Abstract
Transmissible spongiform encephalopathies (TSEs) represent a group of fatal neurodegenerative disorders that can be transmitted by natural infection or inoculation. TSEs include scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle, and Creutzfeldt-Jakob disease (CJD) in humans. The emergence of a variant form of CJD (vCJD), which has been associated with BSE, produced strong pressure to search for effective treatments with new drugs. Up to now, however, TSEs have proved incurable, although many efforts have been made both in vitro and in vivo to search for potent therapeutic and prophylactic compounds. For this purpose, we analyzed a compound library consisting of 10,000 compounds with a cell-based high-throughput screening assay dealing with scrapie-infected scrapie mouse brain and ScN2A cells and identified a new class of inhibitors consisting of 3,5-diphenylpyrazole (DPP) derivatives. The most effective DPP derivative showed half-maximal inhibition of PrPSc formation at concentrations (IC50) of 0.6 and 1.2 μM, respectively. This compound was subsequently subjected to a number of animal experiments using scrapie-infected wild-type C57BL/6 and transgenic Tga20 mice. The DPP derivative induced a significant increase of incubation time both in therapeutic and prophylactic experiments. The onset of the prion disease was delayed by 37 days after intraperitoneal and 42 days after oral application, respectively. In summary, we demonstrate a high in vitro efficiency of DPP derivatives against prion infections that was substantiated in vivo for one of these compounds. These results indicate that the novel class of DPP compounds should comprise excellent candidates for future therapeutic studies.
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) or prion diseases are incurable neurodegenerative disorders that include Creutzfeldt-Jakob disease (CJD) in humans (62), chronic wasting disease in cervids (CWD), scrapie in sheep and goats, and bovine spongiform encephalopathy (BSE) in bovines (15, 54). Human prion diseases include sporadic CJD, inherited forms such as Gerstmann-Sträussler-Scheinker syndrome and fatal familial insomnia, and also acquired forms, which are caused by accidental inoculation (iatrogenic CJD) or by ingestion (kuru) of infectious material (14). During the last decade a variant form of CJD (vCJD) in young adults has been reported (2, 29). After transmission to mice (6) and primates (40), both neuropathological data and biochemical results for the pathological prion protein yielded compelling evidence for a link between BSE and vCJD.
Although the mechanism remains unclear, the central process in TSEs is the conversion of the physiological cellular prion protein (PrPC) into a disease-associated isoform (PrPSc) (5, 47, 52). PrPC represents a membrane-associated, detergent-soluble, and proteinase K (PK)-sensitive glycoprotein of unknown function (9, 10). During the conversion, PrPC is transformed into a PK-resistant isoform, with a high content of β-sheet structures, which forms detergent-insoluble aggregates and deposits in the brain (31, 51).
Until now, TSEs have proved incurable; therefore, a main objective of this project was to search for potent therapeutic and prophylactic compounds. A large number of compounds were found to inhibit the PrPSc formation with high efficiency in vitro, such as tannic acid (34), curcumin (11, 36), or anti-malaria drugs such as mefloquine (37). These compounds interact directly with PrPSc aggregates, but no in vivo activity could be proven. Other effective compounds were toxic (such as Congo red [7]), displayed only poor penetration of the blood-brain barrier (56), or were only transiently effective (such as RNA interference [17]). Potent inhibitors which act in vitro and in vivo are polyene antibiotics such as amphotericin B (42, 49) and its analogues (1), tetracyclines (20), cyclic tetrapyrole-like porphyrins (12, 50), and polyanions such as pentosan polysulfate (PPS) (8, 21). Other therapeutic approaches were carried out through active immunization using prion peptides (41) or by passive immunization using monoclonal antibodies (60).
Several clinical trials with CJD patients were conducted using PPS progression (48, 61), acyclovir (18, 45), the antiviral drug amantadin (57), quinacrine (27), or flupirtine (46), but without significant effects on the progress of the disease.
In order to find novel anti-prion compound with in vivo activity, we analyzed a large compound library using a cell-based high-throughput screening assa and both scrapie-infected wild-type C57BL/6 and transgenic Tga20 mice. Diphenylpyrazole (DPP) derivatives were discovered as new class of inhibitors that delayed the onset of the prion disease significantly after intraperitoneal or oral application.
MATERIALS AND METHODS
Chemicals.
A compound library (DIVERset 1) was purchased from ChemBridge Corp. (San Diego, CA), and compounds were diluted to 1 to 2 mM stock dilutions in dimethyl sulfoxide (DMSO). For the in vivo experiments, the compounds were either dissolved according to their solubility in 100 or 70% DMSO or were suspended in physiological saline (0.9% NaCl) just before use.
Testing for inhibition of PrPSc accumulation in a cell-based dot blot model.
Approximately 20,000 mouse neuroblastoma (ScN2a) or mouse brain (SMB) cells infected with the Rocky Mountain Laboratory scrapie strain (RML) were suspended in 100 μl of medium and seeded into a Costar 3599 flat-bottom 96-well plate (Corning, Inc., Corning, NY) prior to the addition of the test compound. The 1 to 2 mM solutions of the test compounds were diluted in DMSO prior to being added to the cell medium. A 1-μl portion of each solution was added to the cell medium. After incubation at 37°C for the ScN2a cells and at 35°C for the SMB cells in a CO2 incubator for 3 days, the cultures were lysed and analyzed for PrPres expression.
