Abstract
The role of α1-adrenergic receptors (α1ARs) in cognition and mood is controversial, probably as a result of past use of nonselective agents. α1AAR activation was recently shown to increase neurogenesis, which is linked to cognition and mood. We studied the effects of long-term α1AAR stimulation using transgenic mice engineered to express a constitutively active mutant (CAM) form of the α1AAR. CAM-α1AAR mice showed enhancements in several behavioral models of learning and memory. In contrast, mice that have the α1AAR gene knocked out displayed poor cognitive function. Hippocampal brain slices from CAM-α1AAR mice demonstrated increased basal synaptic transmission, paired-pulse facilitation, and long-term potentiation compared with wild-type (WT) mice. WT mice treated with the α1AAR-selective agonist cirazoline also showed enhanced cognitive functions. In addition, CAM-α1AAR mice exhibited antidepressant and less anxious phenotypes in several behavioral tests compared with WT mice. Furthermore, the lifespan of CAM-α1AAR mice was 10% longer than that of WT mice. Our results suggest that long-term α1AAR stimulation improves synaptic plasticity, cognitive function, mood, and longevity. This may afford a potential therapeutic target for counteracting the decline in cognitive function and mood associated with aging and neurological disorders.
Introduction
Norepinephrine (NE) has been shown to influence a variety of cognitive functions in the brain, from enhancing learning and memory to modulating mood (Sirviö and MacDonald, 1999). NE mediates its effects by selectively binding to and activating adrenergic receptors (ARs), a family of glycosylated integral membrane proteins. AR subtypes are defined according to their pharmacological properties, physiological characteristics, and primary structure and are classified as α1, α2, and β. In the brain, α1-ARs are the least understood.
The function of α1ARs in learning and memory is controversial and has not been clearly defined. Some studies have shown that α1AR stimulation inhibits memory consolidation in chicks and impairs spatial memory in monkeys and rats (Sirviö and MacDonald, 1999). In contrast, other studies suggest that α1AR activation facilitates learning and memory in rodents. Furthermore, α1ARs can promote long-term potentiation (LTP) and long-term depression (LTD) in the hippocampus and may be important modulators of synaptic plasticity in the adult central nervous system (Sirviö and MacDonald, 1999). However, many of these previous studies used high doses of weakly selective α1AR agents, possibly cross-activating other AR subtypes.
We recently showed that long-term stimulation of the α1AAR increases neurogenesis (Gupta et al., 2009). Substantial evidence suggests that newly generated neurons contribute to learning and memory, particularly hippocampus-dependent tasks (Deng et al., 2010). Improved memory performance in aged rats correlates with higher numbers of newly generated neurons in the hippocampus. In addition to modulation of learning and memory, adult neurogenesis has been implicated in the enhancement of hippocampal synaptic plasticity. Increased synaptic plasticity is strongly associated with improved cognition and adult matured hippocampal granule cells possess lower thresholds for the induction of LTP and are more sensitive to excitatory input (Schmidt-Hieber et al., 2004).
The role of α1ARs in mood is also not well understood; however, we have shown that long-term α1AAR stimulation is associated with a decrease in depression- and anxiety-like behavior in mice (Doze et al., 2009). Antidepressants that act through NE and/or serotonin increase neurogenesis, and in some instances, their effectiveness seems to be dependent on neurogenesis (Santarelli et al., 2003). In addition, the time for the clinical effect of antidepressants to occur correlates with the time required for newborn cell migration and functional integration (Malberg et al., 2000). Anxiety and stress are also common risk factors for depression. Long-term stress in rodents has been shown to decrease neurogenesis, which is reversed with antidepressants (Alonso et al., 2004).
