Abstract
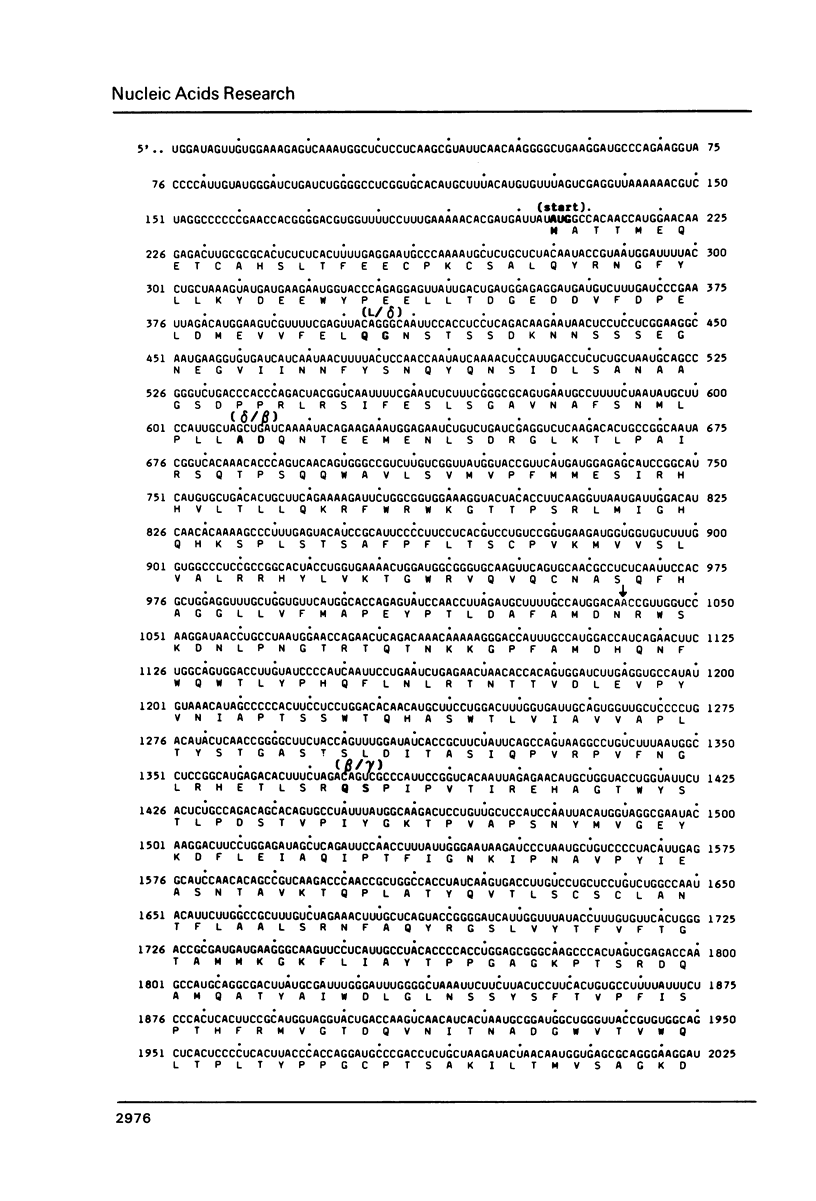
The nucleotide sequence of 7200 bases of encephalomyocarditis (EMC) viral RNA, including the complete polyprotein-coding region, was determined. The polyprotein is encoded within a unique translational reading frame, 6870 bases in length. Protein synthesis begins with the sequence Met-Ala-Thr, and ends with the sequence Leu-Phe-Trp, 126 bases from the 3' end of the RNA. Viral capsid and noncapsid proteins were aligned with the deduced amino acid sequence of the polyprotein. The proteolytic processing map follows the standard 4-3-4 picornaviral pattern except for a short leader peptide (8 kd), which precedes the capsid proteins. Identification of the proteolytic cleavage sites showed that EMC viral protease, p22, has cleavage specificity for gln-gly or gln-ser sequences with adjacent proline residues. The cleavage specificity of the host-coded protease(s) includes both tyr-pro and gln-gly sequences.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Kaesberg P. Determination of the length distribution of poly(A) at the 3' terminus of the virion RNAs of EMC virus, poliovirus, rhinovirus, RAV-61 and CPMV and of mouse globin mRNA. Nucleic Acids Res. 1979 Nov 10;7(5):1195–1204. doi: 10.1093/nar/7.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Beck E., Forss S., Strebel K., Cattaneo R., Feil G. Structure of the FMDV translation initiation site and of the structural proteins. Nucleic Acids Res. 1983 Nov 25;11(22):7873–7885. doi: 10.1093/nar/11.22.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. N., Stephenson P., Rowlands D. J., Brown F. Sequence and location of the poly C tract in aphtho- and cardiovirus RNA. Nucleic Acids Res. 1979 Jun 11;6(7):2381–2390. doi: 10.1093/nar/6.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Harris T. J., Rowlands D. J., Lowe P. A. The nucleotide sequence of cDNA coding for the structural proteins of foot-and-mouth disease virus. Gene. 1982 Feb;17(2):153–161. doi: 10.1016/0378-1119(82)90068-3. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chumakov K. M., Agol V. I. Poly(C) sequence is located near the 5'-end of encephalomyocarditis virus RNA. Biochem Biophys Res Commun. 1976 Jul 26;71(2):551–557. doi: 10.1016/0006-291x(76)90822-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Dorner L. F., Larsen G. R., Wimmer E., Anderson C. W. Identification of the initiation site of poliovirus polyprotein synthesis. J Virol. 1982 Jun;42(3):1017–1028. doi: 10.1128/jvi.42.3.1017-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake N. L., Palmenberg A. C., Ghosh A., Omilianowski D. R., Kaesberg P. Identification of the polyprotein termination site on encephalomyocarditis viral RNA. J Virol. 1982 Feb;41(2):726–729. doi: 10.1128/jvi.41.2.726-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss S., Schaller H. A tandem repeat gene in a picornavirus. Nucleic Acids Res. 1982 Oct 25;10(20):6441–6450. doi: 10.1093/nar/10.20.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Ansorge W. Improvements of DNA sequencing gels. Anal Biochem. 1981 Aug;115(2):450–457. doi: 10.1016/0003-2697(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin YuV, Agol V. I. Proteolytic activity of the nonstructural polypeptide p22 of encephalomyocarditis virus. Biochem Biophys Res Commun. 1981 Feb 27;98(4):952–960. doi: 10.1016/0006-291x(81)91203-1. [DOI] [PubMed] [Google Scholar]
