Abstract
The ability of IFN-γ to enhance graft-versus-leukemia (GVL) activity without direct interaction with leukemia cells was examined by comparing GVL effects against IFN-γ receptor-deficient (GRKO) leukemia between wild-type (WT) and IFN-γ–deficient (GKO) allogeneic hematopoietic cell transplantation (allo-HCT). We established a primary IFN-γ–unresponsive T-cell leukemia model using virally-transduced GRKO B6 mouse bone marrow cells overexpressing Notch1. We first assessed GVL effects in lethally-irradiated B6 mice receiving CD4-depleted allo-HCT from WT or GKO BALB/c donors. Administration of CD4+ cell-depleted allo-HCT from WT, but not GKO, BALB/c donors mediated significant GVL effects against GRKO leukemia. Similar results were obtained in pre-established allogeneic chimeras receiving delayed donor lymphocyte infusion (DLI). Although both WT and GKO DLI achieved significant anti-tumor responses, the former was markedly stronger than the latter. These data indicate that IFN-γ is capable of promoting GVL effects via mechanisms independent of its interaction with leukemia cells.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) remains a major therapy used in the treatment of leukemia patients.1,2 However, its broader clinical application has been limited by a high incidence of GVHD. IFN-γ has been shown to inhibit GVHD, while mediating graft-versus-leukemia (GVL) effects.3–6 Multiple mechanisms were found to contribute to the down-regulation of GVHD by IFN-γ, including stimulating apoptosis and inhibiting cell division of alloreactive donor T cells,7 and preventing tissue damage through interaction with recipient parenchymal cells.8
IFN-γ is known to mediate anti-tumor effects through interaction with IFN-γ receptor (IFN-γR) on tumor cells. IFN-γ signaling in tumor cells inhibits tumor cell expansion by inducing apoptosis and suppressing proliferation, and sensitizes tumor cells to cytotoxic T cells by up-regulating the expression of Fas and MHC molecues.9 These studies indicated that the interaction between IFN-γ and leukemia cells is likely to play an important role in IFN-γ–mediated anti-leukemia effects in allo-HCT recipients. However, it remains unknown whether induction of GVL effects by IFN-γ depends on its signaling in leukemia cells. It has been reported that T cells may mediate anti-tumor effects by producing IFN-γ to inhibit tumor angiogenesis.10 In the present study, we established an IFN-γR–deficient mouse primary leukemia model to determine whether IFN-γ can promote GVL effects in the absence of its interaction with leukemia cells.
Methods
Lin−Sca1+ bone marrow cells (BMCs) were prepared from IFN-γR KO (GRKO) C57BL/6 (B6) mice, transduced with Notch-1 retroviruses (MSCV-ICN/GFP),11 and injected into lethally-irradiated GRKO syngeneic mice to establish IFN-γ–unresponsive leukemia. We explored the effect of IFN-γ on GVL responses against GRKO leukemia in 3 allo-HCT models. In the first 2 models, lethally-irradiated recipient mice received allogeneic BMCs plus unfractionated or CD4-depleted splenocytes from either wild-type (WT) or IFN-γ KO (GKO) donors. The third model involved delayed donor lymphocyte infusion (DLI) in pre-established mixed allogeneic chimeras. Detailed descriptions of all materials and methods can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Protocols involving animals used in this study were approved by the Massachusetts General Hospital Subcommittee of Research Animal Care.
