Maher et al., 2001). With respect to opioid-induced behaviors, this becomes evident when antinociceptive tolerance is assessed by different endpoints (hot plate versus tail-flick tests) in mice lacking β-arrestin2. Although βarr2-KO mice do not develop antinociceptive tolerance to morphine in the hot plate test, they do develop morphine tolerance in the tail-flick test, although to a lesser extent than that experienced by their WT littermates (Bohn et al., 2000, 2002). Because the hot plate test has been shown to predominantly measure opioid inhibition of supraspinal mechanisms involved in the perception of nociceptive stimuli, and the tail-flick test has been shown to assess opioid inhibition of spinal cord-mediated reflexes in response to a nociceptive stimuli, these studies indicate that supraspinal tolerance to morphine is β-arrestin2-dependent and antinociceptive tolerance in the spinal cord has both β-arrestin2-dependent and -independent components.
Likewise, the mechanisms underlying the development of antinociceptive tolerance to DAMGO also differ depending upon the tissue. For instance, intracerebroventricular administration of small molecule GRK inhibitors prevents the development of “rapid antinociceptive tolerance” to intracerebroventricular injection with DAMGO in the tail-flick test (Hull et al., 2010). In contrast, peripheral nociception tests in mice made tolerant to DAMGO suggest that PKC contributes to the development of DAMGO tolerance in peripheral tissues. Ueda et al. (2001) found that when DAMGO was administered via intraplantar injection, intraplantar pretreatment with the PKC inhibitor calphostin C inhibits the development of tolerance measured using a bradykinin-nociceptin pain test. Therefore, the differences between the neuronal populations make it attractive to propose that the cellular mechanisms underlying the development of tolerance to the analgesic effects of opioids may be greatly influenced by the complement of proteins expressed in the immediate vicinity of the μOR and can differ among neuronal cell types.
Although GRKs seem to regulate morphine-induced antinociceptive tolerance in the brain, other regulatory kinases have been shown to play an important role in the development of morphine tolerance at the level of the spinal cord. For example, intrathecal infusion of the PKC inhibitor calphostin C attenuates the development of short-term tolerance to DAMGO in mice (Narita et al., 1995). Likewise, blocking PKC activity with a intrathecal coinfusion of the PKC inhibitor chelerythrine (Granados-Soto et al., 2000) or PKCα oligonucleotides (Hua et al., 2002) with morphine prevents the development of spinal tolerance in rats. Intracerebroventricular injections of PKC inhibitors can also reverse morphine-induced tolerance in the tail-flick antinociception test (Smith et al., 2002, 2003; Gabra et al., 2008). However, inhibitor studies are complicated by the likelihood that they may also act on other kinases that may affect the response. Moreover, the role of PKC in opioid-induced antinociceptive tolerance has been further exemplified using PKC knockout mice. PKCγ-KO develop significantly less spinal tolerance to long-term treatment with morphine (75-mg morphine pellet for 3 days) compared with WT controls as assessed by the tail-flick test (Zeitz et al., 2001).
Collectively, these studies demonstrate that the cellular mechanisms underlying the development of tolerance are quite complex and involve multiple signaling pathways. Moreover, it is not clear in all cases whether the kinases are directly affecting the μOR (by phosphorylation) or if they are intermediary signaling molecules promoting neuroadaptive responses downstream of μOR activation. It is also possible that the regulation of μOR signaling by β-arrestins, GRKs, PKA, JNK, PKC, and CaMKII is all part of the same feedback loop. It is attractive to imagine that through a complex interplay of these common intracellular kinases and signaling proteins, all of these systems may be working in concert to produce antinociceptive tolerance. This idea has been put forth in a review by Garzón et al. (2008), in which the authors propose that many approaches to prevent opioid tolerance may actually be targeting elements of the same regulatory pathways. Finally, different agonists may be activating additional receptor systems to produce their differential effects. Indeed, the fact that agonists at GPCRs are likely to hit multiple targets (or induce the release of additional neurotransmitters that act on other receptors) in vivo remains one of the most challenging aspects of attributing altered behavioral responses to drugs exhibiting functionally selective properties at a particular receptor.