Prior to cell lysis, the cultures were controlled for toxicity of the compound and density of the cell culture in comparison to the controls by light microscopy. After removal of the cell medium, 100 μl of lysis puffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl; 0.5% [wt/vol] deoxycholic acid sodium salt [DOC], 0.5% [vol/vol] Triton X-100) was added to each well for 5 min at room temperature. Using a dot blot apparatus (Sigma-Aldrich), we transferred the cell lysate to an activated polyvinylidene difluoride membrane (Immobilon-P; Millipore) under vacuum pressure and fixed it to the membrane for 1 h at 37°C. The membrane was then incubated simultaneously in lysis puffer and treated with a PK solution (final concentration, 25 μg/ml) for 90 min at 37°C. The membrane was then washed twice with pure water. Denaturation solution (3 M guanidinium thiocyanate, 0.1 M Tris-HCl [pH 8]) was added for 10 min at room temperature and, afterward, the membrane was washed five times with pure water. After the denaturation the membrane was blocked with 5% (wt/vol) nonfat milk containing 0.1% (vol/vol) Tween 20 (Sigma) in phosphate-buffered saline (PBST-milk) for 60 min. The polyclonal antibody (PAb) Ra10 (26, 28) was incubated in the blocking solution with the membrane for 60 min. After three rinses in PBST, the membrane was incubated in 0.2 μg of horseradish peroxidase-conjugated, anti-rabbit IgG (Promega)/ml in PBST-milk at room temperature for 1 h. After an additional rinse in PBST, the bound antibodies were detected by using a chemiluminescence reagent system (ECL; Amersham) and were visualized directly in an image analysis system (Versa Doc; Quantity One, Bio-Rad, Munich, Germany). Together with each dilution series of the compounds, untreated control wells and wells that were treated with suramin, a known PrPSc inhibitor in RML-infected ScN2a and SMB cells, were analyzed.
In addition, we determined the half-maximal inhibitory concentration (50% inhibitory concentration [IC50]) values for each of the effective compounds from dose-response curves after the application of 10-fold dilutions (from 20 μM to 2.0 μM and down to 0.2 μM) of each compound to the corresponding cell line.
Testing for PrPC expression in uninfected N2a cells.
Approximately 20,000 N2a cells were seeded into 96-well-plates. Further processing including compound application, cell lysis and PrPSc detection was carried out as described earlier.
MTT test.
We performed an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] test (Sigma) as a marker for cell viability. Therefore, about 20,000 N2a cells were added to each well of a 96-well plate. Test compounds were added to a final concentration of 20 μM and incubated for 3 days. After that, the MTT test was conducted according to the manufacturer's instructions. In this test, the tetrazolium salt MTT was converted into blue insoluble formazan dye crystals, which had to be dissolved by a suitable extraction mixture. The formazan absorption was quantified through spectrophotometric analysis (570-nm test wavelength; 630-nm reference wavelength).
Proteasome-Glo cell-based assay.
Approximately 20,000 N2a cells were added to each well of a 96-well plate. The cells were allowed to settle before the test compounds were added to a final concentration of 20 μM and incubated for 3 days. After that, a Proteasome-Glo assay was conducted according to the manufacturer's instructions (Promega). In this assay, we measured the activity of the proteasome complex in cultured N2a cells by cleavage of a luminogenic proteasome substrate, Suc-LLVY-aminoluciferin.
Cell-free conversion assay.
This assay was carried out according to the protocol established by Eiden et al. (22) and Kupfer et al. (39). In brief, cell-free conversion was carried out by mixing bacterial recombinant murine 3F4-tagged prion protein with purified PrPSc seeds (mouse-passaged scrapie strain Me7) in an appropriate conversion buffer. After digestion with PK for 1 h at 37°C (end dilution, 30 μg/ml), newly generated PrPres fragments were detected by immunoblotting and incubation with monoclonal antibody (MAb) 3F4 (33). After treatment of the membranes with stripping buffer, PrPSc aggregates were detected by using PAb Ra10. Compounds were added to the conversion reaction to a final concentration of 100 μM and analyzed with regard to inhibition of PrPres formation and dissolution of PrPSc aggregates.
PrPSc detection.
Detection of PrPSc aggregates was examined by phosphotungstic acid (PTA) precipitation and Western blotting, which were carried out according to the protocols established by Wadsworth et al. (59) and Gretzschel et al. (25), with some modifications.
For all samples, 10% (wt/vol) brain homogenates were prepared in 0.42 mM sucrose solution containing 0.5% DOC and 0.5% Nonidet P-40 using a Ribolyser (Hybaid, Heidelberg, Germany). Large cellular debris was removed by centrifugation at 6,000 rpm for 5 min at room temperature. A 200-μl aliquot of the supernatant was adjusted with PK (Boehringer Mannheim) to a final concentration of 50 μg of PK/ml, followed by incubation at 55°C for 1 h. Digestion was stopped by the addition of 4 μl of Pefabloc (Roche, Mannheim, Germany), followed by heating to 95°C for 5 min. In the next step, PTA was added to the samples to give a final concentration of 0.3% (wt/vol). The samples were incubated at 37°C for 60 min with constant agitation before centrifugation at 13,300 rpm for 30 min at room temperature. After careful removal of the supernatant, the pellets were resuspended in various volumes of a loading buffer containing 1% (wt/vol) sodium dodecyl sulfate (SDS), 25 mM Tris-HCl (pH 7.4), 0.5% mercaptoethanol, and 0.001% bromophenol blue and then heated for 5 min at 95°C. The volume of loading buffer was adjusted in order to ensure comparable strengths in the Western blot signals. After a short centrifugation, samples were loaded on 16% Tris-polyacrylamide gels. SDS-PAGE and Western blotting was performed according to standard procedures (22). As a primary antibody, we used MAb SAF70 (Spibio) at a concentration of 0.03 μg/ml.
Immunohistochemistry.
All tissues samples were fixed in 4% buffered formalin, treated for 1 h with 98% formic acid, rinsed for 40 min in tap water and embedded in paraffin. The paraffin-wax tissue sections were mounted on Superfrost Plus slides (Menzel-Gläser) and rehydrated. The subsequent pretreatment included incubation of the slides in 98% formic acid for 15 min, a 5-min rinse in tap water, and inhibition of the endogenous peroxidase activity with 3% H2O2 (Merck) in methanol for 30 min, followed by a 15-min digestion with PK (4 mg/ml; Boehringer Mannheim) at 37°C. The primary MAb ICSM 18 (D-Gen, Ltd.) was applied at a dilution of 1:250 in goat serum, followed by incubation at room temperature for 2 h. Negative-control sections were treated with goat serum alone. As a secondary antibody system, we used the EnVision reagent (Dako) containing a peroxidase-conjugated polymer backbone. The incubation time was 30 min at room temperature. The slides were finally developed in diaminobenzidine tetrahydrochloride (Fluka) and counterstained for 10 min with Mayer's hematoxylin. All sections were examined by light microscopy.
