Abstract
Background
Decitabine was evaluated for its efficacy and safety in Korean patients with myelodysplastic syndrome with IPSS score of 0.5 or over.
Design and Methods
Decitabine 20 mg/m2/day was given intravenously over one hour for five consecutive days every four weeks. The primary end point was overall response rate.
Results
A total of 101 patients were analyzed. The International Prognostic Scoring System risk category was Intermediate-2/High in 47.5%. A median of 5 courses (range 1–18) were delivered. The overall response rate was 55.4% (13 complete responses, one partial response, 23 marrow complete responses, and 19 hematologic improvements). Forty-eight patients (47.5%) showed some hematologic improvement. With a median follow-up duration of 478 days (range 69–595), median overall survival was 17.7 months. Patients who showed hematologic improvement had significantly longer overall survival than those who did not (19.2 vs. 15.9 months, P=0.006 by landmark analysis at six months). The difference in overall survival was evident in the Intermediate-2/High risk group but not in the Intermediate-1 risk group. The progression-free survival and acute myeloid leukemia-free survival were 61.9% and 77.9% at one year, respectively. Among 489 assessable treatment courses, there were 97 fever episodes requiring intravenous antimicrobials.
Conclusions
Decitabine treatment was feasible and effective in Korean patients with myelodysplastic syndrome, and the overall survival was significantly longer in patients showing hematologic improvement.
Keywords: MDS, decitabine, observational study
Introduction
Myelodysplastic syndrome (MDS) is a clonal hematopoietic stem cell disorder characterized by ineffective hematopoiesis and peripheral cytopenias. The natural history of this syndrome ranges from more indolent forms of the disease over years, to those with a rapid evolution to acute myeloid leukemia (AML). MDS shows a high prevalence of tumor suppressor gene hypermethylation1 and DNA methylation is believed to contribute to cancer initiation and progression by gene expression inactivation.2,3
Decitabine (5-aza-2′-deoxycytidine) is a cytosine nucleoside analog which is incorporated into DNA. At lower doses, decitabine induces hypomethylation, which may induce cell differentiation, re-expression of tumor suppressor genes, and inhibition of tumor growth.4,5
The drug is approved for the treatment of myelodysplastic syndrome (MDS) including previously treated or untreated de novo or secondary MDS of all FAB subtypes, and intermediate (INT)-1, INT-2 and high-risk International Prognostic Scoring System (IPSS) groups.6 Possible mechanisms of clinical effects of decitabine include direct cytotoxicity and hypomethylation.1,7
Phase II and III clinical trials have demonstrated the efficacy of decitabine in the treatment of patients with MDS. In an initial phase II trial, decitabine was administered every six weeks as a continuous intravenous infusion of 50 mg/m2/day for three days (150 mg/m2 per course), and response was observed in 54% of 28 evaluable patients.8 Subsequently, a phase II and two phase III trials investigated a modified 3-day regimen, in which decitabine was given intravenously over three hours at a dose of 15 mg/m2 every eight hours for three days (135 mg/m2 per course) and each course was repeated every six weeks. These trials showed overall response rates of 30–49%.9–11 The US Food and Drug Administration approved this 3-day regimen for the treatment of patients with MDS. The alternative 5-day regimen, in which decitabine 20 mg/m2/day is administered as a one hour intravenous infusion for five consecutive days (100 mg/m2 per course) every four weeks, was subsequently also approved. This regimen is the most widely used because it is suitable for treatment in an outpatient clinic and achieved a higher response rate in a randomized trial of three schedules.12 A recent phase II trial using the 5-day regimen showed meaningful clinical benefit for patients with MDS, with more than half of the patients showing improvement.13 These clinical trials demonstrating the efficacy of decitabine in the treatment of patients with MDS were performed in Western countries; there have been few data on the effects of decitabine in Asian MDS patients.14 Thus, we performed a prospective multicenter observational study of the 5-day decitabine regimen in Korean patients with MDS.