The role of α1-ARs or any mammalian G protein-coupled receptor in longevity has not been explored. Models of neurodegeneration have shown shortened lifespan in rodents (Ohsawa et al., 2008). Likewise, human life expectancy after diagnosis with Alzheimer's disease (AD) is approximately half as long as without the disease (Larson et al., 2004). Recent evidence suggests that the longevity gene, sirt1, is linked to the activity of neural stem cells (Libert et al., 2008), providing another association between neurogenesis and longevity.
Transgenic mice engineered to express a CAM-α1AAR and normal mice treated with an α1AAR-selective agonist, cirazoline, were studied to determine the effects of long-term α1AAR stimulation on learning, memory, synaptic plasticity, depression, anxiety, and longevity. We found that long-term α1AAR activation enhances learning and memory, promotes synaptic plasticity, improves mood, and increases lifespan. α1AAR stimulation may offer a new strategy for treating the decline in cognition and mood associated with aging and neurological disorders.
Materials and Methods
Transgenic Mice.
Animals used in this study included transgenic CAM-α1AAR mice that were created on a B6CBA background, α1AAR-KO mice created on a C57BL/6 background, and their respective WT controls. Male and female mice, 182 total, were used for the behavioral tests and 86 total for the longevity studies. Mice were bred and genotyped at the Cleveland Clinic Foundation and were housed and provided veterinary care in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal care facility. Large cohorts of mice were transferred to the University of North Dakota's Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal care facility. Mice were maintained on a 12-h light/dark cycle (lights on 5:00 AM–5:00 PM), housed in 17 × 28 × 13-cm translucent, polycarbonate boxes attached to an automatic watering system (Edstrom Industries, Inc., Waterford, WI) and were provided ad libitum access to pelleted food with 5% fat [Teklad 22/5 Rodent Diet (W) 8640, Harlan, Indianapolis, IN]. Room air was 100% exchanged 12 to 40 times per hour with no recirculation, the temperature was 22°C, and the humidity was 23 to 27%. Mice were identified by ear tags placed at the Cleveland Clinic. The experimental protocols employed in this study conform to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Animal Care and Use Committee at both institutions.
Behavioral Testing.
Behavioral testing was completed when animals were aged 3 to 6 months, except for mice treated long-term with cirazoline, which were aged 6 to 11 months. Tests for learning and memory included the Barnes, Morris water, and multi-T mazes. The Barnes maze was performed between 8:00 AM and 12:00 noon, whereas the other cognitive tests took place between 10:00 AM and 12:00 noon. Tests for depression and anxiety included the tail-suspension test, marble-burying test, elevated zero maze, and light/dark exploration. All mood tests were performed between 12:00 noon and 5:00 PM. The Morris water and multi-T mazes were performed at the Cleveland Clinic Foundation; all other tests were completed at the University of North Dakota. Animals were acclimated in separate cages in the testing room for 30 min before Barnes maze testing and for 1 h before all other tests. Animals were deprived of food and water for the duration of the tests. Lighting was measured using a digital lux meter (Mastech, Fremont, CA) and was held at 40 lux for depression and anxiety tests, with the exception of light/dark exploration, which required 400 lux. Testing equipment was cleaned between trials with alcohol. All testing was video-captured, performed, and analyzed blind to mouse genotype.
Barnes Maze.
The Barnes maze was used to assess spatial learning and memory in mice with a modified protocol. The Barnes maze consisted of a white, flat, circular platform (120 cm in diameter) elevated 140 cm above ground (Med Associates, St. Albans, VT). An escape box (21 × 5.5 × 5 cm), not visible from the top of the maze, was located under one of 40 holes (5 cm in diameter) evenly spaced 3.5 cm apart around the perimeter. Three visual cues were placed on a black curtain surrounding the maze and their locations in relation to the escape box remained the same throughout the experiment. Two floodlights (1700 total lux) and four evenly spaced fans above the maze provided aversion.