X. Conclusions
The studies discussed in this review indicate that the nature of μOR signaling and regulation are functions of both the agonist acting at the receptor and the cellular environment in which it is expressed. Biochemical and electrophysiological studies demonstrate that ligand-directed signaling and regulation of the μOR cannot entirely be explained by the intrinsic efficacy of the agonist. Instead, these studies demonstrate that opioid ligands may bias the receptor toward or against interactions with specific intracellular proteins, such as G proteins, kinases, and β-arrestins, and these different interactions result in the differential signaling, phosphorylation, desensitization, and internalization of the μOR. The in vivo studies suggest that ligand-directed signaling may have major implications for μOR-mediated physiological responses, including the development of analgesic tolerance. Although the regulatory events leading to tolerance may be complex, scaffolding molecules such as β-arrestins, as well as other adaptors involved in the phosphorylation and dephosphorylation of the receptors, are certain to affect receptor trafficking and the ability to restore sensitivity to further agonist activation. Although many of the studies published have focused on processes involved in μOR desensitization, it may be that facilitation of receptor resensitization, whether via an internalization process or through dephosphorylation at the membrane, is a key means to reverse or prevent the development of tolerance. Broadening our understanding of how different μOR agonists induce distinct trafficking and signaling events and relating these events to the behavioral effects of the drugs will be instrumental to the development of new opioid therapeutics with enhanced analgesic properties but limited adverse side effects. Toward this goal, ultimately, it will be essential to understand how the receptor is regulated in the tissues and neuronal populations that directly control each of the behavioral responses to opioids narcotics.
Acknowledgments
This research was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grants DA18860, DA014600, DA025259] (to L.M.B.) [Grant DA021952] (to K.M.R.).
Authorship Contributions
Conducted experiments: Raehal, Groer, and Bohn.
Wrote or contributed to the writing of the manuscript: Raehal, Schmid, Groer, and Bohn.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.111.004598.
- CaMKII
- calcium/calmodulin kinase II
- CaV
- voltage-gated calcium channel
- CHO
- Chinese hamster ovary
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- DRG
- dorsal root ganglion
- ERK1/2
- extracellular regulated kinase 1/2
- FRET
- fluorescence resonance energy transfer
- GF109203X
- 3-[1-[3-(dimethylaminopropyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione monohydrochloride
- Gö6976
- 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole
- GPCR
- G protein-coupled receptor
- GRIN1
- glutamate receptor subunit ζ 1
- GRK
- G protein-coupled receptor kinase
- GTPγS
- guanosine 5′-O-(3-thio)triphosphate
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- JNK
- c-jun N-terminal kinase
- Kir3
- G protein-coupled inwardly rectifying potassium channel
- KO
- knockout
- μOR
- μ opioid receptor
- MOR
- μ opioid receptor
- MEF
- mouse embryonic fibroblast
- PKA
- protein kinase A
- PKC
- protein kinase C
- Ro32-0432
- 3-[(8S)-8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl[-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione hydrochloride
- siRNA
- small interfering RNA
- WT
- wild type.
References
- Abbadie C, Gultekin SH, Pasternak GW. (2000a) Immunohistochemical localization of the carboxy terminus of the novel mu opioid receptor splice variant MOR-1C within the human spinal cord. Neuroreport 11:1953–1957 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan Y, Drake CT, Pasternak GW. (2000b) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100:141–153 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan YX, Pasternak GW. (2000c) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol 419:244–256 [DOI] [PubMed] [Google Scholar]
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. (2003) Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA 100:1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. (2002) mu-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadée W. (1995) Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem 65:1636–1645 [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66:1592–1598 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, Henderson G. (2009a) Role of protein kinase C and mu-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur J Neurosci 29:307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009b) Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2008) A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132:125–136 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2005) An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273 [DOI] [PubMed] [Google Scholar]
- Bohn LM. (2009) Selectivity for G protein or arrestin-mediated signaling, in Functional Selectivity of G Protein-Coupled Receptor Ligands (Neve K. ed) pp 71–85, 1st ed, Humana Press, Totowa, NJ [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. (2004) Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol 66:106–112 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci 22:10494–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278:18776–18784 [DOI] [PubMed] [Google Scholar]
