Abstract
Our previous studies showed that treatment of mice with total body irradiation (TBI) or total lymphoid tissue irradiation (TLI) markedly changes the balance of residual T cell subsets to favor CD4+CD44hi natural killer T (NKT) cells due to differential resistance of the latter subset to cell death. The object of the current study was to further elucidate the changed balance and mechanisms of differential radioresistance of T cell subsets after graded doses of TBI. The experimental results show that CD4+ T cells were markedly more resistant than CD8+ T cells, and CD44hi T cells including NKT cells and memory T cells were markedly more resistant than CD44lo (naïve) T cells. The memory T cells immunized to alloantigens persisted even after myeloabloative (1,000cGy) TBI, and were able to prevent engraftment of bone marrow transplants. Although T cell death after 1,000cGy was prevented in p53−/− mice, there was progressive T cell death in p53−/− mice at higher doses. Whereas, p53 dependent T cell death changed the balance of subsets, the p53 independent T cell death did not. In conclusion, resistance of CD44hi T cells to p53 dependent cell death results in the persistence of immunological memory after TBI, and can explain the immune mediated rejection of marrow transplants in sensitized recipients.
Keywords: Memory T cells persistence, Irradiation, Bone Marrow Transplantation
Introduction
In vivo irradiation is used as a standard therapy for treating many human cancers, and as a conditioning regimen for bone marrow transplantation. Total body irradiation (TBI) prevents rejection of allogeneic donor cells by residual host T cells after bone marrow transplantation(1, 2), and the engrafted donor T cells mediate graft anti-tumor activity, and tumor cell eradication (3–6). However, the risk of failure of engraftment is increased in recipients that are highly sensitized to alloantigens by multiple transfusions (7, 8).
The effects of TBI on immunity have been studied extensively in preclinical and clinical studies, and profound lymphodepletion due to the marked sensitivity of lymphocytes to irradiation is well known. TBI also causes activation of antigen presenting cells, and can augment innate immunity and the anti-tumor function of adoptively transferred T cells directed to tumor antigens (9, 10). Recent studies have shown that the depletion of T cell subsets by TBI or total lymphoid irradiation (TLI) is not uniform, and is instead selective due to the differential sensitivity of the subsets to irradiation induced cell death(9, 11, 12). The selective depletion results in an altered balance of T cell subsets after non-myeloablative or myeloablative irradiation favoring regulatory T cells including CD4+CD44hi natural killer T (NKT) cells (9, 11). The acceptance of allogeneic bone marrow transplants after non-myeloablative TBI or TLI is not due to lymphodepletion alone, since acceptance is dependent upon the presence of residual NKT cells that suppress alloreactivity of residual host conventional T cells (13, 14).
The selective effect of irradiation on T cell subsets is in part explained by their differential expression of anti-apoptotic proteins. The NKT cell subset in mice has been reported to express high levels of anti-apoptotic proteins such as Bcl-2 constitutively, and to upregulate these proteins after exposure to irradiation or to glucocorticoids (12, 15, 16). The NKT cell subset, which is a minority subset accounting for 2–3% of all T cells in untreated mice, becomes the dominant subset after high doses of either TBI or TLI(12, 13, 17). Although NKT cells are a considerably lower percentage of all T cells in humans as compared to mice, the percentage increases markedly after TLI and anti-thymocyte globulin (ATG) conditioning in humans given allogeneic hematopoietic cell transplants (18).
The object of the current study was to further elucidate the mechanisms underlying the changes in the balance of a broad range of T cell subsets after TBI including CD4+ and CD8+ T cells, CD4−CD8− T cells, CD44lo naive T cells, and CD44hi T cells that include memory T cells and NKT cells. An additional goal was to determine whether radioresistant memory T cells immunized to donor alloantigens can prevent engraftment of allogeneic bone marrow transplants after myeloablative TBI. The role of p53 in the changed balance was investigated by comparing p53−/− to wild type mice. p53 is known to be activated by irradiation induced damage of DNA and to initiate the p53/Bcl-2 apoptotic pathway that results in DNA fragmentation and cell death (19–21). Accordingly, we studied the radiation sensitivity of T cell subsets expressing low and high levels of intracellular Bcl-2 in untreated mice and after irradiation.
The results of the study show that up to 1,000cGy TBI, T cell death was dependent on the expression of the p53 gene, and that at higher doses of TBI cell death occurred in the absence of the p53 gene expression. Whereas, p53 dependent T cell death resulted in a changed balance of T cell subsets favoring the CD44hi T cells, p53 independent T cell death did not change the balance. CD44hi memory T cells immunized to alloantigens persisted even after myeloablative (1,000cGy) TBI in mice with an intact p53 gene, and were able to prevent engraftment of bone marrow transplants.
Materials and Methods
Animals
Eight to 10 week old male C57BL/6 wild type mice, and 8 week old male C57BL/6 mice that were homozygotic for the inactivated p53 gene (p53−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal protocols were reviewed and approved by the Stanford Administrative Panels on Laboratory Animal Care (APLAC).
Irradiation
TBI was administered to wild type C57BL/6 or p53−/− mice 24, 48, 72 or 120 hrs before analysis of spleen T ell subsets. TBI was performed with a Philips x-ray unit (200 kV, 10mA; Philips Electronic Instruments, Rahway, NJ) at a rate of 84 cGy/min with a 0.5 mm Cu filter, and delivered as a single dose.
Monoclonal antibodies and chemical reagents
Anti-CD44-FITC, anti-NK1.1-PE, anti-TCRαβ-APC, anti-CD4-Cy7PE, anti-CD8-Cy7APC, anti-B220-Cy7APC, anti-Mac1-PE, anti-Gr1-PE, anti-H-2Kd-FITC and anti-CD25-Cy7APC mAbs as well as the FITC-conjugated anti-Bcl-2 antibody reagent kit, BD cytofix/cytoperm kit, and anti-CD16/32 mAb were purchased from BD PharMingen (San Diego, CA). FITC conjugated anti-Foxp3 mAb was purchased from eBioscience (San Diego, CA). Pan T cell isolation kit II was purchased from Miltenyi Biotec (Auburn, CA)
Immunization to alloantigens
Five-week-old male C57BL/6 mice were immunized by i.p. injection of 50 × 106 splenocytes from age matched male BALB/c mice followed by a dose of 50 × 106 splenocytes one week later. Four weeks after immunization, immunized C57BL/6 mice and the splenocytes from these mice were used for further studies.
Cell preparations
For preparation of splenic T cells, single-cell suspensions from mice spleens were filtered through Nitex membranes. Suspensions were stained with cocktail of biotin-conjugated monoclonal antibodies against CD11b, CD11c, CD19, CD45R (B220), CD49b (DX5), CD105, Anti-MHC class II, and Ter-119 and monoclonal anti-biotin antibodies (Miltenyi Biotec, Pan T cell Isolation Kit II), and passed over two consecutive MACS LS-separation columns (Miltenyi Biotec). The purity of T cell preparation was determined by staining with anti-TCR-APC and analyzed by flow cytometry.
