Abstract
We previously reported that the multidrug-resistant (MDR) Acinetobacter baumannii strain MDR-ZJ06, belonging to European clone II, was widely spread in China. In this study, we report the whole-genome sequence of this clinically important strain. A 38.6-kb AbaR-type genomic resistance island (AbaR22) was identified in MDR-ZJ06. AbaR22 has a structure similar to those of the resistance islands found in A. baumannii strains AYE and AB0057, but it contained only a few antibiotic resistance genes. The region of resistant gene accumulation as previously described was not found in AbaR22. In the chromosome of the strain MDR-ZJ06, we identified the gene blaoxa-23 in a composite transposon (Tn2009). Tn2009 shared the backbone with other A. baumannii transponsons that harbor blaoxa-23, but it was bracketed by two ISAba1 elements which were transcribed in the same orientation. MDR-ZJ06 also expressed the armA gene on its plasmid pZJ06, and this gene has the same genetic environment as the armA gene of the Enterobacteriaceae. These results suggest variability of resistance acquisition even in closely related A. baumannii strains.
INTRODUCTION
Acinetobacter baumannii is an important opportunistic pathogen that has caused global outbreaks of nosocomial infection (7, 24). A. baumannii rapidly increases antibiotic resistance due to the presence of mobile genetic elements, such as insertion sequences (ISs), plasmids, and resistance islands (23). Notably, all of these genetic elements vary even among closely related isolates of A. baumannii (1). Resistance islands differ in length and gene content (26). To date, the largest one is AbaR1 in AYE, which contains 45 genes associated with antibiotic, antiseptic, and heavy metal resistance within an 86-kb region (9). In contrast, AbaR2 in ACICU is much shorter (ca. 8.9 kb) and encodes only seven resistance genes, lacking arsenic, mercury, or a tetracycline resistance gene (14).
Our previous studies determined six clones (clones A to F) of imipenem-resistant A. baumannii isolates (IRABs) in China by pulsed-field gel electrophoresis (PFGE). Among these clones, clone C has the overwhelming majority of the isolates (160/342) that have been identified in different cities (9/16) (34). Using multilocus sequence typing (MLST) with seven standard housekeeping loci, gltA, gyrB, gdhB, recA, cpn60, gpi, and rpo, we assigned MDR-ZJ06, an isolate of clone C, to sequence type (ST) 90 (1-3-3-2-2-62-3), which was clustered into clonal complex 22 (3, 34).
In order to determine the genetic basis of multiple-drug resistance of clone C, in this study we completely sequenced MDR-ZJ06 and performed comparative analyses.
MATERIALS AND METHODS
Bacterial strains and susceptibility test.
A. baumannii isolate MDR-ZJ06 was isolated on 20 April 2006 from the bloodstream of a patient hospitalized in the intensive care unit of the first affiliated hospital at Zhejiang University in Hangzhou, China. The patient suffered from acute exacerbations of chronic obstructive pulmonary disease, respiratory failure, and ventilator-associated pneumonia (VAP). MDR-ZJ06 was considered to be the pathogen that caused the bloodstream infection. A. baumannii strains with the same resistant profile as MDR-ZJ06 were also isolated from sputum specimens twice on 15 April and 23 April, so MDR-ZJ06 might also be the causative agent of VAP. Imipenem and cefoperazone-sulbactam were administered successively until this patient died on 27 April 2006. Susceptibility testing for MDR-ZJ06 was performed using the Etest strip according to the manufacturer's instructions. Results were interpreted according to published recommendations (5a). The breakpoint of tigecycline and rifampin for A. baumannii was not available.
High-density pyrosequencing and sequence assembly.
The genomic DNA of A. baumannii MDR-ZJ06 was prepared using Wizard Genomic DNA purification kits (Promega) according to the manufacturer's instructions. The genomic DNA (3 to 5 μg) was fragmented by nebulization, and DNA fragments were subjected to the complete sequencing work flow of the 454 genome sequencer FLX system (Roche, Basel, Switzerland). Initial assembly was performed by the 454 life Sciences software program newbler. Contigs were aligned to reference genomes to construct the scaffolds, and primer pairs were subsequently designed to close the gaps by sequencing PCR products using the dideoxy-mediated chain termination method (ABI3730; Applied Biosystems, Foster City, CA). Two lanes on an Illumina sequencer (Illumina/Solexa; Illumina Inc., San Diego, CA) were used, both of which were single-end runs of 35 bp, and the acquired short reads were mapped to the MDR-ZJ06 genome by using SOAP software tools (17).