Design and Methods
Patients
Study eligibility criteria were: older than 17 years of age, and MDS (de novo or secondary) of any subtype as defined by the WHO classification with an IPSS score of 0.5 or more or chronic myelomonocytic leukemia (CMML). Patients were excluded if they had previously received any hypomethylating therapy or if they had severe coexisting disease. Pregnant or lactating women were also excluded. Patients had to have adequate renal and hepatic function, and good performance status (Eastern Cooperative Oncology Group performance status of 0 to 2). This study was approved by the institutional review boards of all participating centers. Patients provided informed consent before registration on this prospective multicenter observational study. This trial is registered at www.clinicaltrials.gov as #NCT01041846.
Treatment
Decitabine was given intravenously over one hour at a dose of 20 mg/m2 daily for five consecutive days and each course was repeated every four weeks in the outpatient clinic. General guidelines for dose adjustments, treatment intervals, treatment duration, and response assessments by bone marrow examination were provided. No dose escalation of decitabine was allowed. Dose reduction or treatment delay could be determined at the discretion of the treating physicians, but no dose reduction or treatment delay was recommended, at least in the first 3 courses, regardless of blood counts. Exceptions to this were: presence of grade 3 or 4 non-hematologic toxicities, occurrence of life-threatening myelosuppression complications, such as bleeding or infection, or persistent myelosuppression defined by bone marrow hypocellularity without evidence of disease progression over six weeks after start of decitabine treatment. Dose reductions were made to 15 mg/m2/day, 10 mg/m2/day, and 7.5 mg/m2/day. Physicians were advised to continue decitabine treatment for at least 4 courses or until disease progression or death, unacceptable adverse events, or withdrawal from the study by the patient, and to perform bone marrow examination every 2 courses until a complete remission (CR) was confirmed. Prophylactic antimicrobials or hematopoietic growth factors and other supportive care were given at the discretion of the treating physician.
Study end points
The primary end point of this study was overall response rate (ORR). Treatment response was assessed by a central reviewer using modified International Working Group (IWG 2006) response criteria.15 The ORR included rates for CR, marrow CR (mCR), partial response (PR), and hematologic improvements (HI). Secondary end points were adverse events, cytogenetic response and time-to-event outcomes of overall survival (OS), progression-free survival (PFS), and time to AML evolution (TTA), calculated from the initial date of decitabine treatment to the date of death from any cause (OS), and to the date of disease progression or death from MDS (PFS).15 Adverse events were graded according to the Common Toxicity Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Statistical analysis
The χ2 test was used for comparison of categorical variables such as prognostic factors for treatment response. Time-to-event outcomes were calculated using the Kaplan-Meier product limit estimates and were compared by log rank test. When we analyzed the associations between survival and response to decitabine, we used a landmark analysis to avoid the length bias associated with time-dependent variables.16,17 Time zero for this analysis was chosen a priori as six months because all initial responses to decitabine were achieved within six months after the start of decitabine treatment. Outcome differences at landmark time were compared using the log rank test. Multivariate analysis was performed using a stepwise multiple logistic regression for ORR achievement and using Cox’s proportional hazard model for OS. The database was locked one year after enrollment of the last patient, on September 30, 2010.
Results
Patients’ characteristics
A total of 103 patients were enrolled into this study between December 2008 and September 2009 from 24 Korean institutes. Two patients left the study because bone marrow diagnosis review showed idiopathic myelofibrosis and AML, respectively. Thus, 101 patients (68 males and 33 females) were included in the analyses. Median age was 65 years (range 23–80 years) and median duration of MDS was 25 days (range 1–1,797 days). Ninety patients had de novo MDS, 5 had therapy-related MDS, and 6 had MDS evolving from aplastic anemia. WHO subtypes at study entry were refractory anemia (RA; n=4), refractory cytopenia with multilineage dysplasia (RCMD; n=24), RCMD with ringed sideroblasts (n=3), refractory anemia with excess of blasts (RAEB-1; n=26 and RAEB-2; n=32), unclassifiable (n=1), and CMML (n=11). The IPSS risk category was Intermediate (INT)-1 in 52 patients, INT-2 or High in 48, and unknown in one whose IPSS score was at least 0.5 but whose karyotype was not available. Thirty-seven patients had abnormal karyotype before decitabine treatment; IPSS risk stratification of cytogenetic findings was good (n=65), intermediate (n=15), poor (n=19), and unknown (n=1). At the time of decitabine treatment, 33 patients were RBC transfusion-dependent and 27 were platelet transfusion-dependent.