Results and discussion
Development and characterization of an IFN-γ–unresponsive leukemia model
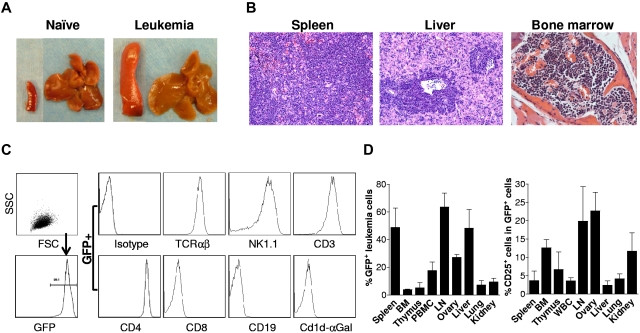
Previous studies have shown that overexpression of the intracellular domain of Notch1 (ICN1) in hematopoietic stem cells results the development of T-cell acute lymphoblastic leukemia (T-ALL).12,13 Most GRKO mice receiving Notch1-transduced GRKO BMCs developed leukemia and became moribund ∼ 7-10 weeks after transplantation (Figure 1A-B). We then expanded the leukemia cells by adoptive cell transfer into syngeneic mice, and cryopreserved the resultant leukemia cell pool (ie, splenocytes with > 95% of GFP+ leukemia cells; Figure 1C) in liquid nitrogen until use. Flow cytometric analysis revealed that the GRKO leukemia cells express TCRαβ, NK1.1, CD3, CD4, but are stained negative for CD8, CD19, and CD1d tetramers loaded with α-galactosylceramide (Figure 1C), indicating that these are T-cell leukemia cells with a CD1d-independent NKT-like cell phenotype. Injection of 5 × 106 GRKO leukemia cells into naive B6 mice resulted in leukemia in all mice. GFP+ leukemia cells were found in almost all tissues examined, including spleen, BM, thymus, blood, lymph nodes (LN), ovary, liver, lung, and kidney (Figure 1D). GFP+ leukemia cells from different tissues were found highly variable in CD25 expression, indicating a heterogeneity of the leukemia cells (Figure 1D).
Figure 1.
Development and characterization of GRKO leukemia. (A-B) Leukemia development in GRKO B6 mice receiving Notch1-overexpressing Lin−Sca1+ GRKO BMCs. Shown are representative spleen and liver tissues from naive and leukemia B6 mice (A) and histology (H&E) of spleen, liver and bone marrow from a representative leukemia mouse (B). (C) B6 mice were injected with leukemia cells (ie, splenocytes from mice receiving Lin−Sca1+ GRKO BMCs), killed when moribund, and GFP+ leukemia cells in the spleen were assessed by flow cytometry. Left panel indicates that the spleen consists of predominantly GFP+ leukemia cells; right panel shows the expression of various cell surface markers on gated GFP+ leukemia cells. (D) Naive B6 mice (n = 4) were injected intravenously with 5 × 106 cryopreserved GRKO leukemia cells and killed 2-3 weeks later for assessing leukemia development. Shown are percentages (mean ± SD) of GFP+ leukemia cells (left) and of CD25+ leukemia cells (right) in the indicated tissues.
IFN-γ promotes GVL activity through mechanisms independent of its interaction with leukemia
We first assessed GVL effects against GRKO leukemia in lethally-irradiated CBF1 (BALB/c × B6 F1) mice that received allo-HCT (ie, BMCs and splenocytes) from BALB/c donors. Injection of 2 × 104 GRKO leukemia cells led to death of all syngeneic HCT (syn-HCT) controls by 31 days (supplemental Figure 1). In comparison with syn-HCT–treated leukemic recipients, those receiving WT allo-HCT showed significantly prolonged survival (supplemental Figure 1; P < .005). The similar survival rates observed between WT allo-HCT recipients with or without leukemia indicate that complete eradication of leukemia had been achieved by allo-HCT. This was further confirmed by autopsy, in which none of the leukemic recipients of WT allo-HCT showed evidence of tumors (supplemental Table 1). However, the early death caused by GVHD in GKO allo-HCT recipients prohibited assessment of the GVL potential of GKO allo-HCT. All GKO allo-HCT recipients, regardless of whether leukemia cells were injected, succumbed before the death of syn-HCT–treated leukemic recipients (supplemental Figure 1).