Genome annotation.
Gene prediction was performed by two independent software programs, Glimmer and GeneMark (8, 20). Open reading frames (ORFs) that were predicted by both the programs are considered bona fide ones, and discrepant ORFs were then manually verified by identification of putative ribosomal binding sites. tRNA genes were predicted using the tRNAscan-SE tools (19). The RNAmmer1.2 software program was used to predict 5S, 16S, and 23S rRNA in full-genome sequences (16). ISs were characterized by using the IS Finder database (www-is.biotoul.fr). The Phage Finder program was used to identified the prophage or prophage remnant on the chromosome (10). Functional classification was performed by aligning predicted proteins to the COG (cluster of orthologous group) database (28). All of the predicted proteins were compared to the nonredundant (nr) protein database of NCBI (www.ncbi.nlm.nih.gov) using BLASTP with a cutoff E-value of ≤1e−4, identity of ≥35%, and coverage length of ≥80%. To further analyze protein functions, protein domains were screened by the InterProScan software program (22).
Comparative genomics.
Data used in comparative analysis were downloaded from the NCBI database (ftp://ftp.ncbi.nlm.nih.gov/GenBank/genomes/Bacteria/), including complete genome sequences and annotation of A. baumannii isolates AB0057 (GenBank accession no. CP001182), AB307-0294 (CP001172), ATCC 17978 (CP000521), ACICU (CP000863), AYE (CU459141), and SDF (CU468230), as well as the draft assembled genomes of AB0056 (GenBank accession no. ADGZ00000000), AB0058 (ADHA00000000), and AB0059 (ADHB00000000). The bidirectional best-hit (BBH) method and BLASTP algorithm were used to construct the orthologues in different A. baumannii isolates, with a cutoff of ≥50% amino acid similarity and ≥80% coverage in length. Other in-house-developed Perl scripts were used for information integration and graphic presentation.
PCR amplification of genetic environment of blaoxa-23.
The genetic environment of blaoxa-23 was confirmed by PCR amplification and sequence analysis. The three pairs of primers were listed as follows: F1 (5′-GTAATACGGAGCGTCTGACT-3′) and R1 (5′-ACGCTTCTGCATGAGCTTCT-3′), F2 (5′-CAGATGCAGCAGATCCAATG-3′) and R2 (5′-ACCAGGTGCAACTGTTGACT-3′), and F3 (5′-ATCCTGATGCTCGCAATCGT-3′) and R3 (5′-CTGTCTGCGAACACATTCAC-3′). The amplicons were sequenced with an ABI 3730 automatic sequencer using the Sanger chain-termination method.
Analysis of blaampC and adeB gene expression by real-time RT-PCR.
For gene expression studies, total RNA was prepared using the TRIzol Max method (Invitrogen, Carlsbad, CA). Real-time reverse transcriptase PCR (RT-PCR) was performed using 250 ng of DNase-treated RNA, a PrimeScript RT reagent kit (Takara, Japan), and specific internal blaampC and adeB primer pairs. Expression of the endogenous control 16S rRNA gene was used to normalize data. The A. baumannii ATCC 19606 strain was used as the reference strain. Real-time RT-PCRs were carried out using an Opticon 2 real-time PCR detector, and the results were analyzed with the Opticon 2 real-time PCR detection software program.
Nucleotide sequence accession numbers.
The A. baumannii MDR-ZJ06 chromosome and pZJ06 plasmid sequences were submitted to the GenBank database and can be found under accession numbers CP001937 and CP001938, respectively.
RESULTS AND DISCUSSION
Susceptibility profiles.