- Celver J, Xu M, Jin W, Lowe J, Chavkin C. (2004) Distinct domains of the mu-opioid receptor control uncoupling and internalization. Mol Pharmacol 65:528–537 [DOI] [PubMed] [Google Scholar]
- Celver JP, Lowe J, Kovoor A, Gurevich VV, Chavkin C. (2001) Threonine 180 is required for G-protein-coupled receptor kinase 3- and beta-arrestin 2-mediated desensitization of the mu-opioid receptor in Xenopus oocytes. J Biol Chem 276:4894–4900 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Law PY, Loh HH. (1998) Distinct differences between morphine- and [d-Ala2,N-MePhe4,Gly-ol5]-enkephalin-mu-opioid receptor complexes demonstrated by cyclic AMP-dependent protein kinase phosphorylation. J Neurochem 71:231–239 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Prather PL, Yu L, Law PY, Loh HH. (1995) Expression of the mu-opioid receptor in CHO cells: ability of mu-opioid ligands to promote alpha-azidoanilido[32P]GTP labeling of multiple G protein alpha subunits. J Neurochem 64:2534–2543 [DOI] [PubMed] [Google Scholar]
- Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, Mercadante S, Pasternak G, Ventafridda V, and Expert Working Group of the European Association of Palliative Care Network (2001) Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 19:2542–2554 [DOI] [PubMed] [Google Scholar]
- Chu J, Zheng H, Loh HH, Law PY. (2008) Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell Signal 20:1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. (2010) Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization. Cell Signal 22:684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CC, Bruchas MR, Lee ML, Chavkin C. (2010) Phosphorylation of the mu-opioid receptor at tyrosine 166 (Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol 77:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett BJ. (1998) Opioid tolerance: the clinical perspective. Br J Anaesth 81:58–68 [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. (1995) Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci USA 92:10540–10544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF. (1996) Modulatory effects of Gs-coupled excitatory opioid receptor functions on opioid analgesia, tolerance, and dependence. Neurochem Res 21:1347–1351 [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. (1998) Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem 273:685–688 [DOI] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2005) Morphine-Induced mu-opioid receptor desensitization. Mol Pharmacol 68:1127–1132 [DOI] [PubMed] [Google Scholar]
- Deng HB, Yu Y, Pak Y, O'Dowd BF, George SR, Surratt CK, Uhl GR, Wang JB. (2000) Role for the C-terminus in agonist-induced mu opioid receptor phosphorylation and desensitization. Biochemistry 39:5492–5499 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82:1226–1236 [DOI] [PubMed] [Google Scholar]
- El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J Biol Chem 276:12774–12780 [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Höllt V. (2001) Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain research 95:129–137 [DOI] [PubMed] [Google Scholar]
- Fan X, Zhang J, Zhang X, Yue W, Ma L. (2002) Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology 43:809–816 [DOI] [PubMed] [Google Scholar]
- Feng B, Li Z, Wang JB. (2011) Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signaling. Mol Pharmacol 79:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Ménard L, Caron MG. (1996) Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363–366 [DOI] [PubMed] [Google Scholar]
- Frölich N, Dees C, Paetz C, Ren X, Lohse MJ, Nikolaev VO, Zenk MH. (2011) Distinct pharmacological properties of morphine metabolites at Gi-protein and β-arrestin signaling pathways activated by the human μ-opioid receptor. Biochem Pharm 81:1248–1254 [DOI] [PubMed] [Google Scholar]
- Gabra BH, Bailey CP, Kelly E, Smith FL, Henderson G, Dewey WL. (2008) Pre-treatment with a PKC or PKA inhibitor prevents the development of morphine tolerance but not physical dependence in mice. Brain Res 1217:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, Sánchez-Blázquez P. (2008) Do pharmacological approaches that prevent opioid tolerance target different elements in the same regulatory machinery? Curr Drug Abuse Rev 1:222–238 [DOI] [PubMed] [Google Scholar]
- Ge X, Qiu Y, Loh HH, Law PY. (2009) GRIN1 regulates micro-opioid receptor activities by tethering the receptor and G protein in the lipid raft. J Biol Chem 284:36521–36534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL. (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85:395–404 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bartzsch K, Widera A, Becker A, Höllt V, Koch T. (2006) Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology (Berl) 186:177–184 [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. (2007) An opioid agonist that does not induce micro-opioid receptor–arrestin interactions or receptor internalization. Mol Pharmacol 71:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. (2006) The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110:465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. (2005) Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci 25:7847–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. (2003) Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci 23:4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. (2005) Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands. J Med Chem 48:4765–4771 [DOI] [PubMed] [Google Scholar]
- Harris JD. (2008) Management of expected and unexpected opioid-related side effects. Clin J Pain 24 (Suppl 10):S8–S13 [DOI] [PubMed] [Google Scholar]