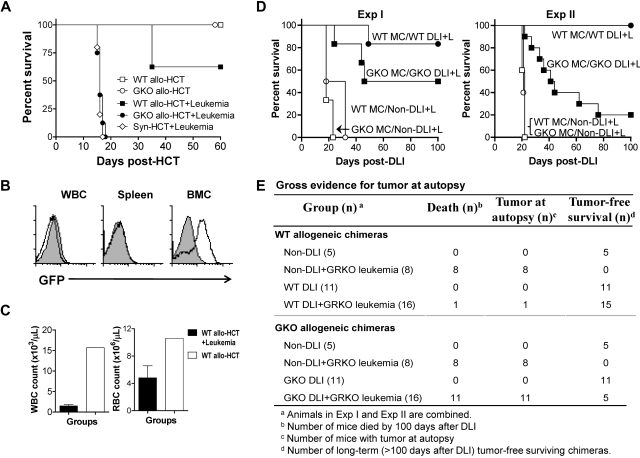
We have previously shown that in a BALB/c-to-B6 allo-HCT model, donor CD4+ cell depletion significantly reduces GVHD mortality, and allows for assessment of GKO donor CD8 T cell–mediated GVL effects.14 Thus, we next assessed GVL effects in B6 mice receiving CD4-depleted allo-HCT from WT or GKO BALB/c donors. As shown in Figure 2A, neither WT nor GKO CD4-depleted allo-HCT induced lethal GVHD in lethally-irradiated B6 recipients. CD4-depleted GKO allo-HCT failed to achieve detectable GVL effects, and all leukemic recipients of GKO allo-HCT died with a similar kinetics as the Syn-HCT controls (Figure 2A). In contrast, leukemic recipients of WT CD4-depleted allo-HCT showed significantly prolonged survival (P < .001 compared with those receiving GKO allo-HCT or Syn-HCT). The data indicate that IFN-γ may promote anti-tumor effects via mechanisms independent of its direct interaction with leukemia cells in lethally-irradiated mice receiving CD4-depleted allo-HCT. Three leukemic recipients of WT allo-HCT were killed when moribund on day 35 after HCT. Interestingly, GFP+ leukemia cells were readily detected in the BM, but hardly detectable in WBCs or spleen of these mice (Figure 2B). Furthermore, these mice had extremely low WBC and RBC counts compared with nonleukemia recipients (Figure 2C). These results indicate that BM tumor and the associated hematopoietic failure is a likely cause of death in leukemic mice receiving CD4-depleted allo-HCT.
Figure 2.
IFN-γ promotes GVL effects against GRKO leukemia. (A-C) Lethally-irradiated B6 mice received T cell–depleted (TCD) B6 BMCs (5 × 106) plus allogeneic TCD BMCs (7.5 × 106) and CD4-dep splenocytes (8.5 × 106) from WT (WT allo-HCT; n = 8) or GKO (GKO allo-HCT; n = 8) BALB/c donors. Three groups of leukemic recipients, including those receiving TCD B6 BMCs alone (Syn-HCT+Leukemia; n = 5), TCD B6 BMCs plus WT BALB/c TCD BMCs and CD4-dep splenocytes (WT allo-HCT+Leukemia; n = 7), or TCD B6 BMCs plus GKO BALB/c TCD BMCs and CD4-dep splenocytes (GKO allo-HCT+Leukemia; n = 7), were injected with 3 × 105 GRKO leukemia cells at the time of HCT. (A) Survival. (B) Flow cytometric analysis of GFP+ cells in WBCs, spleen and BM from representative leukemic (WT allo-HCT+Leukemia; open histogram) and nonleukemic (WT allo-HCT; filled histogram) mice receiving WT CD4-dep allo-HCT. (C) WBC (left) and RBC (right) counts of leukemic (WT allo-HCT+Leukemia) versus nonleukemic (WT allo-HCT) mice receiving WT CD4-dep allo-HCT. (D-E) Lethally-irradiated CBF1 mice were reconstituted with a mixture of TCD CBF1 plus WT or GKO BALB/c mouse BMCs. Eight weeks later, these BM chimeras were administered either GRKO leukemia cells (1 × 106 or 1.5 × 106 cells per mouse for Exp I and Exp II, respectively) alone or along with DLI. DLI was performed by injection of 3.5 × 107 (Exp I) or 4.5 × 107 (Exp II) splenocytes from WT or GKO B6 mice into the WT and GKO BM chimeras, respectively. (D) Survival of WT mixed chimeras that received leukemia cells alone (WT MC/NonDLI+L; ○) or along with WT DLI (WT MC/WT DLI+L; ●), and GKO mixed chimeras that received leukemia cells alone (GKO MC/NonDLI+L; □) or along with GKO DLI (GKO MC/GKO DLI+L; ■). The survival curves of the nonleukemic chimeras (with or without DLI), which all survived long-term (see panel E and supplemental Figure 2), are not shown in the figure for the sake of clarity. (E) Gross evidence for tumor at autopsy. Results from Exp I and Exp II are combined.