Propagation of scrapie strains.
Wild-type C57BL/6 or transgenic Tga20 mice (kindly provided by C. Weissmann, Zürich, Switzerland) which overexpress wild-type mouse PrP and have shortened incubation periods for mouse prions (23) were inoculated either intracerebrally (i.c.) with 30 μl of a 1% homogenate of RML or intraperitoneally (i.p.) with 50 μl of a 1% RML homogenate. The health status of the mice was inspected daily, and their body weights were recorded weekly. After the onset of TSE-associated clinical symptoms (abnormal tail tonus, hind limb paralysis, and weight loss), the animals were euthanized. The incubation time was then calculated as the time between inoculation and death. The brains were removed; one half of each brain was stored at −20°C, and the other half was fixed in 4% natural buffered formalin. Mice that died from intercurrent diseases were excluded from the data. Table 2 gives an overview about the animal experiments.
Table 2.
Animal experiments
| Approach | Treatment | Mice | Inoculation |
|---|---|---|---|
| Primary efficiency test | i.p. | C57BL/6 | i.c. |
| Therapeutic approach | i.p. | Tga 20 | i.p. |
| Oral | Tga 20 | i.p. | |
| Prophylactic approach | Oral | C57BL/6 | i.p. |
Toxicity study.
The toxicity of DMSO solved 2-[5-(3-fluorophenyl)-1H-pyrazol-3-yl]-5-methylphenol (DPP-1; 5 mg/kg/day) was analyzed by application to uninfected C57BL/6 mice.
Therefore, 100 μg of DPP-1 (5 mg/kg/day) was administered i.p. to five wild-type mice daily for a period of 21 days. In addition, five mice were used as dissolving-solution controls, and six mice were left untreated. Abnormalities that might be due to the compound administered were examined by pathological and histopathological analysis of the most relevant tissues (i.e., the liver, kidneys, brain, lungs, heart, muscles, gut, genitalia, spleen, and pancreas).
Intraperitoneal injection.
The daily treatment with 100 μg (5 mg/kg/day) of the DPP-1 compound dissolved in 100% DMSO started 90 days after the i.c. inoculation of C57BL/6 mice with RML and was administered i.p. over a period of 20 days (Primary efficiency test). As a control, inoculated mice were injected with pure DMSO and were used as dissolving solution controls (DMSO-control). For treatment with a benzothiazol derivative (BTD), [2-methoxy-4-({[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]imino}methyl]phenol, the BTD was dissolved in 100% DMSO and administered i.p. Daily treatment with 100 μg of BTD started 14 days after i.p. inoculation of C57BL/6 mice with RML over 8 weeks.
Osmotic pump treatment.
Application of DPP compound was first carried out with osmotic pumps (Alzet, Cupertino, CA), which allowed a continuous administration of the compound over a period of 8 weeks. The DPP compound was dissolved in 70% DMSO (maximum capacity for the osmotic pump membrane) The polyethylene catheter of the pump was inserted into the peritoneum, and the compound was administered over 4 weeks at a flow rate of 0.25 μl/h, which corresponded to 100 μg per mouse per day. The pump worked stably for 40 h after implantation and continuously for 4 weeks. After 4 weeks the pump was replaced by another pump, and the procedure was continued for another 4 weeks. This drug delivery schedule allowed a continuous and undisturbed administration of the test agents without the need for perpetual injections. As a control, 70% DMSO was administered via osmotic pumps (vehicle control).
Oral treatment.
In terms of the oral application approach, we resuspended DPP-1 and BTD in 0.9% NaCl to a concentration of ∼5 mg/ml, which led to a daily dose of 500 μg per mouse. The administration was carried out with feeding needles (Scanbur, BK, Sollentuna, Sweden).
Statistical analysis.
Survival times were analyzed by Kaplan-Meier Survival analysis using a log-rank test to compare the curves. The statistical analysis was performed using SigmaBlot statistical software (San Jose, CA). The survival times are expressed as means ± the standard deviations.
RESULTS
In vitro testing.
To discover efficient anti-prion compounds in a library of 10,000 chemical substances (DIVERset 1; ChemBridge), we established a high-throughput screening assay for PrPSc inhibitors using ScN2a cells and SMB cells in a 96-well format. Using these in vitro systems and a computerized representation of the data, we found a new class of inhibitors comprising 3,5-diphenylpyrazole (DPP) derivatives by structure-activity and cluster analysis (30).
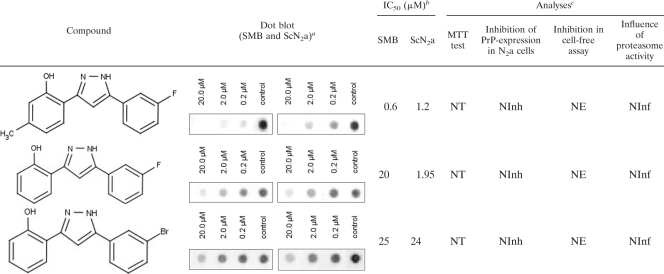
Among these, 2-[5-(3-fluorophenyl)-1H-pyrazol-3-yl]-5-methylphenol, (here designated DPP-1), 2-[5-(3-fluorophenyl)-1H-pyrazol-3-yl]phenol (DPP-2), and 2-[5-(3-bromophenyl)-1H-pyrazol-3-yl]phenol (DPP-3) were the most effective PrPres formation inhibitors. Structures, dot blot results and IC50s of all relevant compounds are summarized in Table 1.
Table 1.