Treatment data
Median number of courses delivered was 5 (range 1–18). Twenty-seven patients (26.7%) discontinued decitabine treatment before 3 courses because of treatment failure (n=10), allogeneic HCT (n=3), adverse effects (n=7), and personal reasons (n=7). Thirty-six patients (35.6%) received more than 6 courses and 15 patients were still on decitabine treatment at the time of analysis. Mean interval (± standard deviation) between treatment courses was 34.0±9.5 days and there was no significant difference in the intervals according to the treatment course number. Decitabine dose modification or treatment delay was made only in 4.2% of patients within the first 3 courses.
Treatment response
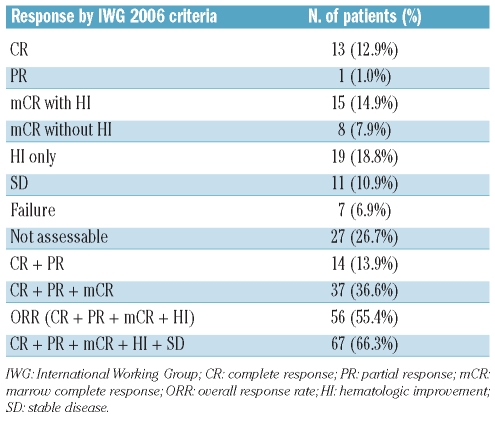
The response was observed in 56 (55.4%; 95% confidence interval [CI], 45.7–65.1%) among 101 patients: 13 CR (12.9%), one PR (1.0%), 15 mCR with HI (14.9%), 8 mCR without HI (7.9%), and 19 HI only (18.8%) (Table 1). Sixty-seven patients (66.3%) showed stable disease (SD) or better. Forty-eight patients (47.5%) experienced HI: 1-lineage improvement in 22, 2-lineage improvement in 15, and 3-lineage improvement in 11. Erythroid improvement (HI-E) was observed in 35 (36.1%) of 97 patients whose initial hemoglobin was less than 11.0 g/dL, platelet improvement (HI-P) in 30 (43.5%) of 69 patients whose initial platelets were less than 100×109/L, and neutrophil improvement in 20 (37.7%) of 53 patients whose initial neutrophils were less than 1×109/L. Cytogenetic response could be evaluated in 17 patients: cytogenetic CR was achieved in 5 (29.4%) and cytogenetic PR in 2 (11.8%).
Table 1.
Response to decitabine treatment.
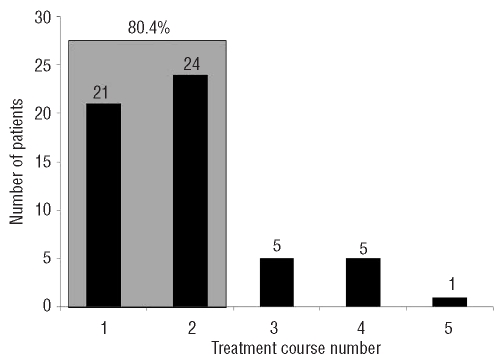
Initial response was detected during the first 2 courses in 45 (80.4%) of 56 patients with response (Figure 1). Median time to response was 1.9 months (range 0.9–5.2 months) and median duration of response was 13.2 months (95% CI, 9.3–17.1 months). Median number of treatment courses to achieve any HI, HI-E, HI-P, and HI-N was 2 (range 1–6), 2 (range 1–5), 1 (range 1–4), and 3 (range 1–8), respectively.
Figure 1.
Time to initial response, according to treatment course, in 56 patients who attained complete response, partial response, marrow complete response or hematologic improvement.