The first 4 days consisted of four learning trials with 30-min intervals between trials. At the start of each trial, a mouse was placed in the center of the maze under a holding chamber for 30 s. When the chamber was lifted, timing of the trial began, and the mouse was allowed up to 300 s to enter the escape box. If a mouse failed to enter after the allowed time, it was gently placed into the hole containing the escape box for 30 s. Memory trials were conducted on days 1, 4, 5, and 8 (transgenic mice) and days 1, 4, and 6 (cirazoline-treated mice) after the 4 days of training. The procedure for the memory trials was the same as learning, except the mice were allowed only one attempt to solve the maze each day. Later analysis included the time to solve, number of errors made, and distance traveled on the maze. Errors were defined as when a mouse poked more than three-quarters of its head into any hole other than the appropriate escape hole. Distance traveled was measured using ANY-maze software (ver. 4.73; Stoelting Co., Wood Dale, IL).
Morris Water Maze.
The Morris water maze was used to assess spatial learning and memory. The maze consisted of an oval tub (76 cm diameter) with 15 cm of water held at 26°C. A stationary platform was placed below the surface near the middle of the tub to allow the mice an escape from the water. An identical free-floating platform was placed next to the stationary platform and remained there for the duration of training and testing. The free-floating platform did not provide an escape. The tub was aligned with visual cues, red stickers placed inside, that remained constant throughout the experiment. Each mouse was placed at one end of the tub and observed until it climbed onto the stationary platform, at which time it was removed. If a mouse did not find or remember the correct stationary platform in 300 s, it was guided to the platform and allowed to remain there for 30 s before escaping from the water. This procedure was repeated for 6 days to determine learning. The stationary platform and cues were then reversed and mice were tested for memory on day 9. The tests were digitally recorded and later analyzed for the time required to locate the stationary platform during learning and memory trials.
Multi-T Maze.
The multi-T maze (60 × 60 × 16 cm) is a test of spatial working memory. Visual cues were placed along the correct path of the maze, which led to a peanut butter reward and an escape box. Mice were trained five times per day on 5 consecutive days with the incorrect paths blocked, allowing access only to the correct solution. On day 5 of training, mice were timed while they solved the maze with paths unblocked to assess learning. To test memory, the mice were retested on days 1, 4, 5, and 8 after training. Activity was recorded and later analyzed for the time required to solve the maze and the number of errors made. Each time the mouse turned down the wrong path was counted as an error.
Locomotor Activity (Open Field).
The open field test is used to measure spontaneous locomotor activity in rodents. The open field for these experiments was a 41 × 41-cm enclosure with infrared beams of light aimed to form two grids, 2.5 and 7.5 cm above the floor of the enclosure. The mice were placed in the open field and allowed to explore freely for 15 min. An Active8 Open Field Activity System (Harvard Apparatus, Holliston, MA) was used to monitor total activity, distance traveled, and number of rearings. Locomotor activity (total number of beam breaks per minute) was determined using a computer algorithm that calculated the distance traveled per minute during horizontal ambulation.
Tail-Suspension Test.
The tail-suspension test is a learned helplessness model used as a measure of depression in rodents. The test has traditionally been used only to assess antidepressant efficacy. It has gained popularity as a way to assess depression-like behavior in transgenic mice models. Increased mobility in transgenic models mimics the effect seen with antidepressant treatment and is a measure of decreased depression-like behaviors. More commonly, immobility time is reported to express depression-like behavior. The test apparatus was a box made of 1.25-cm white melamine-coated particleboard. Each mouse was suspended by its tail on a hook using 1.25 cm of label tape, 26 cm from the bottom of the box, for 6 min. Each test was digitally recorded and later analyzed for the time spent immobile. Data were excluded if the mouse climbed their tail ≥20% of the time (72 s).
Marble-Burying Test.
The marble-burying test is as a measure of obsessive-compulsive type anxiety in mice. The test was performed in 17 × 28 × 13-cm translucent polycarbonate boxes containing bedding 5 cm deep with 20 marbles positioned in five rows of four. Each mouse was placed in a box for 30 min. Marbles buried, defined as at least two-thirds covered, were counted after the mouse's removal.