Chemical structures off DPP derivatives and in vitro analyses
Left blot, SMB; right blot, ScN2a.
IC50, half-maximal inhibitory concentration.
NT, nontoxic; NInh, no inhibition; NE, no effect; NInf, no influence.
DPP-1 exhibited IC50s of 0.6 μM in SMB cells and 1.2 μM in ScN2a cells, whereas the IC50s of DPP-2 were 20 μM (SMB cells) and 19.5 μM (ScN2a cells), respectively, and the IC50s of DPP-3 were 25 μM (SMB cells) and 24 μM (ScN2a cells). Moreover, all of the compounds lacked cytotoxic effects, as shown by the MTT test.
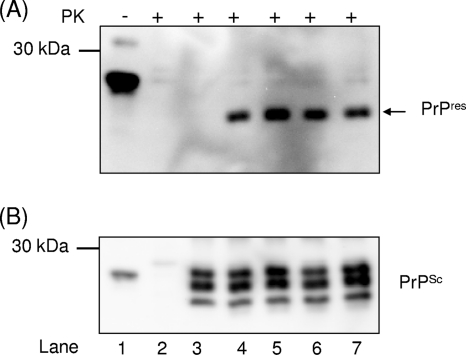
Further analysis of any potential molecular inhibitory mechanism was performed using a cell-free conversion assay. All three compounds had no influence on the PrPres formation (Fig. 1A) or on the dissolution of PrPSc aggregates (Fig. 1B). We were also unable to detect any influence of these compounds on the proteasome activity, as well as on the cellular trafficking and processing of PrPC in N2a cells. Based on its high inhibitory effects for scrapie-infected cells, DPP-1 was selected to be used in in vivo studies.
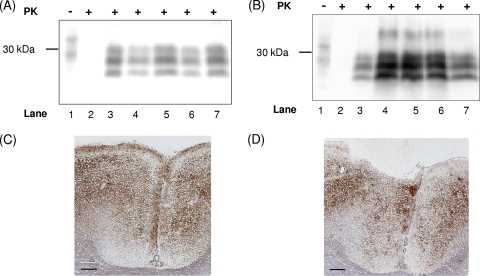
Fig. 1.
DPP derivatives do not interfere with cell-free PrPres formation and do not dissolve existing PrPSc aggregates. (A) Controls were performed as follows: PrPC in a 1:10 dilution without PrPSc or PK (lane 1), PrPC without PrPSc incubated with PK (lane 2), and PrPC incubated with PrPSc but stopped immediately by freezing (t0 control, lane 3). Lane 4 shows the PrPres fragments after incubation for 72 h with scrapie strain Me7 in the presence of 100 μM DPP-1 (lane 5), 100 μM DPP-2 (lane 6), or 100 μM DPP-3 (lane 7). Detection of PrPC and PrPres fragments was carried out with MAb 3F4. (B) The membrane in panel A was stripped and reincubated with PAb Ra10 for the detection of PrPSc seeds in lanes 3 to 7.
In vivo testing.
The anti-prion efficacy of DPP-1 was determined by mouse bioassays using C57BL/6 wild-type and transgenic Tga20 mice. The experimental approach included a toxicity study, as well as three treatment regimes (primary efficiency, prophylactic, and therapeutic) for the mice (Table 2).
Toxicity study.
Following a substantial DPP dose (i.e., 5 mg/day i.p. for a period of 21 days), no pathoanatomical and histopathological alterations were found. The only affections that were observed were a fibrous peritonitis in some of the DPP-1 treated mice, as well as in the DMSO control mice, which was most probably caused by the application.
Primary efficiency test.
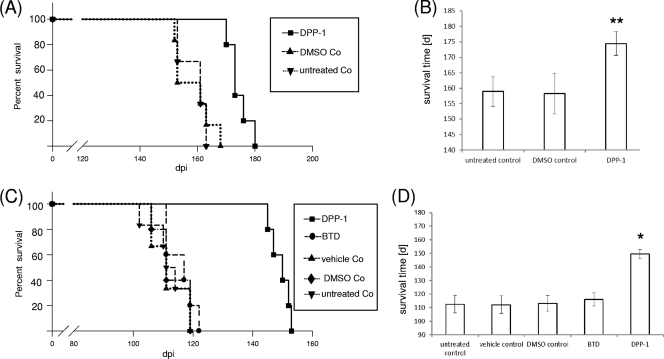
The results of this experiment are summarized in Fig. 2A and B. Untreated control mice had a mean incubation time of 160 ± 4.1 days, which was similar to that of the DMSO control mice (160 ± 6.5 days). DPP-1 significantly prolonged the mean incubation time for 14 days (from 160 ± 4.1 days to 174 ± 3.8 days). Based on these findings, the therapeutic and prophylactic effects of DPP-1 were further evaluated in animal experiments. Moreover, we included a BTD for which we have recently found an anti-prion effect in vitro and in vivo (24).
Fig. 2.
DPP-1 prolongs the incubation time in scrapie-infected mice after i.p. treatment. (A) Primary efficiency test. Kaplan-Meier survival analysis of i.c. scrapie-infected C57BL/6 mice was performed after i.p. treatment with DPP-1. The treatment groups included untreated controls (▾), DMSO controls (▴), and DPP-1-treated mice (■). (B) Mean survival times ± the standard deviations as determined by the primary efficiency test. Comparison of DPP-1 versus untreated controls and DMSO controls was carried out by using the log-rank test (**, P < 0.01). (C) Intraperitoneal therapy. Kaplan-Meier survival analysis of i.p. scrapie-infected Tga20 mice was performed after i.p. treatment with BTD or DPP-1. The treatment groups included untreated controls (▾), vehicle controls (▴), DMSO controls (◆), benzothiazol (BTD)-treated mice (●), and DPP-1-treated mice (■). (D) Mean survival times in i.p. therapy ± the standard deviation. Comparison of DPP-1-treated versus untreated controls, DMSO controls, vehicle control, and BTD-treated mice was carried out by using the log-rank test (*, P < 0.05).