- He L, Whistler JL. (2005) An opioids cocktail that reduces morphine tolerance and dependence. Curr Biol 15:1028–1033 [DOI] [PubMed] [Google Scholar]
- Hua XY, Moore A, Malkmus S, Murray SF, Dean N, Yaksh TL, Butler M. (2002) Inhibition of spinal protein kinase Calpha expression by an antisense oligonucleotide attenuates morphine infusion-induced tolerance. Neuroscience 113:99–107 [DOI] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. (2010) The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different efficacy. J Pharmacol Exp Ther 332:1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Ueda H. (2000) Protein kinase C-mediated acute tolerance to peripheral mu-opioid analgesia in the bradykinin-nociception test in mice. J Pharmacol Exp Ther 293:662–669 [PubMed] [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. (2006) Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol 70:676–685 [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271:19021–19024 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2005) New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov 4:919–927 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2007) Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci 28:407–415 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2009) Biased agonism. F1000 Biol Rep 1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302 [DOI] [PubMed] [Google Scholar]
- Kieffer BL. (1999) Opioids: first lessons from knockout mice. Trends Pharmacol Sci 20:19–26 [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Liang Y, Schulz S, Beyer A, Schröder H, Höllt V. (2004) Phopsholipase D2 modulates agonist-induced mu-opioid receptor desensitization and resensitization. J Neurochem 88:680–688 [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. (2003) ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem 278:9979–9985 [DOI] [PubMed] [Google Scholar]
- Koch T, Kroslak T, Mayer P, Raulf E, Höllt V. (1997) Site mutation in the rat mu-opioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J Neurochem 69:1767–1770 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schröder H, Kahl E, Höllt V. (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem 276:31408–31414 [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol 67:280–287 [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. (2001) beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA 98:1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver JP, Wu A, Chavkin C. (1998) Agonist induced homologous desensitization of mu-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Mol Pharmacol 54:704–711 [PubMed] [Google Scholar]
- Kramer HK, Simon EJ. (1999) Role of protein kinase C (PKC) in agonist-induced mu opioid receptor down-regulation: I. PKC translocation to the membrane of SH-SY5Y neuroblastoma cells is induced by mu-opioid agonists. J Neurochem 72:585–593 [DOI] [PubMed] [Google Scholar]
- Kreek MJ. (1973) Medical safety and side effects of methadone in tolerant individuals. JAMA 223:665–668 [PubMed] [Google Scholar]
- Kumar P, Sunkaraneni S, Sirohi S, Dighe SV, Walker EA, Yoburn BC. (2008) Hydromorphone efficacy and treatment protocol impact on tolerance and mu-opioid receptor regulation. Eur J Pharmacol 597:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY. (2011) Opioid receptor signal transduction mechanisms, in The Opioid Receptors (Pasternak GW. ed) pp 195–237, Springer Science, New York [Google Scholar]
- Li Y, Liu X, Liu C, Kang J, Yang J, Pei G, Wu C. (2009) Improvement of morphine-mediated analgesia by inhibition of beta-arrestin 2 expression in mice periaqueductal gray matter. Int J Mol Sci 10:954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Daaka Y, Lefkowitz RJ. (1998) Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor 1 receptor. J Biol Chem 273:31640–31643 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Della Rocca GJ, Lefkowitz RJ. (1997) G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem 272:31648–31656 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. (1999) Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283:655–661 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. (2002) The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115:455–465 [DOI] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. (2006) Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem 281:34515–34524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. (2009) Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl) 207:413–422 [DOI] [PubMed] [Google Scholar]
- Maher CE, Eisenach JC, Pan HL, Xiao R, Childers SR. (2001) Chronic intrathecal morphine administration produces homologous mu receptor/G-protein desensitization specifically in spinal cord. Brain Res 895:1–8 [DOI] [PubMed] [Google Scholar]
- Mailman RB. (2007) GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massotte D, Brillet K, Kieffer B, Milligan G. (2002) Agonists activate Gi1 alpha or Gi2 alpha fused to the human mu opioid receptor differently. J Neurochem 81:1372–1382 [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. (2000) Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290:1574–1577 [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. (2010) mu-Opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova I. (2010) Pain market. Nat Rev Drug Discov 9:589–590 [DOI] [PubMed] [Google Scholar]