Elevated Zero Maze.
The elevated zero maze is designed to assess anxiety in rodents. The maze was 61 cm in diameter. Aversive stimuli included the height of the maze (50 cm) and light (40 lux). Each mouse was placed next to and facing a closed quadrant and allowed to explore the maze for 10 min. Activity was digitally recorded and later analyzed for time spent in and the number of entries to the open sections.
Light/Dark Exploration.
Light/dark exploration is used as a measure of anxiety in rodents. The light/dark apparatus consisted of a 35 × 40 × 40-cm box with a partition creating a 35 × 40 × 14-cm dark compartment (0 lux) connected to the light side (400 lux) by a 7.5 × 7.5-cm opening. Each mouse was placed in the light side of the box facing away from the opening to the dark side. Activity was digitally recorded for 10 min and later analyzed for the time spent in the light side and transitions to the dark side.
Hippocampal Slice Preparation.
Mice were weighed, deeply anesthetized with isoflurane, and then immediately decapitated. The brain was removed quickly and placed in ice-cold oxygenated choline chloride solution (110 mM choline chloride, 25 mM NaHCO3, 25 mM dextrose, 11.6 mM sodium ascorbate, 7 mM MgSO4, 3.1 mM sodium pyruvate, 2.5 mM KCl, 1.25 mM NaH2PO4, and 0.5 mM CaCl2). While the brains were submerged, hippocampi were removed and placed on a tissue chopper. Coronal brain slices were cut 400 μm thick and immediately transferred to an oxygenated holding chamber filled with oxygenated artificial cerebrospinal fluid solution (119 mM NaCl, 26.2 mM NaHCO3, 11 mM dextrose, 5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, and 1 mM NaH2PO4) warmed to 33°C in a water bath for approximately 30 min. Slices were then removed from the water bath and allowed to cool to room temperature (22°C). After 15 min, the entorhinal cortex and CA3 region of each slice was quickly removed. Slices were then returned to the holding chamber and allowed an acclimation period of approximately 2 h (not including incubation time and time to remove the entorhinal cortex and CA3). Slices were transferred to recording chambers in preparation for electrophysiological recordings, where they were constantly perfused with oxygenated artificial cerebrospinal fluid at a rate of 1.5 ml/min at 24°C. For these experiments, 22- to 24-month-old mice were used to examine the effects of long-term α1AAR stimulation on synaptic transmission and plasticity in aged mice.
Electrophysiology Recordings.
Glass micropipettes were backfilled with 3 M NaCl solution for the recording electrodes and subsequently placed in the stratum radiatum of the CA1 region. Evoked field excitatory postsynaptic potentials (fEPSPs) were recorded and measured using a BVC-700A Cornerstone amplifier (Dagan Corporation, Minneapolis, MN) in current clamp mode with 100× gain. An ISO-flex stimulator (A.M.P.I., Jerusalem, Israel) paired with a 7.5-cm bipolar tungsten-stimulating electrode (World Precision Instruments, Sarasota, FL) was used for presynaptic stimulation of the Schaffer collateral-commissural fibers in the stratum radiatum, between the CA3 region and the recording electrode. Signals were converted from analog to digital using an Axon Digidata1440A Data Acquisition System (Molecular Devices Inc., Sunnyvale, CA), and electronic cycling and noise were filtered using a HumBug 50/60 Hz noise eliminator (Quest Scientific, Vancouver, BC, Canada). Recordings were made using Clampex (ver. 10.2; Molecular Devices). Basal synaptic transmission was assessed by determining input-output curves, generated by applying a stepwise 5 μA increase in stimulation intensity, with a range of 5 to 80 μA. Responses were elicited every 20 s with duration of 100 μs per pulse. For the subsequent experiments, the stimulus was set to approximately 50% of the maximal response. Short-term plasticity was investigated by assessing paired-pulse facilitation (PPF) by applying two pulses with interpulse intervals of 35, 50, 75, 100, 150, 200, and 300 ms. A baseline response of 30 min was recorded immediately after PPF, which was followed by θ-burst stimulation (TBS; 10 trains, each train of four pulses at 100 Hz, intertrain interval of 200 ms, total train duration of 40 ms) given at 80% maximal response to induce LTP. fEPSPs were then recorded at 50% maximal response every 20 s for 90 min.