Therapeutic approach.
In a first set of experiments, a therapeutic approach was applied using transgenic Tga20 mice that had been i.p. challenged with the RML strain. DPP-1 was administered by continuous subcutaneous infusion using osmotic pumps. Under these conditions DPP-1 prolonged the mean incubation time of scrapie in the mice by 37 days compared to that in untreated control mice (from 113 ± 6.4 days to 149 ± 3 days). The mean value for the vehicle control (70% DMSO) was 112 ± 6.6 days (Fig. 2C and D).
Portions (100 μg) of BTD (per mouse and day) were given daily by i.p. injection since the BTD is soluble only in 100% DMSO, which is not tolerated by the osmotic pumps. Application of the DMSO control without BTD had no effect on the incubation time (113 ± 5.6 days) compared to the untreated control (113 ± 6.4 days) and also the treatment with benzothiazol caused only a minor prolongation of the incubation time (116 ± 5 days) (Fig. 2C and D).
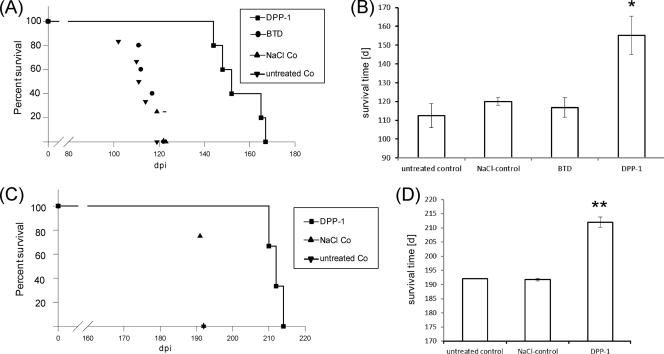
In a second set of experiments DPP-1 and benzothiazol were administered daily to Tga20 mice by using oral feeding needles over a period of 8 weeks, starting 14 days after i.p. inoculation. The animals treated with benzothiazol showed a mean incubation time of 117 ± 5.0 days, and the NaCl control group a mean value of 120 ± 2 days. In contrast, treatment with DPP-1 significantly increased the incubation time by 42 days (155 × 10) compared to the untreated control (113 ± 6.4 days) (Fig. 3A and B).
Fig. 3.
DPP-1 prolongs incubation time in scrapie-infected mice after oral treatment. (A) Oral treatment. Kaplan-Meier survival analysis of i.p. scrapie-infected Tga20 mice was performed after oral treatment with DPP-1 or benzothiazol (BTD). The treatment groups included untreated controls (▾), NaCl controls (▴), benzothiazol (BTD)-treated mice (●), and DPP-1-treated mice (■). (B) Mean survival times in primary efficiency test ± the standard deviation. Comparison of DPP-1 versus untreated controls, NaCl controls, and BTD-treated mice was carried out by using the log-rank test (*, P < 0.05). (C) Oral prophylaxis. Kaplan-Meier survival analysis of i.p. scrapie-infected C57BL/6 mice was performed after oral treatment with DPP-1. The treatment groups included untreated controls (▾), NaCl controls (▴) and DPP-1-treated mice (■). (D) Mean survival times in oral prophylaxis ± the standard deviation. Comparison of DPP-1-treated versus untreated controls and NaCl controls was carried out by using the log-rank test (**, P < 0.01).
In conclusion, the in vitro inhibitory activity of benzothiazol was not confirmed in vivo, whereas a DPP-1 treatment increased in the scrapie incubation time by ∼37 days (osmotic pumps) or 42 days (oral treatment), respectively. Therefore, DPP-1 should be considered as promising drug candidate for the prophylactic treatment of prion diseases.
Prophylactic approach.
An oral DPP-1 application started 2 weeks before the i.p. inoculation (RML) of wild-type C57BL/6 mice and continued for 6 weeks (overall time of treatment, 8 weeks). DPP-1 was again solubilized in 0.9% NaCl (5 mg/ml), which yielded an intake of 500 μg of DPP-1 (25 mg/kg/day) per mouse effectively.
In this prophylactic approach, DPP-1 also increased the survival time significantly by 20 days compared to the controls (212 ± 1.8 days versus 192 ± 0.5 days) (Fig. 3C and D).
Biochemical and immunohistological analysis.
The PrPSc accumulation in brains of compound-treated and untreated terminally sick mice was analyzed by Western blotting and by immunohistochemistry. Western blotting did not reveal any differences in the glycosylation pattern or molecular weight of PrPSc between DPP-1-treated and control mice (Fig. 4A and B). The same applied for benzothiazol (data not shown). The brain tissues of treated and untreated mice at the terminal stage of disease revealed typical disease specific PrPSc accumulation pattern (Fig. 4C and D).
Fig. 4.
PrPSc accumulation in the brains of selected mice challenged with RML. Immunoblot analysis of RML-infected mouse brain homogenate. (A) Control experiments were carried out using PrPC without PK (lane 1), PrPC digested with PK (lane 2), or PrPSc digested with PK (lane 3). Lanes 4 and 5 show PrPSc fragments of mice treated with osmotic pumps infused with DPP-1 (lane 4) or DMSO control (lane 5). Lanes 6 and 7 display PK-digested PrPSc fragments from orally treated mice (postinoculation) either with (lane 6) or without (lane 7) DPP-1. (B) Controls were established similar to those in Fig. 4A: PrPC without K (lane 1), PrPC digested with PK (lane 2), or PrPSc digested with PK (lane 3). Lanes 4 and 5 show PrPSc fragments derived from i.c.-inoculated mice treated with DPP-1 (lane 4) or not treated (lane 5). Lanes 6 and 7 display PK-digested PrPSc fragments of i.p.-infected mice after oral prophylactic administration of DPP-1 (lane 6) compared to the NaCl controls (lane 7). Detection of PrPC or PrPSc was carried out with MAb SAF70 and MAb RGS-His for the detection of molecular mass markers. PrPSc immunohistochemistry of brain tissue was performed. (C and D) Cortices of DPP-1-treated (C) and untreated (D) i.p.-infected C57BL/6 mice (oral prophylaxis) at the terminal stage of disease. MAb ICSM 18, 1:250; bar, 200 μm.