Adoptive immunized T cell transfer and bone marrow transplantation
In brief, immunized C57BL/6 mice were lethally irradiated (1,000 cGy) from a 200 Kv x-ray source. Immediately after, splenic T cells were purified from these irradiated immunized mice and 5×106 were injected into syngenic C57BL/6 hosts via tail vein. Hosts were also lethally irradiated (1,000 cGy) within 2 h before injection. Control irradiated C57BL/6 host mice received the same number of purified splenic T cells from non-immunized wild type C57BL/6 mice. The next day, all C57BL/6 hosts given the adoptive T cell transfer from immunized or non-immunized syngeneic mice were injected with 5×106 donor bone marrow cells from BALB/c mice via tail vein. In additional control groups, mice were given TBI 1,000 cGy only, or TBI 1,000 cGy and bone marrow transplant within 24 hours (5×106 cells/mouse, i.v.), or TBI 1,000 cGy and a bone marrow transplant after two i.p. injections of BALB/c splenocytes (50 ×106 cells, i.p. on day -35 and -28 before bone marrow transplantation). Mice were kept on antibiotic water (25g/ml neomycin/0.3 U/ml polymyxin B; Sigma-Aldrich) for the first 28 days. Survival was monitored daily and body weight was measured weekly and chimerism was measured in the blood at 4 weeks after transplantation.
Immunofluorescent staining and flow cytometry analysis
Single spleen cell suspensions lysed with ammonium chloride buffer were prepared in phosphate-buffered saline (PBS) with 1% calf serum, pre-incubated with anti-CD16/32 mAb to prevent non-specific binding via FcRII/III interactions, and then incubated with the appropriate mAbs as described in detail previously (22). Propidium iodide (Sigma, St Louis, MO) was added prior to analysis to exclude dead cells. To analyze Bcl-2 intracellular expression, all cells were first incubated with the appropriate anti-surface receptor mAbs, then fixed and permeabilized with BD cytofix/cytoperm kit for intracellular staining. Ethidium monoazide bromide (EMA) (Invitrogen, Carlsbad, CA) was added prior to fixation and permeabilization to exclude dead cells. Thresholds for Bcl-2 staining were determined by using isotype-matched irrelevant mAb. All analyses were performed on a modified dual laser LSRScan (BD Immunocytometry Systems, San Diego, CA) in the Shared FACS Facility (Center for Molecular and Genetic Medicine at Stanford University), using FlowJo software (TreeStar, Ashland, OR) for data analysis as described before (12).
Statistical analysis
Difference in percent and absolute number of immunophenotypic populations of cells were analyzed using with two-tailed Student’s t test using Prism software (GraphPad Software, San Diego, CA). For all tests, p values of 0.05 or less were considered significant.
Results
TBI changes the T cell subset balance in wild type mice such that CD44hi memory T cells and NKT cells become predominant
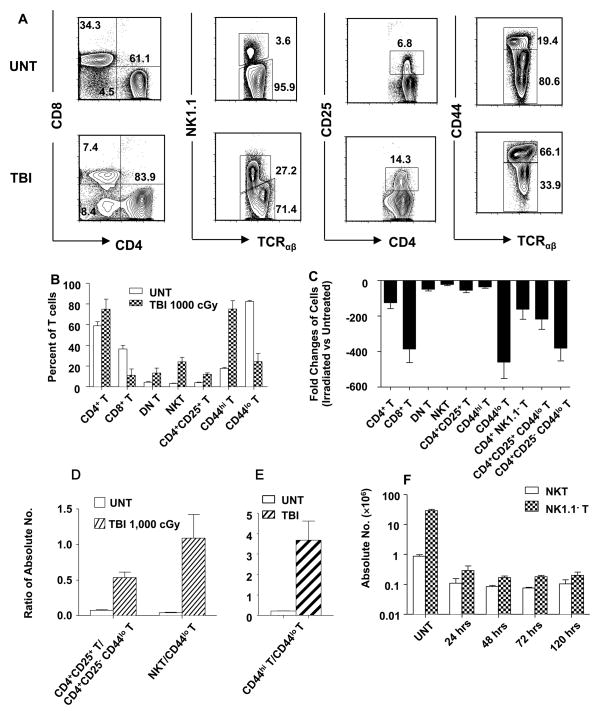
In order to determine the effect of in vivo irradiation on the balance of T cell subsets, groups of wild type male C57BL/6 adult mice were given an approximate 10 fold range of single doses of non-myeloablative (240 and 480 cGy), and myeloablative (1,000, 2,000, and 3,000 cGy) TBI. Spleen cells were stained for subset surface markers 24, 48, 72 and 120 hours later. Figure 1A shows representative examples of two color flow cytometric analyses of gated TCRαβ+ T cells in the spleens of untreated mice and irradiated (1,000 cGy TBI) mice after 24 hours. There was a modest increase in the percentage of total CD4+ T cells from about 61% to 84% after TBI, and an associated decrease in the percentage of total CD8+ T cells from about 34% to 7%. This resulted in a change in the CD4+: CD8+ T cell ratio from about 2:1 to about 11:1. The mean CD4+ and CD8+ T cell percentages of groups of mice are shown in Figure 1B at 24 hours, and there were minimal further changes at 48, 72, and 120 hours (Figure 1F). Changes in T cell subsets in the spleen have been shown to be reflected in the bone marrow and liver previously (12). Yield of T cells in the lymph nodes and blood after TBI were too low to analyze accurately (data not shown).
Figure 1. Effect of Irradiation on different T cell subsets in wild type C57BL/6 mice.
(A) Gated TCRαβ+ spleen cells in untreated wild type mice (upper row) or 24 hours after 1,000 cGy of TBI (lower row), were analyzed by flow cytometry for CD4 versus CD8, NK1.1 versus TCRαβ and CD44 versus TCRαβ. Gated CD4+ TCRαβ+ cells were also analyzed for CD4 versus CD25. (B) The mean percentage of CD4+, CD8+, CD4− CD8−(DN), NK1.1+, CD4+CD25+, CD44hi, and CD44lo TCRαβ+ cells in the spleen are shown by the bars (brackets show standard deviations) before and 24hr after irradiation. There are six to ten mice in each group. The data are representative of three independent experiments. P values comparing untreated and irradiated mice means are: CD4+ (p=0.004), CD8+ (p<0.0001), DN (p=0.001), NKT (p<0.0001), CD4+CD25+ (p<0.0001), CD44hi (p<0.0001), CD44lo (p<0.0001). (C) Mean fold change (ratio of irradiated versus untreated) (±SD) in absolute numbers of different T-cell subsets before and 24 hr after TBI. CD4+ T versus CD4+NK1.1− T (p >0.05), CD4+CD25+ T versus CD4+CD25+CD44lo T (p <0.05), CD4+CD25+CD44lo versus CD4+CD25− CD44lo (p<0.05). (D) Mean ratios of absolute numbers of CD4+CD25+ Treg cells versus CD44lo CD4+CD25− T cells (CD4+ naïve non-Treg), and NKT versus total CD44lo T cells before and 24 hr after TBI. (F) Mean absolute numbers (±SD) of NKT and NK1.1− T cells at different time points after TBI (p<0.0001) for NKT and NK1.1− T at 24 hrs versus untreated. p>0.05 at 24 versus 48, 72, and 120 hrs.