As shown in Table 1, we found that A. baumannii MDR-ZJ06 was resistant to carbapenems, cephalosporins, penicillins, β-lactamase inhibitor combinations, aminoglycosides, quinolones, chloramphenicol, trimethoprim-sulfamethoxazole, and minocycline. It was susceptible only to colistin. The MIC values of tigecycline and rifampin were both 8 mg/liter.
Table 1.
Susceptibility profile of strain MDR-ZJ06
| Antimicrobial drug | MIC (mg/liter) |
|---|---|
| Imipenem | >32 |
| Meropenem | >32 |
| Cefepime | 128 |
| Ceftazidime | 128 |
| Piperacillin | >256 |
| Piperacillin-tazobactam | >256 |
| Ampicillin-sulbactam | 128 |
| Cefoperazone-sulbactam | 64 |
| Aztreonam | 128 |
| Amikacin | >256 |
| Gentamicin | >256 |
| Ciprofloxacin | >32 |
| Minocycline | 32 |
| Colistin | 0.38 |
| Tigecycline | 8 |
| Sulfamethoxazole-trimethoprim | 32 |
| Chloramphenicol | 256 |
| Rifampin | 8 |
Genome annotation and comparative analysis.
The chromosome and plasmid of A. baumannii MDR-ZJ06 were completely sequenced and quality promoted by high-throughput sequencing. The sequencing process is summarized in Table 2. Ab initio methods and comparative analysis were both used to identify the coding regions, and the followed function category showed us the genome features of MDR-ZJ06 that were similar to those of other sequenced A. baumannii strains.
Table 2.
A. baumannii MDR-ZJ06 genome sequencing
| Characteristic for genome assembly | Value |
|---|---|
| No. of total reads | 170,800 |
| No. of assembled reads | 166,353 |
| No. of contigs | 131 |
| Avg contig size (kb) | 34.8 |
| Largest size (kb) | 202 |
| No. of PCR for gap filling | 2,015 |
| No. of short Solexa reads | 7,629,819 |
| No. of Solexa reads mapped to chromosome | 2,983,796 |
The genetic features of the chromosome and plasmid of MDR-ZJ06 are summarized in Table 3. Its genome and plasmid sizes are approximately 4 million and 20,000 bp, respectively. The genome and plasmid were predicted to encode 3,887 and 29 proteins, respectively.
Table 3.
General features of A. baumannii MDR-ZJ06 genome and plasmid
| Element and characteristic | Value |
|---|---|
| Chromosome | |
| Size (bp) | 3,991,133 |
| Coding regions (%) | 87 |
| G+C content (%) | 39 |
| No. of protein-coding sequences | 3,887 |
| No. of tRNA genes | 69 |
| No. of rRNA operons | 6 |
| No. of insertion sequences | 41 |
| Plasmid pZJ06 | |
| Size (bp) | 20,301 |
| Coding regions (%) | 87 |
| G+C content (%) | 47.4 |
| No. of protein-coding sequences | 29 |
| No. of insertion sequences | 6 |
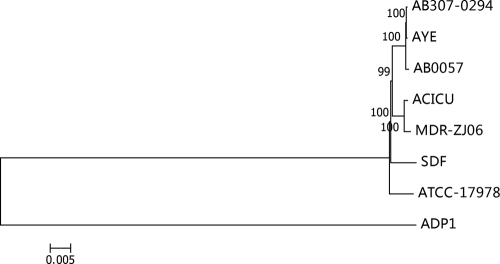
Gene content was comparatively analyzed between completely sequenced A. baumannii strains (Table 4). Sequence analyses indicated that among the sequenced genomes of A. baumannii strains, the genome of MDR-ZJ06 is closest in sequence to that of ACICU, another isolate of the European clone II (ECII) strain. Between MDR-ZJ06 and ACICU, approximately 90% of total genes are conserved. Phylogenetic analysis also revealed their closest relationship (Fig. 1). Comparative analysis between all the completely sequenced or the draft assembled genomes derived 371 ECII lineage-specific genes, most of which were of unknown function. One hundred twenty-three of these genes were common between strains ACICU and MDR-ZJ06, including transposase and prophage genes and those involved in cell wall/membrane/envelope biogenesis.