DISCUSSION
The screening of 10,000 compounds from a chemical compound library by using cell-based assays identified DPPs as potent inhibitors of the PrPSc formation in vitro. The IC50s of the most efficient inhibitor (DPP-1) varied between 0.6 μM in SMB cells and 1.2 μM in ScN2a cells. The protective effect of DPPs was further analyzed in a cell-free conversion assay, but no effect was identified, which indicated an indirect inhibitory mechanism in scrapie-infected cells independent from the protein degradation machinery, because the proteasome activity in vitro was not affected. MTT tests as a marker for cell viability showed no in vitro toxicity of DPPs. Moreover, we examined also the DPP toxicity by mouse treatment in vivo and found no damaging effect. The efficacy of the most potent DPP derivative, DPP-1, was further analyzed in scrapie infected mice and a significant delay of clinical onset was observed. DPPs are most probably able to cross the blood-brain barrier, because the scrapie infection was performed i.c., whereas the compounds were administered i.p.
DPP-1 and another already published anti-prion compound, benzothiazol, were subsequently tested for their therapeutic activity. Scrapie-inoculated Tga20 mice (i.p.) received these compounds i.p. (via osmotic pumps or by i.p. injection) to reveal effects on the spread of the infection from the periphery to the central nervous system. In these experiments only DPP-1 extended the incubation time significantly (i.e., by 36 days).
Benzothiazol derivatives have been shown to effectively bind adenosine receptors and antagonize the neuromodulator adenosine (Hoffmann-La Roche AG, document DE602004004776T2 22.11.2007, EP publication no. 0001628662). Adenosine receptors are involved in the control of neuronal damage and therefore display a specific target for the treatment of neurodegenerative disorders (16). Benzothiazol may therefore only induce general beneficial effects with regard to neuroprotection and neuroinflammation.
Further therapeutic and prophylactic DPP-1 treatments were carried out orally, and significant prolongations of the incubation period were revealed. In contrast, Benzothiazol did not show any effect.
The general molecular mechanisms of prion inhibition by DPPs are unknown to date. DPPs can act as inhibitors of heat shock proteins (Hsps), especially Hsp90 (H. Eggenweiler, M. Wolf, and Merck Patent GmbH, 1,5-Diphenylpyrazoles, WIPO patent application WO/2006/018082, 2005). Hsps belong to the group of molecular chaperones that regulate protein folding and therefore play an essential role during neurodegeneration (55). Normally, chaperones refold damaged proteins or target them to degradation. Under specific conditions, however, they transfer damaged proteins to larger aggregates. This was previously demonstrated for the chaperones GroEL and Hsp104, which stimulated the PrP conversion (19).
On the other hand, inhibition of Hsp90 induces the upregulation of Hsp70 and other Hsps that inhibit the formation of toxic protein aggregates (53). Recently, the inhibition of Hsp90 by novobiocin analogues has been reported to suppress amyloid beta-induced neurodegeneration (3). A similar effect has been demonstrated with the Hsp90 inhibitor geldanamycin, which decreases α-synuclein aggregation in cell culture (44). The interference to cofactors may explain the lack of inhibition in the cell-free conversion assay, which deals only with the interaction of purified prion protein and aggregates devoid of additional components.
Another potential mode of DPP action is the role as antioxidant during the disease. The antioxidative potential of DPP derivatives with regard to inhibition of monoamine oxidase has been demonstrated in several studies (13, 43). During neurodegenerative diseases in mitochondria, an oxidation of numerous proteins by monoamine oxidase occurs. This event is followed by the generation of reactive oxygen species, which ultimately leads to neuronal cell death. Thus, the beneficial effects of DPP may therefore result in a significantly prolonged incubation time in scrapie-infected mice, although the PrP aggregation continues to proceed.
Only a few other compounds have been reported that showed therapeutic effects against TSEs in vivo. PPS has been reported to decrease the PrPSc levels in prion-infected cells (4) and was further analyzed in scrapie-infected mice. Doh-ura et al. (21) showed that direct continuous PPS infusion over 4 weeks with the help of osmotic pumps at a dose of 230 μg/kg/day into the cerebral ventricle significantly prolonged survival time of intracerebrally scrapie infected mice. In addition, PPS was administered to CJD patients, but without observing therapeutic effects (61). Other compounds that have been shown to delay the onset of scrapie after i.c. inoculation were Congo red (32) and amphotericin B (49). In our study we could demonstrate that DPP-1 delayed the onset of disease by a daily i.p. injection (5 mg/kg/day) for 21 days starting 90 days after the i.c. scrapie challenge. In addition, we could monitor an anti-scrapie activity of DPP-1 in i.p.-infected mice when we used a continuous i.p. infusion by starting the therapy at an early stage of the infection (14 days postinoculation) at a dose of 5 mg/kg per day and mouse over 8 weeks. Recently, porphyrin derivates have been described to significantly increase the incubation time when administered i.p. 2 weeks prior to i.p. infection. However, these substances showed only little activity when administered to i.c.-inoculated mice (38). It must therefore be assumed that these compounds are unable to cross the blood-brain barrier.
Several attempts for an oral scrapie therapy had been carried out with previously analyzed in vitro inhibitors such as curcumin (11) and tannic acid, as well as phenolic tea extracts, even at doses of 1,500 mg/kg per day in drinking water (35). However, none of these substances displayed a convincing in vivo effect. Only the oral treatment with pravastin resulted in a mild prolongation of survival time (58). Pravastin, a hydrophilic member of the statins, is known to interfere with cholesterol biosynthesis pathway and harbors general anti-inflammatory and antioxidant effects.