The increase in the percentage of CD4+ T cells is explained by the greater decrease in absolute number of CD8+ T cells than CD4+ T cells as shown in Figure 1C. Whereas the absolute number of total CD4+ T cells decreased by about 100 fold from about 15 × 106 per spleen to about 0.15 × 106 (p<0.0001), the absolute number of CD8+ T cells decreased by about 400 fold from about 7× 106 to about 0.02 × 106 (p<0.0001). The greater resistance of CD4+ T cells to radiation induced cell death was observed even when CD4+ NK1.1− T cells (CD4+ non-NKT cells) were compared to CD8+ T cells (Figure 1C). Previous studies have shown that almost all CD4+ NK1.1+ T cells constitutively express high levels of CD44, and are highly resistant to radiation induced cell death (9, 11, 12). The percentage of CD4−CD8− (DN) T cells increased after irradiation from about 4% to 8% (Figure 1A and B), and the percentage of CD8+ T cells was not significantly different from DN T cells (p>0.05) in irradiated mice. Again, the change in the balance of DN and CD8+ T cells is explained by the greater loss in the absolute number of CD8+ T cells (Figure 1C).
After irradiation, there was almost a seven fold increase in the percentage of NKT cells (NK1.1+TCRαβ+) among all T cells from about 4% to 27% (P<0.0001). (Figure 1A and B). The CD4+CD25+ T cell subset increased from about 7% of CD4+ T cells in untreated mice to about 14% in irradiated mice (p<0.001) because the absolute number of Treg cells decreased to a lesser extent than total CD4+ T cells (Figure 1A, B, and C). More than 80% of the CD4+CD25+ T cells were Treg cells that expressed Foxp3+ as judged by intracellular staining in untreated and irradiated mice (Supplementary Figure 1). The NKT cells and Tregs regulate alloimmunity, and can prevent rejection of allografts and graft versus host disease (13, 23–27). The relative resistance of CD44hi memory T cells to radiation induced cell death as compared that of CD44lo naïve T cells resulted in an increase of the percentage of memory T cells from about 19% to about 66% (p<0.0001) after irradiation (Figure 1A and B). Whereas the CD44hi T cells decreased about 25 fold from about 5×106 to about 0.2×106 cells per spleen after irradiation, the naïve CD44lo T cells decreased about 500 fold from about 20 ×106 to about 0.04×106 (Figure 1C).
Supplementary Figure 2 compares representative T cell subset staining from untreated mice to those given 240, 480, 1,000, 2,000, and 3,000 cGy. There was a graduated change in the balance going from 240 and 480 to that observed at 1,000 cGy. The shifting balance became more extreme at 2,000 and 3,000 cGy, and continued to change in the direction established at the lower doses. The most dramatic changes occurred in the NKT cell subset that gradually increased from about 3% of all T cells in untreated mice to 67% at 3,000 cGy, and in the CD44lo naïve T cell subset that gradually decreased from about 80% to 3% at 3,000 cGy.
Among the CD4+CD44lo T cells, there was greater resistance of the CD4+CD25+ subset to radiation induced loss as compared to the CD4+CD25− subset (p<0.01) (Figure 1C). However, the CD4+CD25+CD44hi cells rose from 33% to about 80% of total CD4+CD25+ T cells after 1,000 cGy due to their greater resistance to loss than CD4+CD25+CD44lo cells (Supplementary Figure 3).
The changes in the balance of splenic regulatory and naïve T cell subsets are apparent by comparing their ratios in untreated versus irradiated mice as shown in Figure 1D and E. Whereas the ratio of CD4+ Treg to naïve CD4+CD25− CD44lo T cells was about 1:13 in untreated mice, the ratio after irradiation was about 1:2 (p<0.0001). Similarly, the ratio of to NKT to naïve non-NKT cells increased from about 1:25 to about 1:1. Since both CD4+ CD25+ T cells and NKT cells are regulatory T cells that can suppress the immune function of conventional naïve T cells (17, 23–27), it is clear that changes in immune function after irradiation especially in the context of allogeneic bone marrow transplantation must take into account both depletion of absolute numbers as well as changes in subset balance. The overall ratio of CD44hi to CD44lo T cells in untreated mice changed from about 1:4 in untreated mice to about 4:1 in irradiated mice (Figure 1E). The changed ratio was similar even when CD44hi and CD44lo non-NKT cells were compared (data not shown). In summary, the combination of NKT cells and memory T cells (both expressing high levels of CD44) accounted for about 80% of splenic T cells after irradiation, and about 20% before irradiation due to their relative radioresistance.
Memory T cells that persist after TBI can reject bone marrow transplants
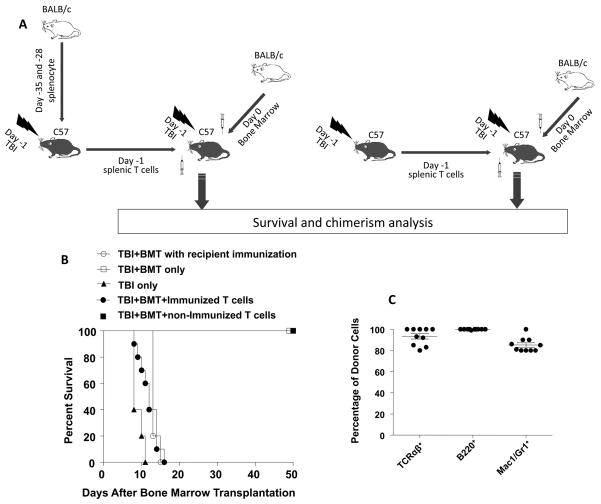
Myeloablative and non-myeloabative TBI has been used in humans to condition recipients of hematopoietic cell transplants for the treatment hematologic malignancies and hemaglobinopathies (1–6). In the case of the hemaglobinopathies and aplastic anemia, it is well known that multiple transfusions given prior to transplantation increases the risk of transplant rejection (7, 8). The rejection is thought to be mediated by radioresistant memory T cells to alloantigens. In order to determine whether memory T cells that per persist after myeloablative TBI (1,000 cGy) in the current study are functional, we developed a bone marrow transplant model in which the C57BL/6 (H-2Kb) recipients conditioned with TBI were rescued with bone marrow transplants from BALB/c (H-2Kd) donor mice. Within 24 hours after TBI, recipients were given an intravenous injection of 5×106 bone marrow cells from the donors, and chimerism of peripheral blood cells and survival were monitored.