Cirazoline Treatment.
Normal, nontransgenic WT mice received bottled water containing cirazoline at 10 mg/l for 2 to 9 months. The water was changed either weekly or biweekly as needed. Food was provided ad libitum. No adverse side effects were noted with this treatment or in the constitutively active mutant (CAM)-α1AAR mice.
Longevity Methods.
Animals in the longevity arm of this study were observed daily but handled only for cage changes, and the date of death logged accordingly. The body weight of a selection of the mice was measured at different ages using a pan electronic scale. Animals that appeared near death (not likely to survive for another 48 h) were euthanized with carbon dioxide; the date of euthanasia was then taken as the best estimate of date of natural death. Factors for this decision were based on clinical signs set forth by The Jackson Laboratory (Bar Harbor, ME) (Yuan et al., 2009). The signs included failure to drink or eat, extreme weight loss over a short period of time, severe weakness based on responsiveness to touch, serious locomotor impairments, or tumors that had ulcerated or were bleeding.
Statistical Analysis.
All results were analyzed using Prism (ver. 5.03; GraphPad Software, San Diego, CA). Statistical comparisons were performed between the transgenic mice and their WT control mice using a Student's unpaired t test. Electrophysiological data were analyzed using Clampfit ver. 10.2 and Prism 5.03. Analysis of input-output curves was done by finding the slope of each fEPSP from 5 to 80 μA, at 5-μA increments. PPF analysis compared the slope of the second elicited fEPSP and divided it by the first elicited fEPSP. Fiber volley amplitude was also analyzed to better assess basal synaptic transmission. Pre- and post-TBS baselines were analyzed by measuring fEPSP slope every 20 s and comparing the average pre-TBS baseline slope with the average post-TBS baseline slope for both CAM-α1AAR and WT mice. fEPSP slopes were expressed as a ratio of the pre-TBS baseline and normalized to the pre-TBS baseline. Data are presented as mean ± S.E.M. Significance levels were taken as p < 0.05, p < 0.01, or p < 0.001.