Table 4.
Comparison of conserved proteins between different A. baumannii strains
| Strain | No. of proteins conserved |
||||||
|---|---|---|---|---|---|---|---|
| ZP6 | ACICU | AYE | AB0057 | AB307 0294 | SDF | ATCC 17978 | |
| ZP6 | 3,887 | 3,312 | 3,038 | 3,062 | 2,979 | 2,245 | 2,065 |
| ACICU | 3,667 | 3,022 | 3,059 | 2,962 | 2,195 | 2,115 | |
| AYE | 3,607 | 3,207 | 3,117 | 2,219 | 2,075 | ||
| AB0057 | 3,790 | 3,124 | 2,160 | 2,104 | |||
| AB307 0294 | 3,451 | 2,128 | 2,083 | ||||
| SDF | 2,913 | 1,563 | |||||
| ATCC 17978 | 3,351 | ||||||
Fig. 1.
Phylogenetic analyses of Acinetobacter baumannii isolates. The phylogenetic tree of eight sequenced Acinetobacter baumannii genomes was constructed according to the orthologous proteins in multigenomes. The 1,200 protein sequences conserved among the sequenced Acinetobacter baumannii genomes were aligned using the software program MUSCLE, and the neighbor-joining tree was constructed using the MEGA4 program. The bootstrap was set to 1,000 replicates with the seed 24,054. The genome of Acinetobacter baylyi ADP1 was used as the outgroup to root the tree. The scale unit is substitutions per site.
Resistance island.
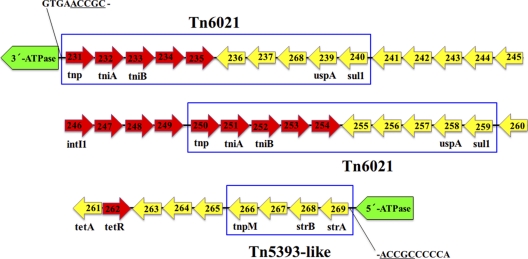
Genome analyses revealed that MDR-ZJ06 has a genomic island (AbaR22) that is inserted into the ATPase gene comM (Fig. 2). AbaR22 contains 40 genes within a 38.683-kb region. The flanked sequences at both ends of AbaR22, including the identical 5-bp direct repeat (5′-ACCGC-3′), were highly similar to those of AbaR1 and the genomic island in A. baumannii SDF (AbaG1) (9).
Fig. 2.
The gene structure of the resistance island AbaR22 in MDR-ZJ06. AbaR22 is inserted into the ATPase gene, and the sequence underlined is the direct repeat. Predicted genes in AbaR22 are displayed as forward/reverse arrows, whose direction is identical to the gene transcription direction. The numbers in the arrows represent the gene locus tags, e.g., 231 is the abbreviated form of ABZJ_00231. The regions marked by the rectangle frames are the transposons (or remnant).
A 16.3-kb backbone of AbaR-type resistance islands, termed Tn6019, has been derived from comparative analysis (25). AbaR22 was basically made of transposon Tn6021. AbaR22 contains two copies of Tn6021, which was identified in the antibiotic-susceptible strain ATCC 17978 in the same location (26). Tn6021 of AbaR22 contains the sul1 resistance genes (ABZJ_00240 and ABZJ_00259) and genes corresponding to universal stress proteins (uspA) (ABZJ_00239 and ABZJ_00258), which share more than 94% amino acid identity with those of ATCC 17978. In addition to sul1 and uspA, transposases encoded within Tn6021 and two putative proteins (ABZJ_00234 and ABZJ_00235) with unknown function were conserved among AbaR22 and the resistance islands in AYE (AbaR1), AB0057 (AbaR3), and ATCC 17978. The tetracycline efflux pump and its regulator genes, tetA and tetR, whose products share the amino acid similarity of ca.46% and 53% with AbaR1 and AbaR3, were also found in AbaR22. However, StrA and StrB were conserved only between AbaR22 and AbaR1, showing ca. 97% amino acid similarity.