In the first series of experiments, conditioned recipients were given either no bone marrow cells, bone marrow cells without recipient immunization, or bone marrow cells after recipient immunization to BALB/c alloantigens by two intraperitoneal injections of 50×106 BALB/c spleen cells (Figure 2B). As expected the recipients given TBI alone all died with marrow aplasia by 12 days, and the unimmunized recipients given TBI and marrow transplants all survived 50 days. The latter recipients had more that 90% donor type cells among blood mononuclear cells as judged by staining for expression of the donor type, H-2Kd receptors at day 28, and subsequent analysis by flow cytometry (data not shown). The immunized recipients failed to engraft, and all died by 14 days (Figure 2B).
Figure 2. Memory T cells that persist after TBI are functional and can reject the graft after allogenic bone marrow transplantation.
(A) Experimental scheme: C57BL/6 mice were immunized with 50 ×106 BALB/c splenocytes i.p. on day -35 and day -28 before i.v. transfer to C57BL/6 bone marrow transplant recipients on day -1. Splenic T cells were harvested and purified from immunized mice 2 hr after a single dose of 1,000cGy TBI. Purified T cells were injected into recipient mice immediately after they were given TBI 1000 cGy. On day 0, recipient mice (●, N=10) received a bone marrow transplant (BMT) (5×106 cells/mouse, i.v.) from allogenic BALB/c mice. Some C57BL/6 hosts (■, N=10) were given the same number of splenic T cells (5×106 cells/mouse, i.v.) from unimmunized C57BL/6 mice before bone marrow transplantation. Control groups were given TBI 1,000 cGy only (▲, N=5), or TBI 1,000 cGy and BMT only (□, N=5) or TBI 1,000 cGy and BMT (5×106/mouse, i.v.) after 2 i.p. injections of BALB/c splenocytes (○, N=5) (50 ×106 cells/mouse, i.p. on day -35 and -28 before bone marrow transplantation). (B) Survival of C57BL/6 recipients after TBI 1,000 cGy and bone marrow transplantation with or without immunization of recipients or with adoptive transferred T cells from immunized or non-immunized syngeneic mice. (C) Peripheral blood chimerism in the recipients at 4 weeks after transplantation in the group given non-immunized T cells. The mean percentage of donor type cells among cells in the blood are shown. Horizontal lines show means, and brackets show standard errors.
In order to determine whether C57BL/6 radioresistant memory T cells can prevent engraftment of donor BALB/c marrow transplants, the experimental scheme shown in Figure 2A was used. In this series of experiments, the C57BL/6 recipients were given bone marrow transplants from BALB/c donors on day 0, and an intravenous injection of purified splenic T cells (5×106) on day-1 from C57BL/6 donor mice that were unimmunized or that were immunized to BALB/c alloantigens by 2 injections of BALB/c spleen cells. Just before the harvest of the spleen cells, the C57BL/6 donor mice were given TBI (1,000 cGy) such that only radioresistant T cells transferred to the marrow recipients would persist. As shown in Figure 2C, the marrow transplant recipients injected with the unimmunized T cells accepted the transplants, and survived at least 50 days. The latter recipients developed high levels of chimerism (>85% donor type cells) among TCR+ T cells, B220+ B cells, and Mac-1+/Gr-1+ monocytes and granulocytes in the blood as judged by immunofluorescent staining on day 28(Figure 2C). In contrast, the marrow transplant recipients injected with the immunized T cells failed to engraft, and all died by day16.
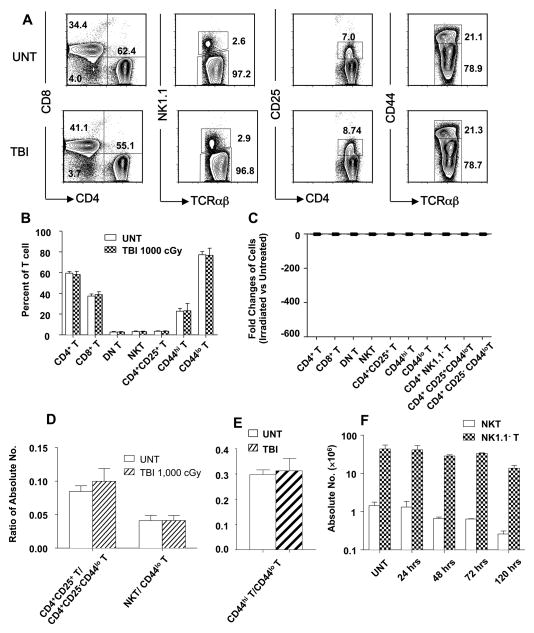
TBI (1,000 cGy) fails to change the balance of T cell subsets in p53−/− mice
Since p53 is a key molecular controller of the p53/Bcl-2 apoptotic pathway (19–21), the effect of p53 gene inactivation on the changes in the balance of T cell subsets after in vivo irradiation was studied using p53−/− C57BL/6 mice. Figure 3A and B show that, in striking contract to the changes observed 24 hrs after irradiation of 1,000 cGy TBI in wild type mice, the same dose of irradiation failed to induce the significant differences in the mean percentages of CD4+ versus CD8+ T cells, NKT cells versus all T cells, CD4+ CD25+ T cells versus all CD4+ T cells, and CD44hi T cells among all T cells (p>0.05 for all comparisons). The lack of significant change in the balance of T cell subsets is explained by the lack of significant differences (less than 2 fold) between the absolute numbers of each T cell subset in untreated p53−/− mice versus irradiated p53−/− mice (p>0.05) examined at 24 hours after 1,000 cGy TBI (Figure 3C). As expected, the lack of significant changes in the absolute numbers of the T cell subsets after 1,000 cGy TBI in p53−/− mice resulted in no significant difference (p>0.05) in the ratios of CD4+ naïve versus CD4+CD25+ T cells, naïve T versus NKT cells, or CD44lo versus CD44hi T cells at 24, 48, and 72 hours (Figure 3D and E). When p53−/− mice were examined at 120 hours after 1,000 cGy TBI, then a significant reduction in the absolute number of both NKT cells (p<0.01) and non-NKT cells (p<0.01) was observed as compared to untreated mice (Figure 1F). However, the reduction in both cases was less than tenfold, and did not result in a significant change in the balance of subsets (p>0.05). The results indicate that there are mild p53 independent contributions to radiation induced cell death that were delayed as compared to p53 dependent contributions.
Figure 3. Effect of Irradiation on different T cell subsets in p53−/− C57BL/6 mice.