- Molinari P, Vezzi V, Sbraccia M, Grò C, Riitano D, Ambrosio C, Casella I, Costa T. (2010) Morphine-like opioids selectively antagonize receptor-arrestin interactions. J Biol Chem 285:12522–12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Suzuki T, Narita M, Dun NJ, Imai S, Yajima Y, Nagase H, Suzuki T, Tseng LF. (2001) Enhanced mu-opioid responses in the spinal cord of mice lacking protein kinase Cgamma isoform. J Biol Chem 276:15409–15414 [DOI] [PubMed] [Google Scholar]
- Narita M, Narita M, Mizoguchi H, Tseng LF. (1995) Inhibition of protein kinase C, but not of protein kinase A, blocks the development of acute antinociceptive tolerance to an intrathecally administered mu-opioid receptor agonist in the mouse. Eur J Pharmacol 280:R1–R3 [DOI] [PubMed] [Google Scholar]
- Noble F, Cox BM. (1996) Differential desensitization of mu- and delta- opioid receptors in selected neural pathways following chronic morphine treatment. Br J Pharmacol 117:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. (1999) Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274:32248–32257 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- Pak Y, O'Dowd BF, George SR. (1997) Agonist-induced desensitization of the mu opioid receptor is determined by threonine 394 preceded by acidic amino acids in the COOH-terminal tail. J Biol Chem 272:24961–24965 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, Rossi G, Pasternak GW. (1999) Identification and characterization of three new alternatively spliced mu-opioid receptor isoforms. Mol Pharmacol 56:396–403 [DOI] [PubMed] [Google Scholar]
- Patel MB, Patel CN, Rajashekara V, Yoburn BC. (2002) Opioid agonists differentially regulate mu-opioid receptors and trafficking proteins in vivo. Mol Pharmacol 62:1464–1470 [DOI] [PubMed] [Google Scholar]
- Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. (2007) Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol 563:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. (1998) A mitogen-activated protein kinase pathway is required for mu-opioid receptor desensitization. J Biol Chem 273:12402–12406 [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. (2007) Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 69:511–534 [DOI] [PubMed] [Google Scholar]
- Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. (1996) Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem 271:6403–6410 [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Sieja A, Starowicz K, Maj M, Bilecki W, Przewlocki R. (2002) Knockdown of spinal opioid receptors by antisense targeting beta-arrestin reduces morphine tolerance and allodynia in rat. Neurosci Lett 325:107–110 [DOI] [PubMed] [Google Scholar]
- Quillan JM, Carlson KW, Song C, Wang D, Sadée W. (2002) Differential effects of mu-opioid receptor ligands on Ca2+ signaling. J Pharmacol Exp Ther 302:1002–1012 [DOI] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. (2011) Recovery from μ-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci 31:4434–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. (2005) Mu opioid receptor regulation and opioids responsiveness. AAPS J 7:E587–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. (2011) The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Schmid CL, Medvedev IO, Gainetdinov RR, Premont RT, Bohn LM. (2009) Morphine-induced physiological and behavioral responses in mice lacking G protein-coupled receptor kinase 6. Drug Alcohol Depend 104:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95:S91–S117 [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2007) Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 21:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidak Z, Blake-Palmer K, Hay DL, Northup JK, Glass M. (2006) Differential activation of G-proteins by mu-opioid receptor agonists. Br J Pharmacol 147:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, et al. (2006) Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47:1131–1139 [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, García-España A, Garzón J. (1995) In vivo injection of antisense oligodeoxynucleotides to G alpha subunits and supraspinal analgesia evoked by mu and delta opioid agonists. J Pharmacol Exp Ther 275:1590–1596 [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Gómez-Serranillos P, Garzón J. (2001) Agonists determine the pattern of G-protein activation in mu-opioid receptor-mediated supraspinal analgesia. Brain Res Bull 54:229–235 [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Díaz M, DeAntonio I, Garzón J. (1999) Endomorphin-1 and endomorphin-2 show differences in their activation of mu opioid receptor-regulated G proteins in supraspinal antinociception in mice. J Pharmacol Exp Ther 291:12–18 [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. (2009) Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther 121:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. (2010) Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci 30:13513–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA 105:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V. (2004) Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J 23:3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. (1996) Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Scoggins KL, Cassidy MP, Smith LA, Dewey WL, Smith FL, Selley DE. (2007) Region-dependent attenuation of mu opioid receptor-mediated G-protein activation in mouse CNS as a function of morphine tolerance. Br J Pharmacol 151:1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. (2000) Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci 20:4555–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Walker EA, Yoburn BC. (2008) The analgesic efficacy of fentanyl: relationship to tolerance and mu-opioid