Additionally, AbaR22 contains a truncated Tn5393-like transposon, which is highly similar to that in AbaR1. In this location, AbaR22 and AbaR1 contain the homologues of the aminoglycoside resistance genes strAB (ABZJ_00268 and ABZJ_00269) and the transposase gene tnpM (ABZJ_00261), whose products share more than 97% and 66% amino acid identity, respectively.
In summary, AbaR22 in MDR-ZJ06 is similar to the resistance islands in European clone I (ECI) strains and was divergent from AbaR2 in ECII strain ACICU. With a few genes (such as strA, strB, sul1, tetA, and tetR) associated with antibiotic resistance being found, AbaR22 clearly lacks the multiple antibiotic resistance regions as previously described (26).
blaoxa-23 and its genetic environment.
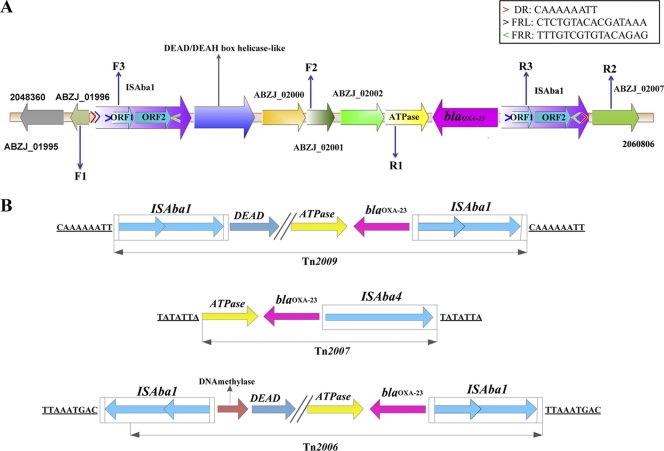
The blaoxa-23 gene, associated with carbapenems resistance, has been identified in A. baumannii isolates around the world (5). Several genetic structures surrounding blaoxa-23, such as the plasmid-mediated composite transposons Tn2006 and Tn2007 and the genomic island AbaR4 (2, 6), have been found in A. baumannii isolates. In MDR-ZJ06, chromosome location of blaoxa-23 was previously predicted from the results of Southern blotting and conjugation (34) and was confirmed in this study. Genome analyses revealed that blaoxa-23 is located in an 8.3-kb transposon (designated Tn2009) that is inserted into a cluster of genes involved in P pilus assembly, with a 9-bp target site duplication (5′-CAAAAAATT-3′). Tn2009 is flanked by two ISAba1 elements, in both of which a 16-bp inverted repeat was found (5′-CTCTGTACACGATAAA-3′) (Fig. 3). Interestingly, two copies of ISAba1 in Tn2009 are transcribed in the same orientation, whereas those in Tn2006 and AbaR4 are opposite (6).
Fig. 3.
Genetic structure of Tn2009 in Acinetobacter baumannii MDR-ZJ06. The detailed structure of Tn2009 (A) and a comparison of blaoxa-23-containing transposons (B) are shown. DR, direct repeat; FRL/FRR, invert repeat. Primer pairs (F1 and R1, F2 and R2, and F3 and R3) were used to determine the structure. The underlined sequences are the direct repeats flanking the insertion segments.
Compared to the sequence of Tn2006, there is an additional 2-kb segment in Tn2009 between the truncated DEAD/DEAH box helicase-like gene and the ATPase gene, encoding three putative proteins. Notably, the DEAD/DEAH box helicase-like gene and these hypothetical ORFs are all absent in Tn2007 and AbaR4.
Plasmid pZJ06 and the armA gene.