DLI is known to mediate strong anti-lymphohematopoietic GVH reactions (LGVHR), which preferentially eliminate recipient lymphohematopoietic cells (as shown by rapid conversion to full donor chimerism) and leukemia cells without GVHD in pre-established mixed chimeras.15–18 We recently showed that IFN-γ was able to enhance LGVHR and GVL effects, while inhibiting GVHD in mixed allogeneic chimeras after delayed DLI.6 In this study, allogeneic chimeras were prepared by injecting mixed TCD BMCs from CBF1 and WT or GKO BALB/c mice into lethally-irradiated CBF1 mice. Eight weeks later, mixed chimeras were administered GRKO leukemia cells and DLI was performed by injecting WT and GKO BALB/c splenocytes into the chimeras that had initially received, respectively, WT or GKO BALB/c BMCs. Although GKO DLI-treated leukemic chimeras showed improved survival compared with non-DLI controls (P < .005; Figure 2D), their survival rates were significantly lower than those administered WT DLI (Figure 2D/Exp II; P < .0005). Because mixed chimeras that received similar numbers of donor splenocytes without leukemia cells survived long-term, the leukemia, and not GVHD, was presumed to be the cause of death in these mice. This was further confirmed by necropsy, in which tumors were found in all leukemic recipients who had died before euthanasia (Figure 2E). GKO DLI-treated chimeras also showed significantly less efficient conversion from mixed to full donor chimerism when compared with those who were administered WT DLI (supplemental Figure 2), a finding consistent with our previous results.6 These data indicate that IFN-γ can promote GVL effects of DLI against IFN-γ-unresponsive leukemia (ie, without direct interaction with leukemia) in mixed allogeneic chimeras.
Although leukemia and tumor cell lines are substantially different from primary leukemia cells, these cell lines have been widely used to investigate GVL effects because of the lack of suitable primary leukemia models. Notch1 plays an important role in normal hematopoiesis,19–21 and its aberrant signaling is associated with T-cell leukemogenesis.12,13 The present study demonstrates that leukemia cells derived from Notch1-overexpressing BMCs may provide a more clinically-relevant primary leukemia model for evaluating GVL effects. This approach also allows for the generation of leukemia cells with genetic manipulations that may facilitate future mechanistic studies. IFN-γ is known to mediate anti-tumor effects by directly signaling through IFN-γR on tumor cells.9,22 Although mechanisms remain unclear, the present study shows that IFN-γ can also enhance GVL effects against GRKO leukemia, indicating that IFN-γ, at least in the leukemia model tested here, may mediate anti-leukemia effects through mechanisms independent of its interaction with leukemia cells. These data provide new insights into the role of IFN-γ in regulating GVHD- and GVL-associated alloreactivity of allo-HCT.
Supplementary Material
Acknowledgments
The authors thank Ms Shumei Wang for technical support and Mr Orlando Moreno for outstanding animal husbandry.
This work was supported by grants from the National Institutes of Health (5P01CA111519-020002 and RC1 HL100117 to Y.G.Y.), NSFC (81090410, 30825017 and 2011CB964800 to T.C.), and the Ministry of Health of China (a Key Clinical Program 2010 to GW). Y.Y. and T.L. are partially supported by awards from the China Scholarship Council. T.C. is a recipient of the scholar award from the Leukemia & Lymphoma Society.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.Y. designed and performed experiments, analyzed data, and drafted the manuscript; H.W. designed and performed experiments; H.Y. performed viral transduction experiments; B-Y.Y. performed statistical analysis; T.L. performed experiments; G.W. contributed to the development of the project and edited the manuscript; T.C. contributed to the development of the project, designed viral transduction experiments and edited the manuscript; and Y-G.Y. conceived the research project, designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Guang Yang, Columbia Center for Translational Immunology, Columbia University Medical Center, 630 West 168th St, Mail Box 127, New York, NY 10032; e-mail: yy2324@columbia.edu; or Guanjun Wang, First Bethune Hospital of Jilin University, 71 Xinmin Street, Changchun, Jilin, China 130021; e-mail: guanjunwang2006@163.com.