Results
Long-Term Stimulation of the α1AAR Enhances Learning and Memory.
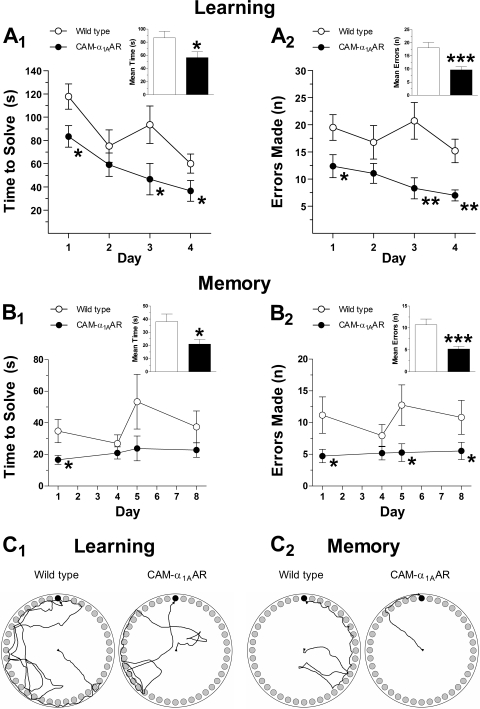
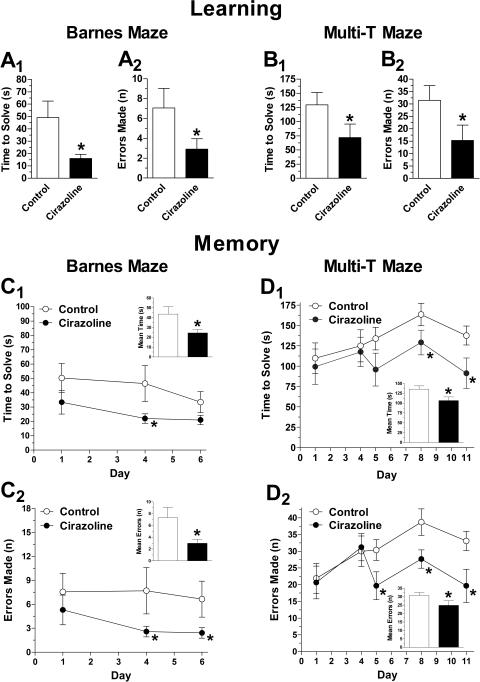
Learning and memory were assessed using the Barnes, Morris water, and multi-T mazes. The Barnes maze is widely accepted as a hippocampus-dependent task of spatial learning and memory. The time to solve, number of errors, and distance traveled are inversely correlated with learning and memory. As shown in Fig. 1, CAM-α1AAR mice (n = 15) showed enhanced cognition compared with WT mice (n = 17). During learning trials, CAM-α1AAR mice took less time to solve the maze (56 ± 8.9 s) (Fig. 1A1, inset) compared with WT mice (87 ± 9.8 s) [t(30) = 2.3, p < 0.05]. CAM-α1AAR mice also made fewer errors during learning trials (9.7 ± 1.1) (Fig. 1A2, inset) than the WT mice (18 ± 2.0) [t(30) = 3.5, p < 0.001]. During memory trials, the CAM-α1AAR mice better remembered the escape box's location, shown by a decreased mean solve time (21 ± 3.5 s) (Fig. 1B1, inset) compared with the WT mice (38 ± 5.8 s) [t(30) = 2.4, p < 0.05]. CAM-α1AAR mice also made fewer errors (5.2 ± 0.6) (Fig. 1B2, inset) than the WT mice (11 ± 1.3) [t(30) = 3.6, p < 0.001]. For both learning and memory trials, CAM-α1AAR mice traveled a shorter distance than WT mice (4.3 ± 0.47 versus 5.2 ± 0.51 m for learning, p = 0.10; 2.3 ± 0.29 versus 2.6 ± 0.38 m for memory, p = 0.29) (data not shown).
Fig. 1.
Long-term α1AAR stimulation improves cognitive performance in the Barnes maze. During learning trials, CAM-α1AAR mice (n = 17) took less time to solve the maze (A1) and made fewer errors (A2) compared with the WT mice (n = 15). During memory trials, CAM-α1AAR mice took less time to solve the maze (B1) and made fewer errors (B2) compared with WT mice. Schematic drawings represent paths traveled during learning (C1) and memory (C2) trials of the WT and CAM-α1AAR mice. The bar graph insets show the mean solve time and errors during learning and memory trials. Statistically significant at *, p < 0.05; **, p < 0.01; or ***, p < 0.001.
