Abstract
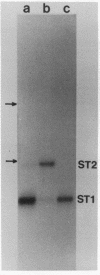
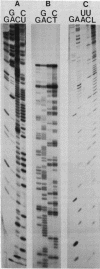
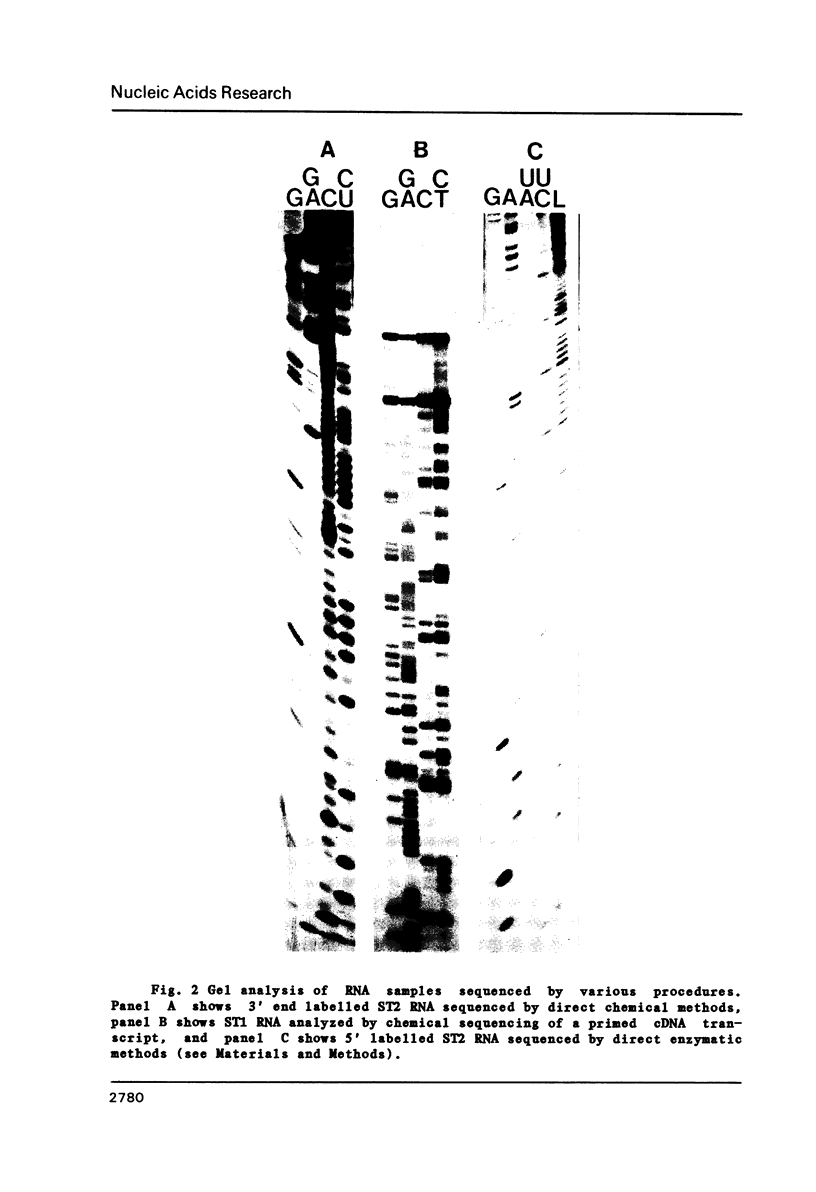
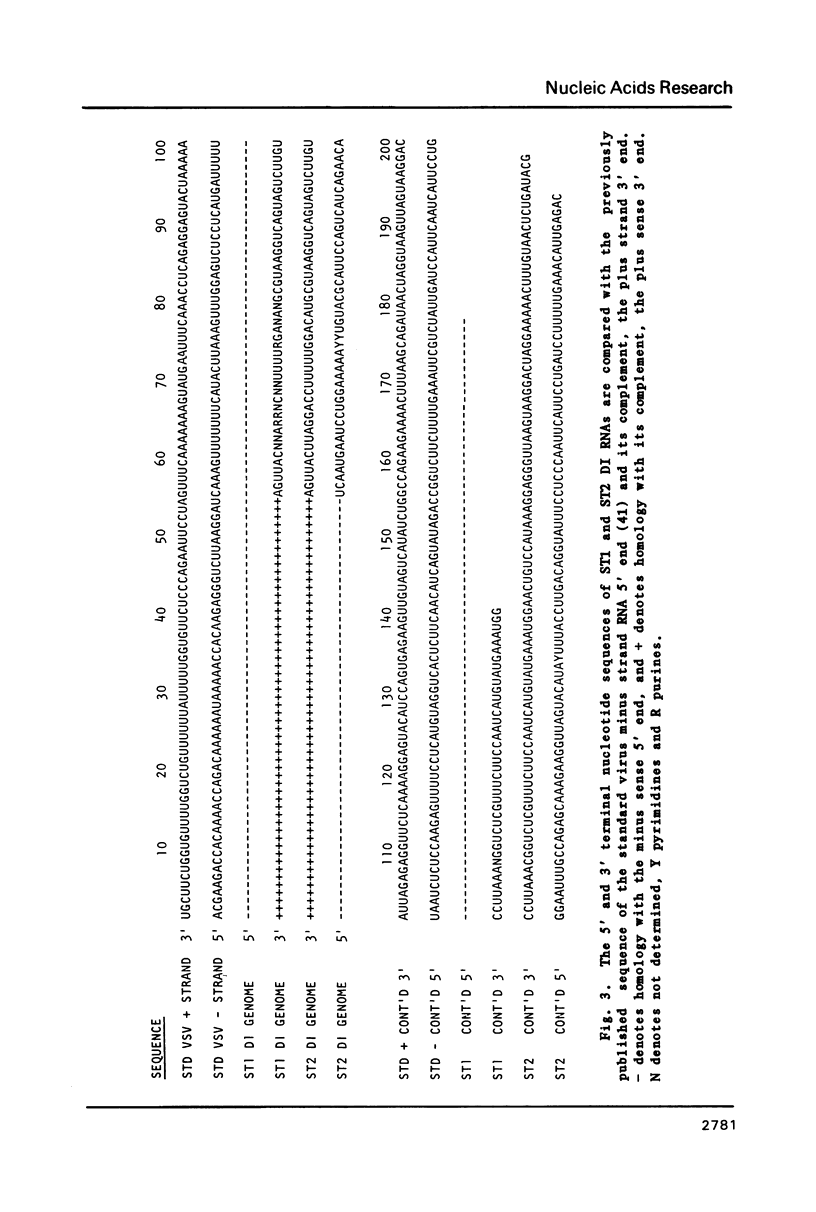
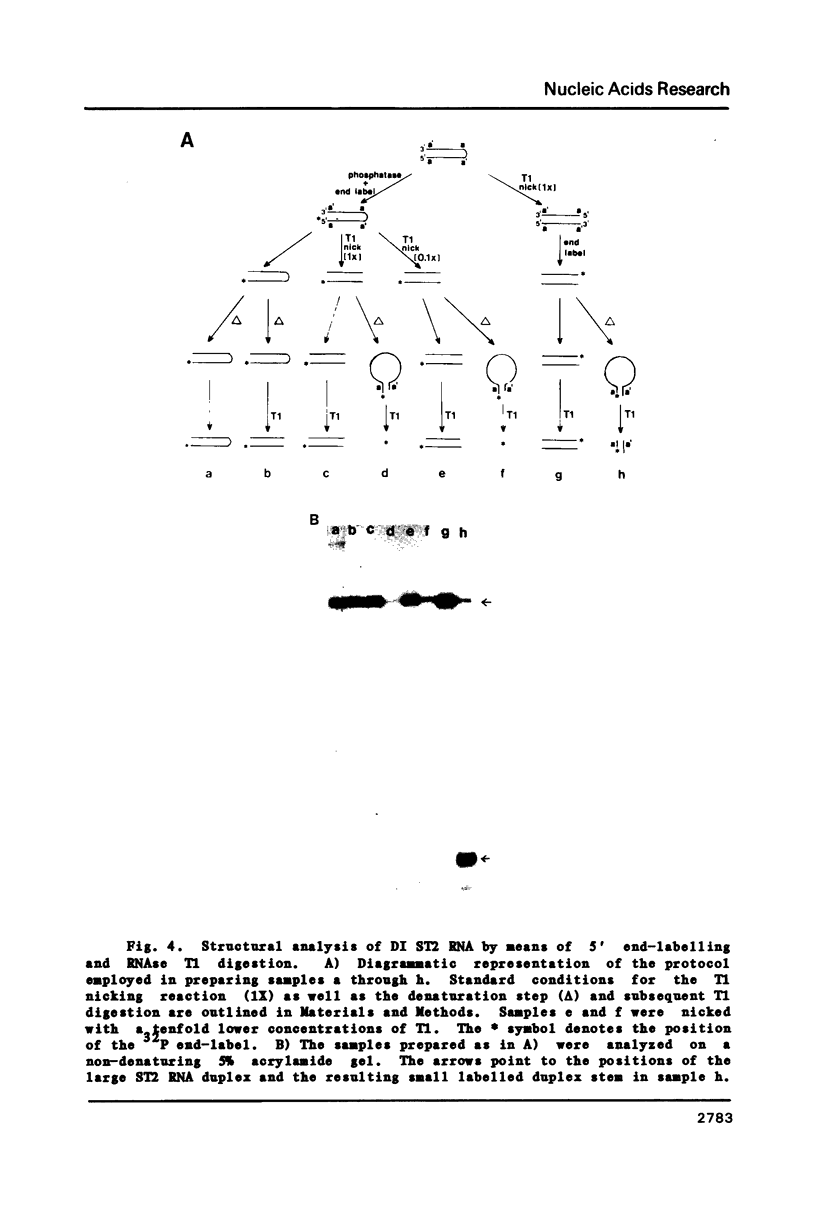
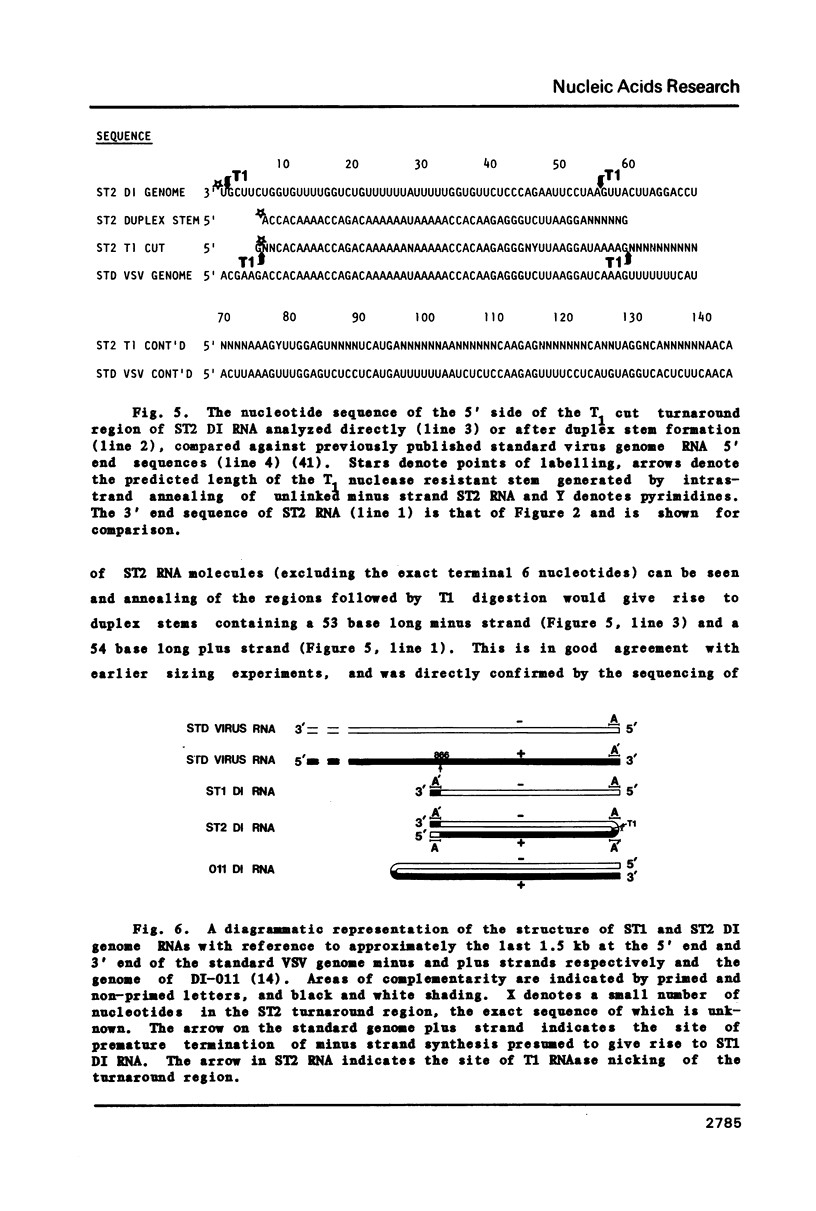
The genome structure and terminal sequences of a 'copyback' defective interfering (DI) particle ST1, and a novel complexly rearranged 'snapback' DI particle ST2 of vesicular stomatitis virus have been determined. The ST1 DI genome RNA possesses 54 base long inverted complementary termini, the 5' end of which is homologous to the standard virus genome 5' end. Following this region of inverted complementarity the DI RNA 5' end continues to be homologous to standard virus RNA 5' sequences, whereas the 3' end diverges into sequences within the virus L gene internal sequences. ST2 DI genome RNA does not contain colinear covalently linked plus and minus sense RNA copies of the standard infectious virus RNA 5' terminus as predicted from the prototype snapback DI structure, but instead appears to be a hairpin copy of the ST1 DI RNA genome. This is the first evidence suggesting that DI particles may be generated from RNA templates other than the standard virus RNA. Generation models and the implications of these findings for RNA virus evolution are discussed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De