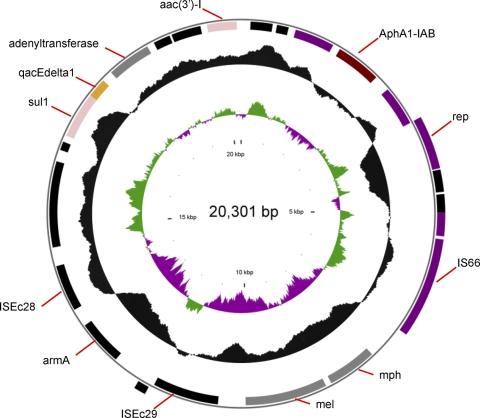
Plasmid pZJ06 contains a 16S rRNA methylase gene (armA), an aminoglycoside 3′-phosphotransferase gene [aph(3′)-I], a macrolide efflux protein-coding gene (mel), and a class I integron, which contained aminoglycoside acetyltransferase (aacC1) and adenyltransferase (aadA1) (Fig. 4). pZJ06-carried armA is located in Tn1548, and this kind of structure was also identified in a plasmid of A. baumannii (EU014811), as well as a variety of plasmids of the Enterobacteriaceae, such as pKT51748 of Klebsiella pneumoniae (GenBank accession no. FJ715937), pMUR050 of Escherichia coli (AY522431), pCOP1 of E. coli (FJ187822), pCTX-M3 of Citrobacter freundii (AF550415), and pKSMA0710 of Serratia marcescens (FJ917355). The armA genetic environment of MDR-ZJ06 is also identical to those of Klebsiella oxytoca and K. pneumoniae, which were isolated from the same region as MDR-ZJ06 (15, 33).
Fig. 4.
Circular map of plasmid pZJ06. The two outer circles represent ORFs in the plus (outside) and minus (inside) orientations, respectively. The two inner circles represent the G+C content plotted against the average G+C content of 47.4% (black circle) and GC skew information (green and purple circles).
Posttranscriptional rRNA methylation by 16S rRNA methylase has been associated with a high level of resistance to aminoglycosides in the Enterobacteriaceae (12, 31). About 66.4% of IRABs in China are armA positive and are resistant to aminoglycosides (32). The present work found that plasmid-carried armA is present in a dominant clone of IRABs in China, suggesting that the plasmid confers the widespread ArmA methylase-mediated aminoglycoside resistance in A. baumannii isolates. According to the methods described by Bertini et al. (4), the replicon of pZJ06 was classified in the Rep-3 group of A. baumannii with a similarity of ca. 80%, suggesting that pZJ06 may be particularly associated with A. baumannii. Nevertheless, the Tn1548-harboring armA gene in pZJ06 is identical to that of plasmids of members of the Enterobacteriaceae isolated from the worldwide regions, suggesting the possibility of Tn1548-mediated horizontal transfer of the armA gene between plasmids of the Enterobacteriaceae and Acinetobacter spp.
Other resistance mechanisms in MDR-ZJ06.
Our genome analyses have also identified some other genes associated with resistance to β-lactams, aminoglycosides, quinolones, tetracyclines, chloramphenicol, and sulfonamide, including a cephalosporinase gene, an aminoglycoside degradation enzyme-coding gene, a 16S rRNA methylase gene, multidrug efflux genes, and others (Table 5).
Table 5.
Antimicrobial resistance-associated genes in MDR-ZJ06 genome
| Antimicrobial class | Enzyme class/family | Coding gene(s) | Locus tag(s); genetic location |
|---|---|---|---|
| β-Lactamases | Intrinsic cephalosporinase | blaadc-30 | ABZJ_02776; chromosome |
| Class D OXA enzymes | blaoxa-23 | ABZJ_02004; chromosome | |
| blaoxa-66 | ABZJ_01736; chromosome | ||
| Enzymatic degradation | aac(2′)-Ib | ABZJ_00200; chromosome | |
| aph(6)-Id | ABZJ_00268; chromosome | ||
| aph(3″)-Ib | ABZJ_00269; chromosome | ||
| aac(6)-Ib | ABZJ_01297; chromosome and integron association | ||
| Aminoglycosides | Enzymatic degradation | aadA1 | ABZJ_01300; chromosome and integron association |
| aadA1 | pABZJ_00026; plasmid and integron association | ||
| aacC1 | pABZJ_00029; plasmid and integron association | ||
| 16S rRNA gene methylase | aphA1-IAB | pABZJ_00004; plasmid and integron association | |
| armA | pABZJ_00017; plasmid | ||
| Tetracyclines | Efflux | tetA | ABZJ_00261; chromosome |
| Quinolone | DNA gyrase mutations | gyrA (Ser-Ler) mutation at position 83 | ABZJ_02465; chromosome |