Taken together, we report here on a new class of in vivo active anti-prion compounds, the 3,5-diphenylpyrazoles (DPPs are covered by patent application PCT/EP2009/004144), which work following i.p. and oral application using both therapeutic and prophylactic experimental setups. Based on our results, DPP-1 in particular should be considered as a novel promising candidate for intervention in prion diseases.
ACKNOWLEDGMENTS
This study was supported financially by the German Ministry for Education and Research, as well as by the EU Commission (NoE Neuroprion).
We thank Anne Balkema-Buschmann and Dan Balkema for critically reading the manuscript and Baerbel Hammerschmidt, Anja M. Oelschlegel, and Katrin Werner for their technical assistance.
Footnotes
Published ahead of print on 11 July 2011.
REFERENCES
- 1. Adjou K. T., Seman M. 2002. What are the prospects for pharmacological treatment of prion disease? Therapie 57:123–127 [PubMed] [Google Scholar]
- 2. Andrews N. J., et al. 2003. Deaths from variant Creutzfeldt-Jakob disease in the UK. Lancet 361:751–752 [DOI] [PubMed] [Google Scholar]
- 3. Ansar S., et al. 2007. A non-toxic Hsp90 inhibitor protects neurons from Aβ-induced toxicity. Bioorg. Med. Chem. Lett. 17:1984–1990 [DOI] [PubMed] [Google Scholar]
- 4. Birkett C. R., et al. 2001. Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J. 20:3351–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown D. R. 2002. Mayhem of the multiple mechanisms: modeling neurodegeneration in prion disease. J. Neurochem. 58:1720–1725 [DOI] [PubMed] [Google Scholar]
- 6. Bruce M. E., et al. 1997. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501 [DOI] [PubMed] [Google Scholar]
- 7. Caughey B., Race R. E. 1992. Potent inhibition of scrapie-associated PrP accumulation by Congo red. J. Neurochem. 59:768–771 [DOI] [PubMed] [Google Scholar]
- 8. Caughey B., Raymond G. J. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caughey B., Race R. E. 1994. Scrapie-associated PrP accumulation and its inhibition: revisiting the amyloid-glycosaminoglycan connection. Ann. N. Y. Acad. Sci. 724:290–295 [DOI] [PubMed] [Google Scholar]
- 10. Caughey B., Lansbury P. T. 2003. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 26:267–298 [DOI] [PubMed] [Google Scholar]
- 11. Caughey B., et al. 2003. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 77:5499–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caughey W. S., Raymond L. D., Horiuchi M., Caughey B. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. U. S. A. 95:12117–12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chimenti F., et al. 2004. Synthesis and selective inhibitory activity of 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives against monoamine oxidase. J. Med. Chem. 47:2071–2074 [DOI] [PubMed] [Google Scholar]
- 14. Collinge J. 1997. Human prion diseases and bovine spongiform encephalopathy (BSE). Hum. Mol. Genet. 6:1699–1705 [DOI] [PubMed] [Google Scholar]
- 15. Collinge J. 2001. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24:519–550 [DOI] [PubMed] [Google Scholar]
- 16. Cunha R. A. 2008. Caffeine, adenosine receptors, memory and Alzheimer disease. Med. Clin. 131:790–795 [DOI] [PubMed] [Google Scholar]
- 17. Daude N., Marella M., Chabry J. 2003. Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J. Cell Sci. 116:2775–2779 [DOI] [PubMed] [Google Scholar]
- 18. David A. S., Grant R., Ballantyne J. P. 1984. Unsuccessful treatment of Creutzfeldt-Jakob disease with acyclovir. Lancet i:512–513 [DOI] [PubMed] [Google Scholar]
- 19. DebBurman S. K., Raymond G. J., Caughey B., Lindquist S. 1997. Chaperone-supervised conversion of prion protein to its protease-resistant form. Proc. Natl. Acad. Sci. U. S. A. 94:13938–13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Luigi A., et al. 2008. The efficacy of tetracyclines in peripheral and intracerebral prion infection. PLoS One 3:e1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doh-ura K., et al. 2004. Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models. J. Virol. 78:4999–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eiden M., et al. 2006. Synergistic and strain-specific effects of bovine spongiform encephalopathy and scrapie prions in the cell-free conversion of recombinant prion protein. J. Gen. Virol. 87:3753–3761 [DOI] [PubMed] [Google Scholar]
- 23. Fischer M., et al. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255–1264 [PMC free article] [PubMed] [Google Scholar]
- 24. Geissen M., et al. 2011. From cell culture to mouse model: identification of two new inhibitors of prion disease. Chem. Med. Chem. doi:10.1002/cmdc.201100119 [DOI] [PubMed] [Google Scholar]
- 25. Gretzschel A., et al. 2005. Strain typing of German transmissible spongiform encephalopathies field cases in small ruminants by biochemical methods. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:55–63 [DOI] [PubMed] [Google Scholar]
- 26. Groschup M. H., Harmeyer S., Pfaff E. 1997. Antigenic features of prion proteins of sheep and of other mammalian species. J. Immunol. Methods 207:89–101 [DOI] [PubMed] [Google Scholar]
- 27. Haik S., et al. 2004. Compassionate use of quinacrine in Creutzfeldt-Jakob disease fails to show significant effects. Neurology 63:2413–2415 [DOI] [PubMed] [Google Scholar]
- 28. Hardt M., Baron T., Groschup M. H. 2000. A comparative study of immunohistochemical methods for detecting abnormal prion protein with monoclonal and polyclonal antibodies. J. Comp. Pathol. 122:43–53 [DOI] [PubMed] [Google Scholar]
- 29. Hill A. F., et al. 1997. The same prion strain causes vCJD and BSE. Nature 389:448–526 [DOI] [PubMed] [Google Scholar]
- 30. Hirschberger T. 2007. Entwicklung von Medikamenten gegen Prionkrankheiten. Ph.D. dissertation Fakultät für Physik, Ludwig-Maximilians-Universität München, Munich, Germany [Google Scholar]