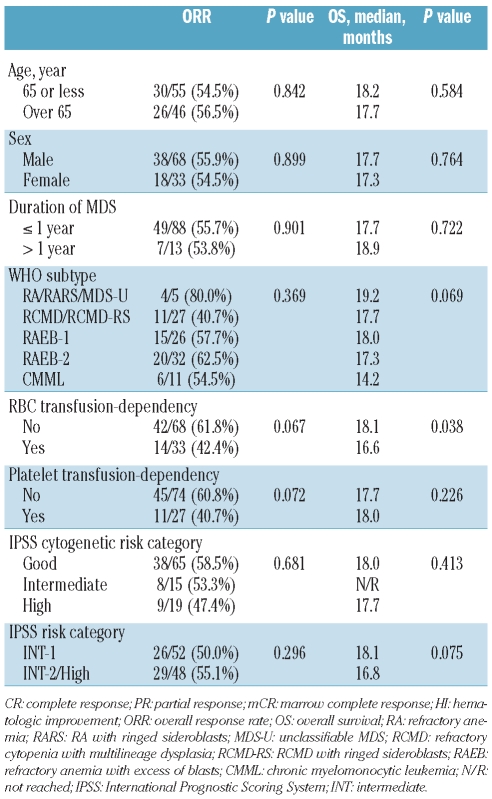
There was no significant difference in ORR according to baseline patients’ characteristics. Although patients who were RBC or platelet transfusion-dependent showed a tendency of lower ORR than those who were not (Table 2), stepwise multiple logistic regression analysis did not identify any independent prognostic factor for ORR.
Table 2.
Prognostic factor analysis for overall response (CR/PR/mCR/HI) and overall survival.
Adverse events
Averse events were evaluated for the first 8 courses for all patients, for a total of 489 courses. Major adverse events were cytopenia and cytopenia-related infection. Grade III or IV anemia (51.5%), neutropenia (80.2%), and thrombocytopenia (53.0%) were frequently observed. Febrile episodes requiring intravenous antibiotics and hospitalization occurred in 97 (19.8%) of 489 cycles evaluated: febrile neutropenia in 55, pneumonia in 12, fungal infection in 12, urinary tract infections in 4, cellulitis in 4, tonsillitis/sinusitis in 3, viral infection in 2, septicemia in 2, abscess in 2, and tuberculosis in one. There was a significant difference in the incidence of a febrile episode according to the treatment course number: 28.7% during the first 3 courses versus 9.6% during the 4th to 8th courses (P<0.001). A total of 13 episodes of grade III bleeding were observed: 12 episodes within the first 3 courses and one in the 6th course. There was no life-threatening bleeding episode. Grade III or higher non-hematologic toxicities were infrequent and reversible.
Survival data
With a median follow-up duration among surviving patients of 478 days (range 69–595 days), 44 patients died, 42 showed disease progression, and 19 progressed to AML. Median overall survival was 17.7 months (95% CI, 16.6–18.9 months). Probabilities for OS, PFS, and AML-free survival at one year were 74.8%, 61.9%, and 77.9%, respectively.
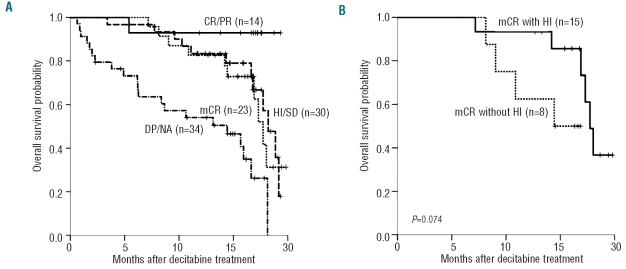
Baseline characteristics except RBC transfusion-dependency, did not have a significant impact on OS (Table 2). Patients with RBC transfusion-dependency at baseline had inferior survival than those without (median OS 16.6 vs. 18.1 months; P=0.038) and RBC transfusion-dependency was the only prognostic factor for OS in Cox’s proportional hazard model (hazard ratio 1.937; 95% CI, 1.043–3.596; P=0.036). Patients who reached CR or PR had a meaningful survival (only one of 14 patients died), while patients with mCR showed similar OS to those with HI or SD (median OS 17.7 vs. 18.2 months; Figure 2A). Patients who progressed or who could not be assessed had the poorest survival (median OS 14.4 months). Among 23 patients with mCR, OS differed according to presence of accompanying HI (17.7 vs. 14.4 months; P=0.074 by landmark analysis at six months; Figure 2B). Thus, achievement of HI appeared to be important for OS and we analyzed the impact of HI on survival both in all patients and according to IPSS risk subgroup (lower risk including IPSS INT-1 and higher risk including IPSS INT-2/High). Patients who achieved HI had significantly longer OS than those who did not (median OS 19.2 vs. 15.9 months; P=0.006 by land-mark analysis at six months; Figure 3A). In a subgroup analysis, achievement of HI had significant impact on OS in higher risk patients (median OS 19.2 vs. 13.1 months; P=0.008 by landmark analysis at six months; Figure 3B), whereas it did not in lower risk patients (median OS 18.0 vs. 18.1 months; P=0.205 by landmark analysis at six months; Figure 3C).