| Chloramphenicol | Acetyltransferase | catB6 | ABZJ_01329; chromosome |
| catB6 | ABZJ_01299; chromosome and integron association | ||
| Sulfonamide | Dihydropteroate synthase | sul1 | ABZJ_03130, ABZJ_01302, ABZJ_00240, ABZJ_00259; chromosome |
| Efflux pumps | RND family | adeABC | ABZJ_02017, ABZJ_02018, ABZJ_02019; chromosome |
| adeIJK | ABZJ_03188, ABZJ_03189, ABZJ_03190; chromosome | ||
| abeM | ABZJ_00435; chromosome | ||
| adeT | ABZJ_03885; chromosome |
IS elements are associated with the genome gain/loss of genes, especially resistant genes. Apart from IS elements, ISAba1 could also provide strong promoters to upregulate the expression of antibiotic resistance genes (21, 27, 29). IS30, ISAba6, and ISAba1 were found upstream of blaampC in A. baumannii isolates ACICU, SDF, and AYE, respectively. In this study, ISAba1 was found to be present in the same location in MDR-ZJ06. ISAba1 has been shown to play a role in upregulation of blaampC in A. baumannii isolates (13). We found that blaampC expression was more than 26-fold higher in MDR-ZJ06 than in ATCC 19606 (data not shown).
A class 1 integron, including the integrase gene (ABZJ_01295) and three resistance genes, aac(6)-Ib (ABZJ_01297), catB8 (ABZJ_01299), and aadA1 (ABZJ_01300), was also found in the chromosome of MDR-ZJ06, in addition to AbaR22 and the plasmid. These genetic elements may also play a role in the drug resistance of MDR-ZJ06.
Multidrug efflux pumps and porins may play roles in A. baumannii antimicrobial resistance (30). In this study, our genome analyses revealed the porins CarO (ABZJ_03018) and OprD-like proteins (ABZJ_00224, ABZJ_01013, ABZJ_01357, and ABZJ_02177), but these genes were complete and not disrupted. The coding genes of transporters AdeABC, AdeIJK, AdeT, and AbeM were also in MDR-ZJ06. The AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-A. baumannii complex (30). The overexpression of the adeB gene was also detected in strain MDR-ZJ06 (data not shown), which might result in the increased MIC to tigecycline. The roles of effluxes and porins in drug resistance in MDR-ZJ06 need to be further defined.
The production of pili is essential for biofilm formation by this clinical strain (11). These pili are the products of the csuA/BABCDE operon. Interestingly, the csuA/B operon, which is present in the A. baumannii strains ACICU, ATCC 19606, AB0057, AYE, and ATCC 17978, was not found in MDR-ZJ06. The P pilus assembly protein PapD, a homolog of the staphylococcal biofilm-associated protein that is conserved among Acinetobacter strains, was found in MDR-ZJ06 (ABZJ_01996), indicating that this protein may play a role in MDR-ZJ06 adhesion and its biofilm formation (18).
Conclusions.
Genomic analyses reveal that MDR-ZJ06, a widespread A. baumannii isolate in China, is genetically closest to the strain ACICU among completely sequenced A. baumannii isolates, indicating a worldwide spread of ECII. Accumulation of resistance genes was not detected in the genomic island AbaR22 in MDR-ZJ06, whereas some drug resistance genes are present in IS elements and the plasmid, suggesting that IS elements and the plasmid but not AbaR22 appear to be important in acquisition of resistance genes in this strain. This work may lay an important molecular foundation for future study of the mechanism of drug resistance in A. baumannii.
ACKNOWLEDGMENTS
This work was supported by research grants from the Ministry of Health of the People's Republic of China (no. 200802107), National Natural Science Foundation of China (no. NSFC30970113), and Natural Science Foundation of Zhejiang Province (no. 2007C23006).