(A) Gated TCRαβ+ cells in untreated p53−/− mice (upper row) or in p53−/− mice 24 hours after 1,000 cGy of TBI (lower row) were analyzed for CD4 versus CD8, NK1.1 versus TCRαβ and CD44 versus TCRαβ, gated CD4+ TCRαβ+ cells were analyzed for CD4 versus CD25 also. (B) The mean percentages of CD4+, CD8+, NK1.1+, CD4+CD25+, CD44hi, and CD44lo TCRαβ+cells are shown. P value comparing untreated and irradiated mice means were >0.05 for all groups. (C) Mean fold change in ratio of absolute numbers of different T-cell subsets before and after TBI. All changes were less than 2 fold (p>0.05). (D) Mean ratios of absolute numbers of CD4+CD25+ T versus CD44lo CD4+CD25− T cells (CD4+ naïve), and NKT versus total naïve CD44lo T cells before and after TBI. P>0.05 for all groups. (E) Mean ratios of absolute numbers of CD44hi T versus CD44lo T cells before and after TBI (p>0.05). (F) Comparison of mean (±SD) absolute numbers of NKT and NK1.1− T cells at different time points. P>0.05 for untreated versus irradiated mice at 24, 48, 72 hrs. P<0.05 for NKT and NK1.1− T of untreated versus 120 hrs. There are six to ten mice in each group. The data are representative of three independent experiments.
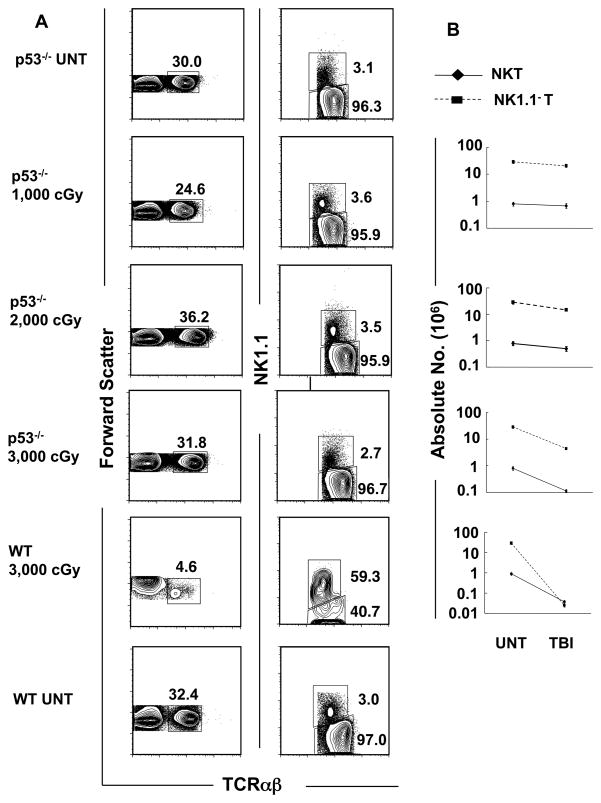
Supralethal TBI induces T cell death but does not change the balance of T cell subsets in p53−/− mice
In further experiments, p53−/− mice were given 2,000 and 3,000 cGy TBI. The effects on the absolute numbers of T cells, and T cell subset balance was studied. The mean absolute numbers of NKT cells and non-NKT cells in the spleen were not significantly different (p>0.05) between untreated mice and those irradiated with 1,000 or 2,000cGy TBI (Figure 4A and B). However, there was about a tenfold reduction in both types of T cells after 3,000 cGy as compared with untreated p53−/− mice (p<0.05 for both NKT and non-NKT cells) (Figure 4B). Since this p53 independent cell loss induced by radiation was similar in both subsets of T cells, their percentages among total T cells did not change after TBI compared to untreated mice.
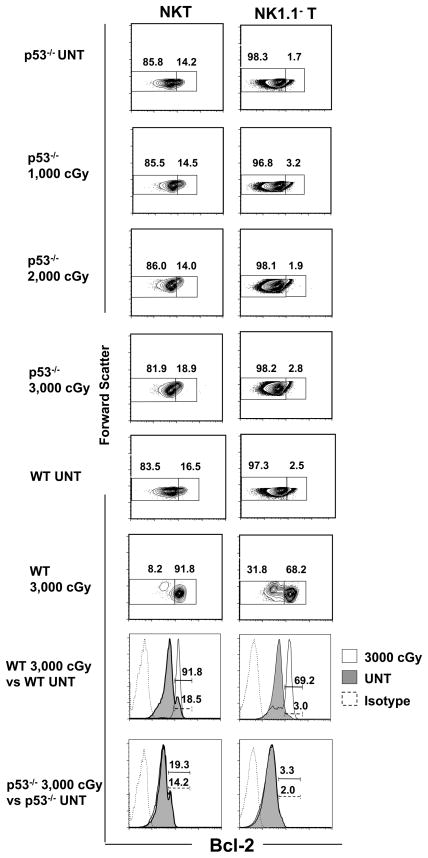
Figure 4. The NKT cell subset fails to become predominant among all T cells after TBI in p53−/− mice.
(A) Upper panels show representative flow cytometric analyses of staining for TCRαβ versus forward scatter or NK1.1 versus TCRαβ on gated TCRαβ+ cells in untreated p53−/− mice or 24hrs after 1000, 2000 or 3000 cGy of TBI. The bottom panels show similar analyses in untreated wild type (WT) mice or 24 hours after 3000 cGy of TBI for comparison. Boxes enclose TCRαβ+ T or NK1.1+ TCRαβ+ cells. The mean percentage ± SD of NKT cells among all T cells in the spleen of untreated mice was 2.8 ± 0.4, and 3.2 ± 0.4; p>0.05, 3.2 ± 0.5; p>0.05, and 2.5 ± 0.4; p>0.05 in mice given 1,000, 2,000, 3,000 cGy respectively. (B) The mean (±SD) absolute numbers of NK T cells and NK1.1− T cells (non-NKT cells) in the spleen of untreated mice and after each dose of irradiation are shown on a logarithmic scale. There are 5 to 10 mice per group. The data are representative of three independent experiments.
The percentage of total T cells among the spleen cells from the untreated p53−/− mice was about 30%, and the percentage after 1,000 to 3,000 cGy was in the range of about 25% to 32% (mean 31% ± 4, p>0.05). Similarly, the percentage of NKT cells among all T cells was about 3% in untreated mice and was in the range of 2% to 3% after 3,000 cGy (mean 3% ± 0.4, p>0.05). The lack of change was in sharp contrast with the results in wild type mice, since 3,000 cGy induced a decrease in the percentage of total T cells from about 30% to about 5% and an increase in the percentage of NKT cells among all T cells to about 59% (Figure 4A). Figure 4B shows that the absolute number of non-NKT cells fell about 50 times more than the NKT cells in the wild type mice given 3,000 cGy TBI (mean fold reduction 52 ± 9, p<0.001), and resulted in the major change in the balance of subsets. The results indicate that the p53 independent killing that is likely due to breaks in the double stranded DNA (28) causes little or no change in the percentage of total T cells in the spleen or in the balance of the T cell subsets, whereas the p53 dependent killing causes a marked change in the percentage of total T cells and in the balance.