References
- 1.Blazar BR, Murphy WJ. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD). Philos Trans R Soc Lond B Biol Sci. 2005;360(1461):1747–1767. doi: 10.1098/rstb.2005.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sykes M, Spitzer TR. Non-myeloblative induction of mixed hematopoietic chimerism: application to transplantation tolerance and hematologic malignancies in experimental and clinical studies. Cancer Treat Res. 2002;110:79–99. doi: 10.1007/978-1-4615-0919-6_4. [DOI] [PubMed] [Google Scholar]
- 3.Yang YG, Dey B, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102(12):2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brok HP, Heidt PJ, Van der Meide PH, Zurcher C, Vossen JM. Interferon-gamma prevents graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Immunol. 1993;151(11):6451–6459. [PubMed] [Google Scholar]
- 5.Murphy WJ, Welniak LA, Taub DD, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102(9):1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Asavaroengchai W, Yong Yeap B, et al. Paradoxical effects of IFN-gamma in graft-versus-host disease reflect promotion of lymphohematopoietic graft-versus-host reactions and inhibition of epithelial tissue injury. Blood. 2009;113(15):3612–3619. doi: 10.1182/blood-2008-07-168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asavaroengchai W, Wang H, Wang S, et al. An essential role for IFN-gamma in regulation of alloreactive CD8 T cells following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13(1):46–55. doi: 10.1016/j.bbmt.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burman AC, Banovic T, Kuns RD, et al. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110(3):1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda H, Old LJ, Schreiber RD. The roles of IFNgamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 10.Qin Z, Schwartzkopff J, Pradera F, et al. A critical requirement of interferon-gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63(14):4095–4100. [PubMed] [Google Scholar]
- 11.Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-Induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93(3):838–848. [PubMed] [Google Scholar]
- 12.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 13.Demarest RM, Ratti F, Capobianco AJ. It's T-ALL about Notch. Oncogene. 2008;27(38):5082–5091. doi: 10.1038/onc.2008.222. [DOI] [PubMed] [Google Scholar]
- 14.Yang YG, Qi J, Wang MG, Sykes M. Donor-derived interferon gamma separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood. 2002;99(11):4207–4215. doi: 10.1182/blood.v99.11.4207. [DOI] [PubMed] [Google Scholar]
- 15.Sykes M, Sheard MA, Sachs DH. Graft-versus-host-related immunosuppression is induced in mixed chimeras by alloresponses against either host or donor lymphohematopoietic cells. J Exp Med. 1988;168(6):2391–2396. doi: 10.1084/jem.168.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelot MR, Pearson DA, Swenson K, et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based non-myeloablative conditioning regimen. Biol Blood Marrow Transplant. 1999;5(3):133–143. doi: 10.1053/bbmt.1999.v5.pm10392959. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer TR, McAfee S, Sackstein R, et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000;6(3A):309–320. doi: 10.1016/s1083-8791(00)70056-5. [DOI] [PubMed] [Google Scholar]
- 18.Mapara MY, Kim YM, Wang SP, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100(5):1903–1909. doi: 10.1182/blood-2002-01-0023. [DOI] [PubMed] [Google Scholar]
- 19.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 20.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 21.Radtke F, Wilson A, Mancini SJC, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5(3):247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 22.Messingham KAN, Badovinac VP, Jabbari A, Harty JT. A Role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to listeria monocytogenes. J Immunol. 2007;179(4):2457–2466. doi: 10.4049/jimmunol.179.4.2457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.