receptor regulation. Pharmacol Biochem Behav 91:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Javed R, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. (2002) Prolonged reversal of morphine tolerance with no reversal of dependence by protein kinase C inhibitors. Brain Res 958:28–35 [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Dewey WL. (2003) The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res 985:78–88 [DOI] [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. (2001) mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav 69:233–237 [DOI] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, von Zastrow M, Evans C, Brecha NC. (1996) Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci USA 93:9241–9246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Yaksh TL. (1989) Potency of infused spinal antinociceptive agents is inversely related to magnitude of tolerance after continuous infusion. J Pharmacol Exp Ther 250:1–8 [PubMed] [Google Scholar]
- Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C. (2004) G-protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br J Pharmacol 141:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, Hiemstra J, Partilla JS, Dersch CM, Rothman RB, et al. (2008) Herkinorin analogues with differential beta-arrestin-2 interactions. J Med Chem 51:2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Matsumoto T. (2001) Protein kinase C-mediated inhibition of mu-opioid receptor internalization and its involvement in the development of acute tolerance to peripheral mu-agonist analgesia. J Neurosci 21:2967–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Urs NM, Daigle TL, Caron MG. (2010) A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology 36:551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG. (2001) Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience 108:467–477 [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422 [DOI] [PubMed] [Google Scholar]
- Virk MS, Williams JT. (2008) Agonist-specific regulation of mu-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol 73:1301–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Young AM. (2001) Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 154:131–142 [DOI] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. (2007) Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci 27:5092–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sadée W, Quillan JM. (1999) Calmodulin binding to G protein-coupling domain of opioid receptors. J Biol Chem 274:22081–22088 [DOI] [PubMed] [Google Scholar]
- Wang HL. (2000) A cluster of Ser/Thr residues at the C-terminus of mu-opioid receptor is required for G protein-coupled receptor kinase 2-mediated desensitization. Neuropharmacology 39:353–363 [DOI] [PubMed] [Google Scholar]
- Wang HL, Chang WT, Hsu CY, Huang PC, Chow YW, Li AH. (2002) Identification of two C-terminal amino acids, Ser(355) and Thr(357), required for short-term homologous desensitization of mu-opioid receptors. Biochem Pharmacol 64:257–266 [DOI] [PubMed] [Google Scholar]
- Wang HY, Burns LH. (2006) Gbetagamma that interacts with adenylyl cyclase in opioid tolerance originates from a Gs protein. J Neurobiol 66:1302–1310 [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E, Olmstead MC, Burns LH. (2005) Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience 135:247–261 [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. (1998) Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA 95:9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. (1999) Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23:737–746 [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343 [DOI] [PubMed] [Google Scholar]
- Wolf R, Koch T, Schulz S, Klutzny M, Schröder H, Raulf E, Bühling F, Höllt V. (1999) Replacement of threonine 394 by alanine facilitates internalization and resensitization of the rat mu opioid receptor. Mol Pharmacol 55:263–268 [DOI] [PubMed] [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. (2007) A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse 61:166–175 [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Purohit V, Patel K, Zhang Q. (2004) Opioid agonist and antagonist treatment differentially regulates immunoreactive mu-opioid receptors and dynamin-2 in vivo. Eur J Pharmacol 498:87–96 [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB. (1997) Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem 272:28869–28874 [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. (2008) Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of N-methyl-d-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem 283:17194–171204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain 94:245–253 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Qiu Y, Loh HH, Law PY. (2008a) Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci USA 105:9421–9426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Zeng Y, Loh HH, Law PY. (2010a) Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J Biol Chem 285:21994–22002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Zhang Y, Loh HH, Law PY. (2011) Modulating μ-opioid receptor phosphorylation switches agonist-dependent signaling as reflected in PCKε activation and dendritic spine stability. J Biol Chem 286:12724–12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008b) Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol 73:178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY. (2010b) mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol 77:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]