Figure 2.
(A) Overall survival according to treatment response type. (B) Overall survival in patients who achieved mCR with or without HI. P value by landmark analysis with the landmark set at six months from the initial date of decitabine treatment. CR: complete response; PR: partial response; HI: hematologic improvement; SD: stable disease; mCR: marrow complete response; DP: disease progression; NA: not assessable.
Figure 3.
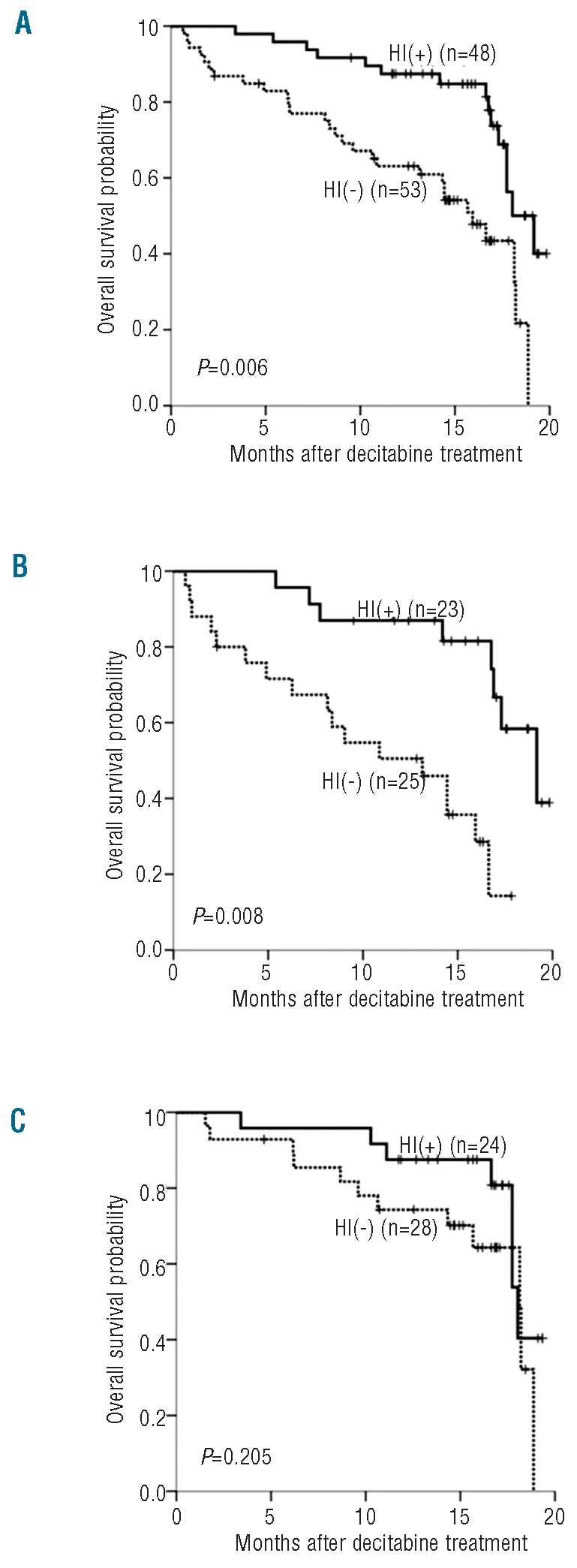
Overall survival according to achievement of HI. (A) Total patients. (B) Higher risk patients (IPSS INT-2/High risk). (C) Lower risk patients (IPSS INT-1 risk). P value by landmark analysis with the landmark set at six months from the initial date of decitabine treatment. HI: hematologic improvement; IPSS: International Prognostic Scoring System; INT: intermediate.