Footnotes
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Adams M. D., Chan E. R., Molyneaux N. D., Bonomo R. A. 2010. Genome-wide analysis of divergence of antibiotic resistance determinants in closely related isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams M. D., et al. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartual S. G., et al. 2005. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 43:4382–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertini A., et al. 2010. Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:4168–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown S., Amyes S. 2006. OXA (beta)-lactamases in Acinetobacter: the story so far. J. Antimicrob. Chemother. 57:1–3 [DOI] [PubMed] [Google Scholar]
- 5a. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-S21 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Corvec S., Poirel L., Naas T., Drugeon H., Nordmann P. 2007. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis K. A., Moran K. A., McAllister C. K., Gray P. J. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 11:1218–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delcher A. L., Harmon D., Kasif S., White O., Salzberg S. L. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636–4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fournier P. E., et al. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fouts D. E. 2006. Phage_Finder: automated identification and classification of prophage regions in complete bacterial genome sequences. Nucleic Acids Res. 34:5839–5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaddy J. A., Actis L. A. 2009. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 4:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galimand M., Courvalin P., Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heritier C., Poirel L., Nordmann P. 2006. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12:123–130 [DOI] [PubMed] [Google Scholar]
- 14. Iacono M., et al. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 52:2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Y., et al. 2010. Complete nucleotide sequence of multi-drug resistance plasmid pKP048 of Klebsiella pneumoniae encoding blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54:3967–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lagesen K., et al. 2007. RNAmmer: consistent and rapid annotation of rRNA genes. Nucleic Acids Res. 35:3100–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li R., Li Y., Kristiansen K., Wang J. 2008. SOAP: short oligonucleotide alignment program. Bioinformatics 24:713–714 [DOI] [PubMed] [Google Scholar]
- 18. Loehfelm T. W., Luke N. R., Campagnari A. A. 2008. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 190:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lowe T. M., Eddy S. R. 1997. tRNAscan-SE: a program for improved detection of tRNA genes in genomic sequence. Nucleic Acids Res. 25:955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lukashin A. V., Borodovsky M. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mugnier P. D., Poirel L., Nordmann P. 2009. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii. J. Bacteriol. 191:2414–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulder N., Apweiler R. 2007. InterPro and InterProScan: tools for protein sequence classification and comparison. Methods Mol. Biol. 396:59–70 [DOI] [PubMed] [Google Scholar]
- 23. Peleg A. Y., Seifert H., Paterson D. L. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez F., et al. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Post V., Hall R. M. 2009. AbaR5, a large multiple-antibiotic resistance region found in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:2667–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Post V., White P. A., Hall R. M. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1162–1170 [DOI] [PubMed] [Google Scholar]
- 27. Segal H., Garny S., Elisha B. G. 2005. Is IS(ABA-1) customized for Acinetobacter? FEMS Microbiol. Lett. 243:425–429 [DOI] [PubMed] [Google Scholar]
- 28. Tatusov R. L., Koonin E. V., Lipman D. J. 1997. A genomic perspective on protein families. Science 278:631–637 [DOI] [PubMed] [Google Scholar]
- 29. Turton J. F., et al. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258:72–77 [DOI] [PubMed] [Google Scholar]
- 30. Vila J., Marti S., Sanchez-Cespedes J. 2007. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 59:1210–1215 [DOI] [PubMed] [Google Scholar]
- 31. Yokoyama K., et al. 2003. Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet 362:1888–1893 [DOI] [PubMed] [Google Scholar]
- 32. Yu Y. S., Zhou H., Yang Q., Chen Y. G., Li L. J. 2007. Widespread occurrence of aminoglycoside resistance due to ArmA methylase in imipenem-resistant Acinetobacter baumannii isolates in China. J. Antimicrob. Chemother. 60:454–455 [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y., et al. 2008. Plasmid-borne armA methylase gene, together with blaCTX-M-15 and blaTEM-1, in a Klebsiella oxytoca isolate from China. J. Med. Microbiol. 57:1273–1276 [DOI] [PubMed] [Google Scholar]
- 34. Zhou H., Yang Q., Yu Y. S., Wei Z. Q., Li L. J. 2007. Clonal spread of imipenem-resistant Acinetobacter baumannii among different cities of China. J. Clin. Microbiol. 45:4054–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]