Increased expression of Bcl-2 in NKT and non-NKT cells after irradiation is p53 dependent
The intracellular expression of Bcl-2 in T cell subsets was compared in untreated wild type and p53−/− mice and in both types of mice given graded doses of TBI. Figure 5 (bottom 2 panels) shows representative one color staining for Bcl-2 in gated NK1.1− TCRαβ+ and NK1.1+ TCRαβ+ T cells. In the wild type untreated mice, two peaks of staining were observed in the NKT cells; a large dull peak, and a small bright peak that accounted for about 18% (mean 18% ± 3%, n=10) of cells. Both peaks were clearly brighter than the isotype control staining. After administration of 3,000 cGy TBI, there was a dramatic shift to brighter staining, and about 92% (mean 85% ± 8%, n=10) of cells were contained in a single bright peak. The pattern observed with non-NKT cells in untreated mice failed to show a distinct bright peak, and about 3% (mean 3% ± 1%, n=10) of cells stained brightly using the threshold used to distinguish the bright and dull NKT cells. After 3,000cGy, the non-NKT cells showed a marked shift in bright staining for Bcl-2 and a clear bright peak containing about 69% (mean 54% ± 18%, n=10) of cells was observed. When the experiments were repeated using untreated p53−/− mice, the staining pattern again showed two peaks with about 14% (mean 13% ± 1%, n=6) of cells in the bright peak. However, after 3,000 cGy there was little shift in the Bcl-2 staining, and the bright peak contained about 19% (mean 18% ± 2%, n=10) of cells. Similarly, there was little shift in the staining of Bcl-2 in the non-NKT cells after 3,000 cGy TBI as compared to the untreated mice.
Figure 5. Intracellular Bcl-2 expression fails to increase in NKT and NK1.1− T cells that survive after irradiation in p53−/− mice.
(A) Splenocytes were harvested from untreated p53−/− or wild type mice or from mice 24 hrs after TBI, and stained for NK1.1 vs TCRαβ, fixed and permeabilized, and stained for intracellular Bcl-2. Dead cells were excluded from the analysis by adding the dye EMA to the staining mixture, and gating on EMA− cells. Bottom panels show that thresholds for Bcl-2hi cells were set between the two peaks of Bcl-2 staining after one color analysis of gated NKT cells from untreated wild type or p53−/− mice shown in shaded profile. One color profile of staining with irrelevant isotype matched mAb is shown by dashed line, and profile for Bcl-2 staining after 3,000 cGy TBI is shown by solid line. The left panels show the representative analyses of light scatter versus Bcl-2 amongst gated NK1.1+ TCRαβ+ T cells and the right columns show the analyses amongst gated NK1.1− TCRαβ+ T cells in p53−/− and WT mice. Boxes enclosed either Bcl-2lo (left) or Bcl-2hi (right) cells, and percentages in boxes are shown. The mean percentage of Bcl-2hi NKT cells among NKT cells in untreated p53−/− mice was 13.3 % ± 0.8%, and increased to 14% ± 1%; p>0.05, 15% ± 2%, p>0.05, 17.8% ± 0.5% in mice given 1,000, 2,000 and 3,000 cGy respectively (p>0.05 for UNT versus TBI). The mean percentages among NKT cells in untreated wild type mice was 15% ± 3%, and after 3,000 cGy was 85% ± 8% (p<0.0001). The mean percentage among non-NKT cells in untreated wild type mice was 3% ± 1%, and after 3,000 cGy was 53%± 17% (p<0.0001). There were 5 to 10 mice per group. The data are representative of three independent experiments.
Additional p53−/− mice were given graded doses of 1,000, 2,000, and 3,000 cGy TBI and the gated NKT cells and non-NKT cells were analyzed for bright Bcl-2 staining versus forward scatter to better delineate shifts in a discrete population of cells. As shown in Figure 5 upper panels, the range of bright Bcl-2 staining was about 14–19% for NKT cells and about 2 to 3% for non-NKT cells in the untreated p53−/− mice, and all groups of irradiated p53−/− mice using the threshold developed from the one color staining analysis. The NKT cells and non-NKT cells from wild type mice developed a marked shift in staining that was maximum after 3,000 cGy, and resulted in two clearly distinct populations of Bcl-2lo and Bcl-2hi cells with concentric contours (Figure 5, third row from bottom). The mean percentage of Bcl-2hi non-NKT cells (mean 2% ± 1%) and NKT cells (mean 17% ± 0.6%) in p53−/− mice given 3,000 cGy were significantly different (p<0.0001) from that (mean 53% ± 18% and 85% ± 8% respectively) of wild type mice.
The marked increase in Bcl-2 staining intensity in the wild type mice after irradiation is accounted for by either the selective survival of Bcl-2hi as compared to Bcl-2lo cells, and/or to the upregulation of Bcl-2 expression after exposure to irradiation. Our previous study showed that upregulation of Bcl-2 after irradiation contributed to increased Bcl-2 staining in CD4+ NKT cells, but did not contribute to increased staining in non-NKT cells (12).
Comparison of survival of Bcl-2hi and Bcl-2lo T cell subsets after irradiation
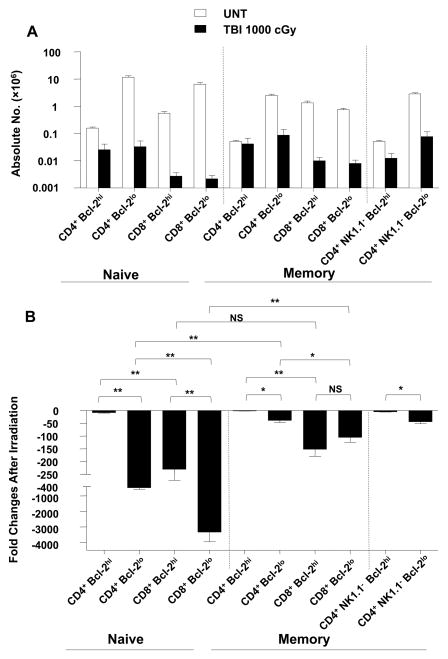
The changes in the absolute numbers of splenic Bcl-2hi and Bcl-2lo T cell subsets was determined after 1,000 cGy TBI. Figure 6A shows the changes in CD44lo (naïve) T cells, and CD44hi (memory) T cells among CD4+ and CD8+ T cells. Figure 5B shows the fold decrease of the T cells after irradiation. Among the CD4+ naïve T cells, the Bcl-2hi cells decreased about 10 fold whereas the Bcl-2lo cells decreased about 400 fold (p<0.001). Unexpectedly, the Bcl-2hi CD8+ naïve T cells decreased about 200 fold, and the Bcl-2lo CD8+ naïve T cells fell dramatically by about 3,500 fold (p<0.001). Thus, the high levels of expression of Bcl-2 were associated with considerately greater radioresistance within the CD4+ and CD8+ naïve T cells. However, there were marked differences in the radioresistance of Bcl-2lo CD4+ versus CD8+ naïve T cells or Bcl-2hi CD4+ versus CD8+ naïve T cells that were independent of the level of expression of intracellular Bcl-2.