Allogeneic hematopoietic cell transplantation
A total of 15 patients received allogeneic HCT after a median 4 courses (range 2–12) of decitabine treatment. Median duration from start of decitabine treatment to HCT was 147 days (range 103–501 days). At the time of HCT, 4 patients were in remission (one PR, 3 mCR), one in HI, 6 in SD or DP, and 3 in AML progression. After median follow-up duration of 252 days (range 36–434 days) among surviving patients, 5 patients died from AML progression (n=3) or transplant-related mortality (n=2). Median survival after allogeneic HCT was 323 days (95% CI, 306–340 days).
Discussion
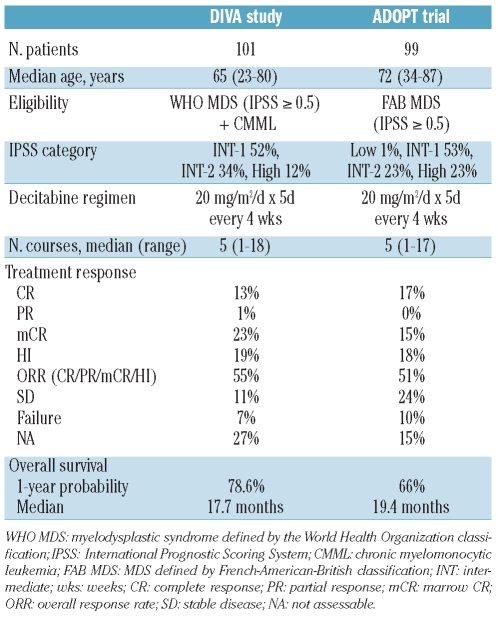
Although the effectiveness of hypomethylating agents in MDS has been reported from several prospective phase II and III clinical trials in Western countries,9,10,12,13,18–20 a prospective study has not yet been carried out in Asia. Published papers have shown possible differences in biological features of MDS between patients from Western countries21–23 and those from Asia.24–26 Asian MDS patients are younger and have a much lower incidence of isolated 5q- syndrome. DIVA (current study) is the first prospective study to demonstrate the effectiveness of hypomethylating agents in Asian MDS patients. Our study results are similar to those of the ADOPT trial, which was a multi-center, phase II, clinical trial from the United States to investigate the 5-day outpatient regimen of decitabine in MDS.13 Table 3 summarizes the eligibility criteria, decitabine regimen, and treatment results of both studies. Both studies used the same 5-day regimen, which seemed to result in better response rates and survival than the 3-day regimen.27
Table 3.
Comparison of DIVA study (current study) with ADOPT trial.13
In our study, achievement of HI was important for overall survival (Figure 3). This finding suggests that decitabine treatment might prolong survival in patients achieving HI. However, our study was not a randomized trial and did not have a non-treated control group; response to decitabine could just have been a marker for better risk disease. The effects of HI on survival were significant in patients with higher risk disease (IPSS INT-2/High risk), but not in those with lower risk disease (IPSS INT-1 risk). Our findings appear to be in line with the results from previous randomized trials, which have demonstrated significant prolongation of overall survival or time to AML/death by hypomethylating agents in higher risk MDS, but not in lower risk disease.9,18 Therefore, the goal of hypomethylating therapy in MDS must be tailored to the disease risk group: survival prolongation in higher risk MDS and improvement of cytopenia in lower risk MDS.
Standardized response criteria in MDS was proposed by the International Working Group (IWG) in 200028 and the criteria were modified by the same group in 2006.15 The modified IWG response criteria (IWG 2006) provided a new definition of mCR which indicated a reduction in bone marrow myeloblasts to 5% or less and a decrease by 50% or more from pre-treatment levels. However, clinical significance of mCR has not been well evaluated. In our study, 23 patients (22.8%) satisfied the response criteria for mCR: 15 of 23 mCRs were accompanied with HI and 8 were not. Patients with mCR showed inferior survival to those with CR or PR and similar survival to those with HI or SD. Survival of patients with mCR differed according to presence of HI (Figure 2). Thus, mCR itself appeared not to have as much clinical significance on patients’ outcomes as HI in non-transplant settings, although mCR may be of value in allogeneic HCT recipients.13 The issue of mCR should be examined in future trials.