- Hall L., Rueckert R. R. Infection of mouse fibroblasts by cardioviruses: premature uncoating and its prevention by elevated pH and magnesium chloride. Virology. 1971 Jan;43(1):152–165. doi: 10.1016/0042-6822(71)90233-9. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Brown F. The location of the ploy(C) tract in the RNA of foot-and-mouth disease virus. J Gen Virol. 1976 Dec;33(3):493–501. doi: 10.1099/0022-1317-33-3-493. [DOI] [PubMed] [Google Scholar]
- Harris T. J. The nucleotide sequence at the 5' end of foot and mouth disease virus RNA. Nucleic Acids Res. 1979 Dec 11;7(7):1765–1785. doi: 10.1093/nar/7.7.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazachkov YuA, Chernovskaya T. V., Siyanova EYu, Svitkin YuV, Ugarova TYu, Agol V. I. Leader polypeptides encoded in the 5'-region of the encephalomyocarditis virus genome. FEBS Lett. 1982 May 17;141(2):153–156. doi: 10.1016/0014-5793(82)80035-5. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Palmenberg A. C., Golini F., Wimmer E., Rueckert R. R. Picornaviral VPg sequences are contained in the replicase precursor. J Virol. 1980 Aug;35(2):414–419. doi: 10.1128/jvi.35.2.414-419.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Rueckert R. R. Evidence for intramolecular self-cleavage of picornaviral replicase precursors. J Virol. 1982 Jan;41(1):244–249. doi: 10.1128/jvi.41.1.244-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Morgan D. O., Moore D. M., Grubman M. J., Card J., Fischer T., Weddell G., Dowbenko D., Yansura D. Identification of amino acid and nucleotide sequence of the foot-and-mouth disease virus RNA polymerase. Virology. 1983 Apr 30;126(2):614–623. doi: 10.1016/s0042-6822(83)80017-8. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert R. R., Pallansch M. A. Preparation and characterization of encephalomyocarditis (EMC) virus. Methods Enzymol. 1981;78(Pt A):315–325. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. Y., Shih D. S. Cleavage of the capsid protein precursors of encephalomyocarditis virus in rabbit reticulocyte lysates. J Virol. 1981 Dec;40(3):942–945. doi: 10.1128/jvi.40.3.942-945.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E. The initiation of protein synthesis directed by the RNA from encephalomyocarditis virus. Eur J Biochem. 1973 Mar 1;33(2):301–313. doi: 10.1111/j.1432-1033.1973.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Rueckert R. Capsid polypeptides of mouse Elberfeld virus. I. Amino acid compositions and molar ratios in the virion. J Virol. 1972 Sep;10(3):347–355. doi: 10.1128/jvi.10.3.347-355.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian A. B., Drygin Y. F., Chumakov K. M., Bogdanov A. A. The structure of the covalent linkage between proteins and RNA in encephalomyocarditis virus. Nucleic Acids Res. 1980 Aug 25;8(16):3729–3742. doi: 10.1093/nar/8.16.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. IV. Amino- and carboxyl-terminal analyses of the major capsid polypeptides. Virology. 1976 May;71(1):111–121. doi: 10.1016/0042-6822(76)90098-2. [DOI] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the mengo virion. III. Purification and amino acid compositions of the major capsid polypeptides. Virology. 1975 Mar;64(1):228–235. doi: 10.1016/0042-6822(75)90094-x. [DOI] [PubMed] [Google Scholar]