B. K., Perrault J. Sequence signal involved in the generation of an internally deleted defective interfering RNA from vesicular stomatitis virus. Nucleic Acids Res. 1982 Nov 11;10(21):6919–6930. doi: 10.1093/nar/10.21.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide sequences of influenza virus segments 1 and 3 reveal mosaic structure of a small viral RNA segment. Cell. 1982 Feb;28(2):303–313. doi: 10.1016/0092-8674(82)90348-8. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Horodyski F. M., Nichol S. T., Spindler K. R., Holland J. J. Properties of DI particle resistant mutants of vesicular stomatitis virus isolated from persistent infections and from undiluted passages. Cell. 1983 Jul;33(3):801–810. doi: 10.1016/0092-8674(83)90022-3. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P. A., Finch J. T., Winter G., Robertson J. S. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983 Sep;34(2):619–627. doi: 10.1016/0092-8674(83)90394-x. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation of vesicular stomatitis virus defective interfering genomes with different amounts of 5'-terminal complementarity. J Virol. 1982 Feb;41(2):566–574. doi: 10.1128/jvi.41.2.566-574.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Söderlund H., Keränen S., Pettersson R. F., Käriäinen L. 18S defective interfering RNA of Semliki Forest virus contains a triplicated linear repeat. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5353–5357. doi: 10.1073/pnas.78.9.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A. Sequence of 200 nucleotides at the 3'-terminus of the genome RNA of vesicular stomatitis virus. Nucleic Acids Res. 1979 Jul 25;6(10):3199–3211. doi: 10.1093/nar/6.10.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. A., Brownlee G. G. Sequence of DNA complementary to a small RNA segment of influenza virus A/NT/60/68. Nucleic Acids Res. 1981 Apr 24;9(8):1941–1947. doi: 10.1093/nar/9.8.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Characterization of snap-back RNAs in vesicular stomatitis defective interfering virus particles. J Gen Virol. 1978 Jan;38(1):21–34. doi: 10.1099/0022-1317-38-1-21. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Perrault J., Semler B. L. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D. D., Huang A. S. Interference among defective interfering particles of vesicular stomatitis virus. J Virol. 1982 Jan;41(1):210–221. doi: 10.1128/jvi.41.1.210-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Iverson L. Nucleotide sequences from the 3'-ends of vesicular stomatitis virus mRNA's as determined from cloned DNA. J Virol. 1979 Nov;32(2):404–411. doi: 10.1128/jvi.32.2.404-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands D. J. Sequences of vesicular stomatitis virus RNA in the region coding for leader RNA, N protein mRNA, and their junction. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4793–4797. doi: 10.1073/pnas.76.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Herman R. C., Lazzarini R. A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J Virol. 1980 May;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Lazzarini R. A. Structure and origin of a snapback defective interfering particle RNA of vesicular stomatitis virus. J Virol. 1981 Feb;37(2):661–672. doi: 10.1128/jvi.37.2.661-672.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian N., Nayak D. P. Defective interfering influenza RNAs of polymerase 3 gene contain single as well as multiple internal deletions. Virology. 1983 Jan 30;124(2):232–237. doi: 10.1016/0042-6822(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Horodyski F. M., Holland J. J. High multiplicities of infection favor rapid and random evolution of vesicular stomatitis virus. Virology. 1982 May;119(1):96–108. doi: 10.1016/0042-6822(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Yang F., Lazzarini R. A. Analysis of the recombination event generating a vesicular stomatitis virus deletion defective interfering particle. J Virol. 1983 Feb;45(2):766–772. doi: 10.1128/jvi.45.2.766-772.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]