Previous study results suggest that treatment duration may be important in prolonging survival by hypomethylating treatment in MDS. The only randomized clinical trial to prove the survival benefits provided by a hypomethylating agent in higher risk MDS was the AZA-001 trial, in which a median of 9 azacitidine treatment courses were given.18 In comparison to the AZA-001 trial, only a median of 4 decitabine treatment courses were given in the GMDSSG/EORTC 06011 trial, with 40% of patients receiving 2 courses or less; this meant that the trial failed to prove survival benefits of a hypomethylating agent in elderly patients with higher risk MDS.11 In our study, a median of 5 treatment courses were given and 27% of the patients received one or 2 courses. Among reasons given for discontinuation of decitabine treatment before the 3rd course, half were due to adverse effects or personal reasons. Major adverse events were cytopenia and cytopenia-related infection. Effective measures to prevent complications of infection may lead to prolonging the duration of decitabine treatment. In a retrospective study from Korea, antibiotic prophylaxis reduced the incidence of febrile episodes in patients who received decitabine treatment for MDS, especially at earlier cycles and in the presence of severe cytopenia.14 Thus, antibiotic prophylaxis may be a major supportive measure to avoid the early discontinuation of decitabine treatment and may thus help prolong survival.
In conclusion, decitabine treatment was feasible and effective in Korean patients with MDS, and OS was significantly longer in patients showing hematologic improvement.
Acknowledgments
We thank all the DIVA investigators who contributed to this work and provided data for the purpose of this analysis.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Study investigators
Je-Hwan Lee, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea; Jun Ho Jang, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea; Jinny Park, Gachon University Gil Hospital, Gachon University of Medicine and Science School of Medicine,, Incheon, Korea; Seonyang Park, Seoul National University Hospital, Seoul, Korea; Young-Don Joo, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea; Yeo-Kyeoung Kim, Chonnam National University Hwasun Hospital, Jeollanamdo, Korea; Hoon-Gu Kim, Gyeongsang National University Hospital, Jinju, Korea; Chul Won Choi, Korea University Guro Hospital, Seoul, Korea; Sung-Hyun Kim, Dong-A University Hospital, Busan, Korea; Seong Kyu Park, Soonchunhyang University Bucheon Hospital, Bucheon, Korea; Eunkyung Park, Chung-Ang University Hospital, Seoul, Korea; Yoo Hong Min, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea; Seung-Hyun Nam, Seoul Veterans Hospital, Seoul, Korea; Hyun Woo Lee, Ajou University Hospital, Suwon, Korea; Min Kyoung Kim, Yeungnam University Medical Center, Daegu, Korea; Chul Soo Kim, Inha University Hospital, Incheon, Korea; JaeYong Kwak, Chonbuk National University Hospital, Jeonju, Korea; Hun-Mo Ryoo, Daegu Catholic University Medical Center, Daegu, Korea; Ho Young Kim, Hallym University Sacred Heart Hospital, Anyang, Korea; Byung Soo Kim, Korea University Anam Hospital, Seoul, Korea; Yang-Soo Kim, Kosin University Gospel Hospital, Busan, Korea; Soo-Mee Bang, Seoul National University Bundang Hospital, Bundang, Korea; Chu Myong Seong, Ewha Womans University Mokdong Hospital, Seoul, Korea; KiHyeong Lee, Chungbuk National University Hospital, Cheongju, Korea.