Figure 6. The effect of radiation on the absolute numbers of Bcl-2hi and Bcl-2lo cells in naïve and memory CD4+ and CD8+ T cell subsets in wild type mice.
(A) Mean (±SD) absolute numbers of Bcl-2lo and Bcl-2hi cells in CD4+ and CD8+ naïve and memory T cell subsets in the spleen of wild type mice are shown 24hrs after 1000 cGy TBI versus untreated mice. Dotted line on left separates naïve (CD44lo) and memory (CD44hi) cells without gating on NK1.1− cells. Dotted line on right separates memory (CD44hi) cells with or without gating for NK1.1− cells, since NKT cells are all CD44hi. (B) Fold change in absolute numbers of Bcl-2hi and Bcl-2lo cells in CD4+ and CD8+ T cell subsets in panel A comparing untreated versus irradiated mice. There were six to ten mice in each group. The data are representative of three independent experiments. **, P<0.001; *, P<0.01; NS, not significant (p>0.05).
A similar analysis of change in the absolute numbers of Bcl-2hi and Bcl-2lo CD44hi (memory T cells) among CD4+ and CD8+ T cells is shown also in Figure 6. The Bcl-2lo CD4+ memory T cells were more radioresistance than the Bcl-2lo CD4+ naïve T cells (p<0.001). Bcl-2lo CD4+ naïve T cells fell 400 fold, but Bcl-2lo CD4+ memory T cells (either CD4+ CD44hi or CD4+ NK1.1− CD44hi) fell only 30 fold (Figure 6B). Differences in the CD8+ naïve and memory T cells with low levels of Bcl-2 were even more pronounced (p<0.001). The naïve cells fell about 3,500 fold as compared to about 100 fold among the memory cells. Interestingly, there was no significant difference between the Bcl-2lo and Bcl-2hi CD8+ memory T cell decreases after irradiation (p>0.05) (Figure 6B). In summary, the differences in reduction of memory versus naïve CD4+ or CD8+ T cells could not be accounted for by differences in levels of Bcl-2 expression.
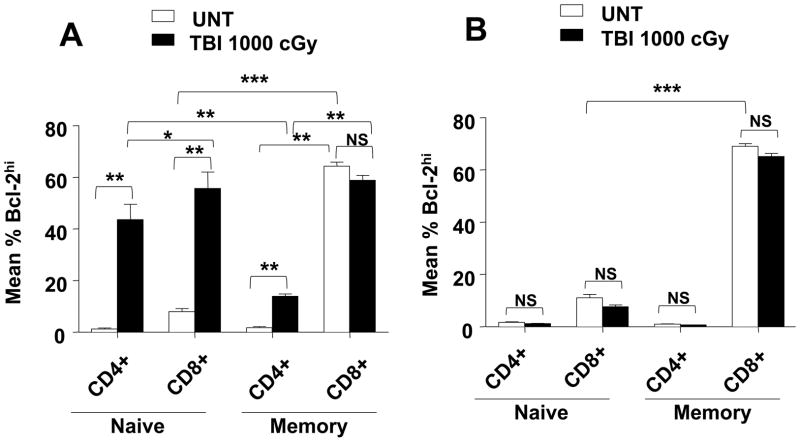
Changes in levels of Bcl-2 of expression in T cell subsets after TBI
Since levels of expression of Bcl-2 can account for the radioresistance of some T cell subsets and not others, it was of interest to determine changes in Bcl-2 levels in the subsets after TBI. We expected that the levels of Bcl-2 would increase markedly among surviving T cells in only those subsets in which high levels of Bcl-2 would provide a survival advantage. Figure 7A shows the mean percentage of Bcl-2hi cells among the different T cell subsets in the spleen of untreated wild type mice and after a single dose of 1,000 cGy TBI. There was a 5 to 20 fold increase in the percentage of Bcl-2hi cells among naïve CD4+ (P<0.001) and naïve CD8+ T cells (p<0.001) respectively after irradiation. The percentage of Bcl-2hi cells among naïve CD4+ cells was significantly lower than that of naïve CD8+ cells (p<0.01) after irradiation. The mean percentage of Bcl-2hi cells also increased about 10 fold (p<0.001) among memory CD4+ T cells after irradiation (Figure 7A). However, there was no statistical difference (p>0.05) between the percentage of Bcl-2 high cells before and after irradiation in the memory CD8+ T cell subset. In part, this was due to the high percentage of Bcl-2hi cells among memory CD8+ T cells before irradiation that was about 30 fold higher than that of memory CD4+ T cells before irradiation (p<0.001). The high percentage of Bcl-2hi cells in unirradiated memory CD8+ T cells was not influenced by the expression of p53, since the high level was also observed in p53−/− mice (Figure 7B). The percentage of Bcl-2hi cells also increased significantly in the Treg and NKT cell subsets in wild type mice after TBI (data not shown). As expected, there were no significant changes (p>0.05) in the percentages of Bcl-2hi ells in naïve and memory CD4+ and CD8+ T cell subsets after irradiation in p53−/− mice (Figure 7B). Thus, all increases in the percentage of Bcl-2hi cells among the CD4+ and CD8+ T cell subsets were dependent on p53 induced apoptosis.
Figure 7. Intracellular expression of Bcl-2 in naïve or memory CD4+ and CD8+ T cell subsets in untreated mice or 24hrs after irradiation in wild type and p53−/− C57BL/6 mice.
(A) Splenocytes were harvested from untreated wild type mice or 24 hrs after 1,000 cGy TBI, stained for CD44lo (naïve) or CD44hi (memory) CD4+ and CD8+ T cell subsets, fixed and permeabilized, and stained for intracellular Bcl-2. The mean percentages of Bcl-2hi cells amongst gated CD44hi or CD44lo in CD4+ and CD8+ T cells are shown. (B) Analysis as in A for p53−/− mice. There were 5 to 10 mice in each group. The data are representative of three independent experiments. ***, P<0.0001; **, P<0.001; *, P<0.01; NS, not significant (p>0.05).
Discussion
In order to more clearly understand the effect of radiation on T cell mediated immunity, we studied changes in the balance of T cell subsets after graded doses of TBI. An important goal of the study was to determine whether radiotherapy induced depletion of T cell subsets was uniform or selective. We found that at doses up to about 3,000 cGy in wild type mice there was a selective loss of T cell subsets that changed their balance with CD8+ T cells being more sensitive than CD4+ T cells, and CD44lo naïve T cells being more sensitive than CD44hi memory T cells and NKT cells. The CD4+ NKT cells were the least sensitive as reported previously (12). Memory T cells that were immunized to alloantigens persisted after myeloablative (1,000cGy) TBI, and were able to prevent engraftment of bone marrow transplants after adoptive transfer to transplant recipients. In the clinical setting of bone marrow transplantation, in which almost all donors are HLA matched with recipients, multiple blood transfusions of recipients can results in an increased risk of graft rejection especially in patients with hemaglobinopathies with chronic anemia(7, 8). This is due to white blood cells in the transfusions that express minor histocompatibility antigens and immunize recipients to cross reactive minor antigens in the donor graft. In the current study, recipients were immunized to both major and minor histocompatibility antigens in the donor grafts based on the stains under investigation. The conclusion that memory T cells directed to alloantigens are radioresistant and can contribute to rejection is likely to be applicable to minor antigens in humans who receive more exposures to antigens over a longer period of time than in the mouse model.