- 31. Hope J., et al. 1986. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 5:2591–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ingrosso L., Ladogana A., Pocchiari M. 1995. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 69:506–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kascsak R. J., et al. 1987. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 61:3688–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kocisko D. A., et al. 2003. New inhibitors of scrapie-associated prion protein formation in a library of 2,000 drugs and natural products. J. Virol. 77:10288–10294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kocisko D. A., Morrey J. D., Race R. E., Chen J., Caughey B. 2004. Evaluation of new cell culture inhibitors of protease-resistant prion protein against scrapie infection in mice. J. Gen. Virol. 85:2479–2483 [DOI] [PubMed] [Google Scholar]
- 36. Kocisko D. A., et al. 2005. Comparison of protease-resistant prion protein inhibitors in cell cultures infected with two strains of mouse and sheep scrapie. Neurosci. Lett. 388:106–111 [DOI] [PubMed] [Google Scholar]
- 37. Kocisko D. A., Caughey B. 2006. Mefloquine, an antimalaria drug with antiprion activity in vitro, lacks activity in vivo. J. Virol. 80:1044–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kocisko D. A., et al. 2006. A porphyrin increases survival time of mice after intracerebral prion infection. Antimicrob. Agents Chemother. 50:759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kupfer L., Eiden M., Buschmann A., Groschup M. H. 2007. Amino acid sequence and prion strain specific effects on the in vitro and in vivo convertibility of ovine/murine and bovine/murine prion protein chimeras. Biochim. Biophys. Acta 6:704–713 [DOI] [PubMed] [Google Scholar]
- 40. Lasmézas C. I., et al. 1996. BSE transmission to macaques. Nature 381:743–744 [DOI] [PubMed] [Google Scholar]
- 41. Magri G., et al. 2005. Decrease in pathology and progression of scrapie after immunisation with synthetic prion protein peptides in hamsters. Vaccine 23:2862–2868 [DOI] [PubMed] [Google Scholar]
- 42. Mange A., Milhavet O., McMahon H. E., Casanova D., Lehmann S. 2000. Effect of amphotericin B on wild-type and mutated prion proteins in cultured cells: putative mechanism of action in transmissible spongiform encephalopathies. J. Neurochem. 74:754–762 [DOI] [PubMed] [Google Scholar]
- 43. Manna F., et al. 2002. Inhibition of amine oxidases activity by 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. Bioorg. Med. Chem. Lett. 12:3629–3633 [DOI] [PubMed] [Google Scholar]
- 44. McLean P. J., Klucken J., Shin Y., Hyman B. T. 2004. Geldanamycin induces Hsp70 and prevents α-synuclein aggregation and toxicity in vitro. Biochem. Biophys. Res. Commun. 321:665–669 [DOI] [PubMed] [Google Scholar]
- 45. Newman P. K. 1984. Acyclovir in Creutzfeldt-Jakob disease. Lancet i:793. [DOI] [PubMed] [Google Scholar]
- 46. Otto M., et al. 2004. Efficacy of flupirtine on cognitive function in patients with CJD: a double-blind study. Neurology 62:714–718 [DOI] [PubMed] [Google Scholar]
- 47. Pan T., et al. 2005. An aggregation-specific enzyme-linked immunosorbent assay: detection of conformational differences between recombinant PrP protein dimers and PrPSc aggregates. J. Virol. 79:12355–12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parry A., Baker I., Stacey R., Wimalaratna S. 2007. Long term survival in a patient with variant Creutzfeld-Jakob disease treated with intraventricular pentosan polysulphate. J. Neurol. Neurosurg. Psychiatr. 78:733–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pocchiari M., Schmittinger S., Masullo C. 1987. Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J. Gen. Virol. 68:219–223 [DOI] [PubMed] [Google Scholar]
- 50. Priola S. A., Raines A., Caughey W. S. 2000. Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503–1506 [DOI] [PubMed] [Google Scholar]
- 51. Prusiner S. B., et al. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35:349–358 [DOI] [PubMed] [Google Scholar]
- 52. Prusiner S. B. 1998. The prion diseases. Brain Pathol. 8:499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rochet J. C. 2003. Novel therapeutic strategies for the treatment of protein-misfolding diseases. Expert Rev. Mol. Med. 9:1–34 [DOI] [PubMed] [Google Scholar]
- 54. Smith P. G., Bradley R. 2003. Bovine spongiform encephalopathy (BSE) and its epidemiology. Br. Med. Bull. 66:185–198 [DOI] [PubMed] [Google Scholar]
- 55. Soti C., Csermely P. 2002. Chaperones and aging: role in neurodegeneration and in other civilizational diseases. Neurochem. Int. 41:383–389 [DOI] [PubMed] [Google Scholar]
- 56. Tagliavini F., et al. 1997. Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science 276:1119–1122 [DOI] [PubMed] [Google Scholar]
- 57. Terzano M. G., Montanari E., Calzetti S., Mancia D., Lechi A. 1983. The effect of amantadine on arousal and EEG patterns in Creutzfeldt-Jakob disease. Arch. Neurol. 40:555–559 [DOI] [PubMed] [Google Scholar]
- 58. Vetrugno V., et al. 2009. Oral pravastatin prolongs survival time of scrapie-infected mice. J. Gen. Virol. 90:1775–1780 [DOI] [PubMed] [Google Scholar]
- 59. Wadsworth J. D., et al. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171–180 [DOI] [PubMed] [Google Scholar]
- 60. White A. R., et al. 2003. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 422:80–83 [DOI] [PubMed] [Google Scholar]
- 61. Whittle I. R., Knight R. S., Will R. G. 2006. Unsuccessful intraventricular pentosan polysulphate treatment of variant Creutzfeldt-Jakob disease. Acta Neurochir. 148:677–679 [DOI] [PubMed] [Google Scholar]
- 62. Will R. G., Ironside J. W., Hornlimann B., Zeidler M. 1996. Creutzfeldt-Jakob disease. Lancet 347:65–66 [PubMed] [Google Scholar]