References
- 1.Leone G, Teofili L, Voso MT, Lubbert M. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica. 2002;87(12):1324–41. [PubMed] [Google Scholar]
- 2.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 3.Herman JG. Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol. 1999;9(5):359–67. doi: 10.1006/scbi.1999.0138. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 5.Mund C, Hackanson B, Stresemann C, Lubbert M, Lyko F. Characterization of DNA demethylation effects induced by 5-Aza-2′-deoxycytidine in patients with myelodysplastic syndrome. Cancer Res. 2005;65(16):7086–90. doi: 10.1158/0008-5472.CAN-05-0695. [DOI] [PubMed] [Google Scholar]
- 6.Saba HI. Decitabine in the treatment of myelodysplastic syndromes. Ther Clin Risk Manag. 2007;3(5):807–17. [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto A, Zagonel V. 5-Aza-2′-deoxycytidine (Decitabine) and 5-azacytidine in the treatment of acute myeloid leukemias and myelodysplastic syndromes: past, present and future trends. Leukemia. 1993;7(Suppl 1):51–60. [PubMed] [Google Scholar]
- 8.Wijermans PW, Krulder JW, Huijgens PC, Neve P. Continuous infusion of low-dose 5-Aza-2′-deoxycytidine in elderly patients with high-risk myelodysplastic syndrome. Leukemia. 1997;11(1):1–5. doi: 10.1038/sj.leu.2400526. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 10.Wijermans P, Lubbert M, Verhoef G, Bosly A, Ravoet C, Andre M, et al. Low-dose 5-aza-2′-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18(5):956–62. doi: 10.1200/JCO.2000.18.5.956. [DOI] [PubMed] [Google Scholar]
- 11.Wijermans P, Suciu S, Baila L, Platzbecker U, Giagounidis A, Selleslag D, et al. Low Dose Decitabine Versus Best Supportive Care in Elderly Patients with Intermediate or High Risk MDS Not Eligible for Intensive Chemotherapy: Final Results of the Randomized Phase III Study (06011) of the EORTC Leukemia and German MDS Study Groups. Blood. 2008;112(11) Abstract 226. [Google Scholar]
- 12.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 13.Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27(23):3842–8. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Lee KH, Kim DY, Kim SH, Lim SN, Kim SD, et al. Decreased incidence of febrile episodes with antibiotic prophylaxis in the treatment of decitabine for myelodysplastic syndrome. Leuk Res. 2011;35(4):499–503. doi: 10.1016/j.leukres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24):3913–5. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 18.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons RM, Cosgriff TM, Modi SS, Gersh RH, Hainsworth JD, Cohn AL, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850–6. doi: 10.1200/JCO.2008.17.1058. [DOI] [PubMed] [Google Scholar]
- 20.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895–903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 22.Muller-Berndorff H, Haas PS, Kunzmann R, Schulte-Monting J, Lubbert M. Comparison of five prognostic scoring systems, the French-American-British (FAB) and World Health Organization (WHO) classifications in patients with myelodysplastic syndromes: Results of a single-center analysis. Ann Hematol. 2006;85(8):502–13. doi: 10.1007/s00277-005-0030-z. [DOI] [PubMed] [Google Scholar]
- 23.Navarro I, Ruiz MA, Cabello A, Collado R, Ferrer R, Hueso J, et al. Classification and scoring systems in myelodysplastic syndromes: a retrospective analysis of 311 patients. Leuk Res. 2006;30(8):971–7. doi: 10.1016/j.leukres.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Shin YR, Lee JS, Kim WK, Chi HS, Park CJ, et al. Application of different prognostic scoring systems and comparison of the FAB and WHO classifications in Korean patients with myelodysplastic syndrome. Leukemia. 2003;17(2):305–13. doi: 10.1038/sj.leu.2402798. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda A, Germing U, Jinnai I, Misumi M, Kuendgen A, Knipp S, et al. Difference in clinical features between Japanese and German patients with refractory anemia in myelodysplastic syndromes. Blood. 2005;106(8):2633–40. doi: 10.1182/blood-2005-01-0040. [DOI] [PubMed] [Google Scholar]
- 26.Wang XQ, Ryder J, Gross SA, Lin G, Irons RD. Prospective analysis of clinical and cytogenetic features of 435 cases of MDS diagnosed using the WHO (2001) classification: a prognostic scoring system for predicting survival in RCMD. Int J Hematol. 2009;90(3):361–9. doi: 10.1007/s12185-009-0403-5. [DOI] [PubMed] [Google Scholar]
- 27.Geils GF. Clnical experience of decitabine in elderly patients with myelodysplastic syndrome (MDS) Blood. 2009;114(22) Abstract 2792. [Google Scholar]
- 28.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–4. [PubMed] [Google Scholar]