Since p53 is a major controller of apoptosis after irradiation (19–21), we compared the changes in the balance of T cell subsets in wild type mice to those in p53−/− mice in the current study. When doses of irradiation were up to 1,000 cGy TBI in p53−/− mice, there were no significant reductions in the absolute number any T cell subsets as compared to to unirradiated p53−/− mice after 24 hours. Thus, the inactivation of the p53 gene prevented the early change in the balance of T cell subsets observed in the wild type mice due to the prevention of cell death from apoptosis. Interestingly, when the dose of TBI was increased to 3,000cGy in p53−/− mice, then there was a marked (10 fold) reduction in the absolute number of T cells as compared to unirradiated p53−/− mice. This p53 independent T cell death did not alter the balance of T cell subsets nor change the level of expression of intracellular Bcl-2 within the subsets. Thus, the p53 dependent T cell death resulted in a marked change in the balance of T cell subsets, whereas the p53 independent T cell death did not.
Naïve CD8+ Bcl-2lo CD44lo T cells were the most sensitive to cell loss in wild type mice, and decreased about 3,500 fold after 1,000 cGy TBI, whereas memory CD4+ Bcl-2hi CD44hi T cells decreased less than 10 fold. However, different levels of Bcl-2 could not account for differences in radioresistance of CD4+ versus CD8+ T cells, nor naïve versus memory T cells. The results suggest that anti-apoptotic mechanisms other than Bcl-2 determine radioresistance in the latter subset comparisons, and/or that there were marked differences in the upregulation of pro-apoptotic proteins such as Bax, Bim, Puma, and Noxa after irradiation in these subsets(19, 29, 30). It is of interest that high levels of Bcl-2 or both Bcl-2 and Bim have been reported in long lived memory CD8+ T cells in untreated mice (31, 32).. Our findings that CD44hi CD8+ T cells in untreated mice have 30 fold more Bcl-2hi cells than CD44lo CD8+ T cells is consistent with these reports. Nevertheless, the Bcl-2 levels did not determine the radioresistance among CD44hiCD8+ T cells, since Bcl-2hi and Bcl-2lo cell loss after irradiation was similar. Anti-apoptotic proteins other than Bcl-2 that may affect survival after irradiation include Bcl-xL, NAIP and MyD118 (15, 16, 33), and their impact on T cell subset balance is the subject of continuing investigation.
In conclusion, the current study shows that there is a selective p53 depepndent loss of T cell subsets after a dose of TBI (1,000cGy) that is used for conditioning of bone marrow transplant recipients. The selective loss changes the balance of subsets to favor CD44hi T cells including memory T cells and NKT cells. The radioresistant memory T cells can persist after TBI, and can prevent engraftment of allogeneic bone marrow transplants.
Supplementary Material
Acknowledgments
We thank Glenna Letsinger in preparing the manuscript and Yinping Liu for technical assistance.
Footnotes
Contribution: Z.Y. designed, performed the experiments, analyzed data and wrote the paper; S.S. designed the experiments, analyzed data and wrote the paper; and J.C.J. performed experiments and analyzed data. H. K performed experiments and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Baron F, Storb R, Storer BE, Maris MB, Niederwieser D, Shizuru JA, Chauncey TR, Bruno B, Forman SJ, McSweeney PA, Maziarz RT, Pulsipher MA, Agura ED, Wade J, Sorror M, Maloney DG, Sandmaier BM. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. J Clin Oncol. 2006;24:4150–4157. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- 2.Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM, Maris MB, Storb R. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 3.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA, Radich J, Wagner JL, Minor S, Appelbaum FR, Bensinger WI, Bryant E, Flowers ME, Georges GE, Grumet FC, Kiem HP, Torok-Storb B, Yu C, Blume KG, Storb RF. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 4.Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT, Agura E, Langston AA, Pulsipher M, Storb R, Maloney DG. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 5.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, Maloney DG, Storer B, Lange T, Chauncey T, Deininger M, Ponisch W, Anasetti C, Woolfrey A, Little MT, Blume KG, McSweeney PA, Storb RF. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 6.Valcarcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, Sampol A, Bernal MT, Pinana JL, Vazquez L, Ribera JM, Besalduch J, Moraleda JM, Carrera D, Brunet MS, Perez-Simon JA, Sierra J. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 7.Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, Gluckman E, Good RA, Rimm AA, Rozman C, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73:606–613. [PubMed] [Google Scholar]
- 8.Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, Davies SC, Ohene-Frempong K, Bernaudin F, Matthews DC, Storb R, Sullivan KM. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 9.Ren H, Shen J, Tomiyama-Miyaji C, Watanabe M, Kainuma E, Inoue M, Kuwano Y, Abo T. Augmentation of innate immunity by low-dose irradiation. Cell Immunol. 2006;244:50–56. doi: 10.1016/j.cellimm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104:1565–1573. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 12.Yao Z, Liu Y, Jones J, Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells. Eur J Immunol. 2009;39:763–775. doi: 10.1002/eji.200838657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, Strober S. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 14.Nador RG, Hongo D, Yao Z, Strober S. The Changed Balance of Regulatory and Naive T Cells Promotes Tolerance after TLI and Anti-T-Cell Antibody Conditioning. Am J Transplant. 2009;23:23. doi: 10.1111/j.1600-6143.2009.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamada K, Harada M, Abe K, Li T, Nomoto K. IL-4-producing NK1.1+ T cells are resistant to glucocorticoid-induced apoptosis: implications for the Th1/Th2 balance. J Immunol. 1998;161:1239–1247. [PubMed] [Google Scholar]
- 16.Seino K, Harada M, Taniguchi M. NKT cells are relatively resistant to apoptosis. Trends Immunol. 2004;25:219–221. doi: 10.1016/j.it.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: "natural suppressor" cells. J Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 18.Kohrt HE, Turnbull BB, Heydari K, Shizuru JA, Laport GG, Miklos DB, Johnston LJ, Arai S, Weng WK, Hoppe RT, Lavori PW, Blume KG, Negrin RS, Strober S, Lowsky R. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belka C, Budach W. Anti-apoptotic Bcl-2 proteins: structure, function and relevance for radiation biology. Int J Radiat Biol. 2002;78:643–658. doi: 10.1080/09553000210137680. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993;90:5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 22.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 24.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9:355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 26.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 27.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, Strober S. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 29.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 30.Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, Adams JM, Strasser A, Villunger A. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 32.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. Bcl-2 Allows Effector and Memory CD8+ T Cells To Tolerate Higher Expression of Bim. Journal of immunology. 2011 doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.