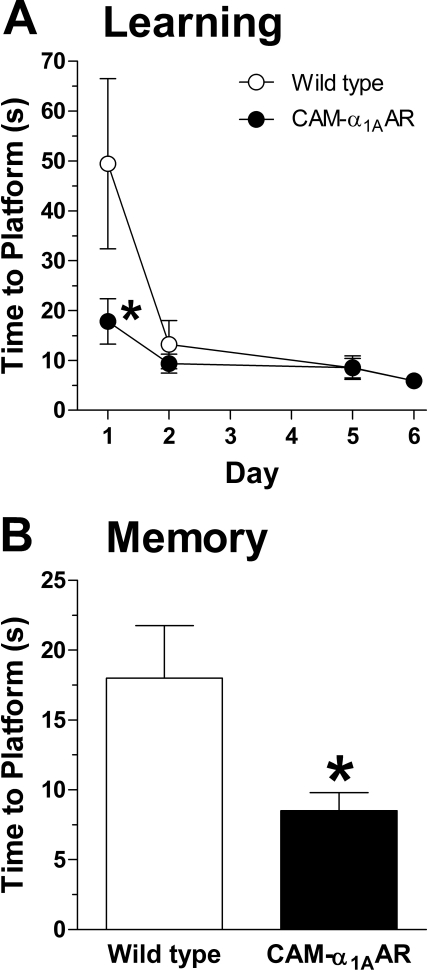
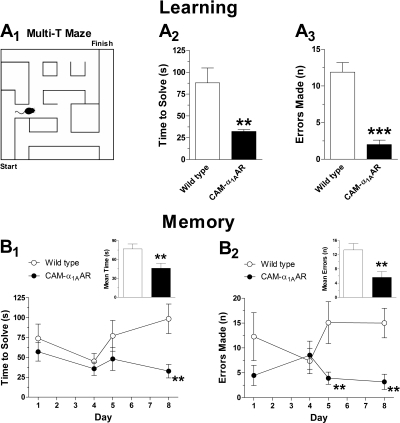
The Morris water and multi-T mazes were used as additional assessments of learning and memory. In the Morris water maze, CAM-α1AAR mice (n = 11) solved the maze in less time (18 ± 4.6 s) than WT mice (n = 11) (50 ± 17 s) [t(20) = 1.8, p < 0.05] during the first day of learning (Fig. 2A). CAM-α1AAR mice also took less time to find the platform (8.5 ± 1.3 s) than WT mice (18 ± 3.8 s) [t(19) = 2.3, p < 0.05] during the memory phase (Fig. 2B). In the multi-T maze (Fig. 3A1), CAM-α1AAR mice (n = 9) took less time to solve the maze during learning trials (32 ± 2.1 s) (Fig. 3A2) than WT mice (n = 9) (88 ± 17 s) [t(16) = 3.2, p < 0.01]. CAM-α1AAR mice also made fewer errors (2.3 ± 0.5) (Fig. 3A3) than the WT mice (12 ± 1.3) [t(16) = 6.9, p < 0.001]. During memory trials, CAM-α1AAR mice solved the multi-T maze in less time (46 ± 7.7 s) (Fig. 3B1, inset) than WT mice (77 ± 7.9 s) [t(16) = 2.8, p < 0.01]. CAM-α1AAR mice also made fewer errors (5.6 ± 1.6) (Fig. 3B2, inset) than WT mice (13 ± 1.8) [t(16) = 3.2, p < 0.01]. Taken together, the results suggest that the CAM-α1AAR mice possess improved cognitive abilities.
Fig. 2.
Learning and memory in the Morris water maze is enhanced with long-term α1AAR activation. A, CAM-α1AAR mice (n = 11) took less time than the WT mice (n = 11) to reach the correct platform on day 1 of the learning phase. B, when the platform and spatial clues were reversed on day 9 to test memory, CAM-α1AAR mice completed the maze in less time than WT mice. Statistically significant at *, p < 0.05.
Fig. 3.
Long-term α1AAR stimulation increases spatial working memory in the multi-T maze. Schematic diagram of Multi-T maze (A1). During the learning phase, CAM-α1AAR mice (n = 9) took less time to solve the maze (A2) and made fewer errors (A3) than the WT mice (n = 11). During memory trials, CAM-α1AAR mice took less time to solve (B1) and made fewer errors (B2) than the WT mice. The bar graph insets show the mean solve time and errors during memory testing. Statistically significant at **, p < 0.01 or ***, p < 0.001.
Aged CAM-α1AAR Mice Have Enhanced Synaptic Plasticity.
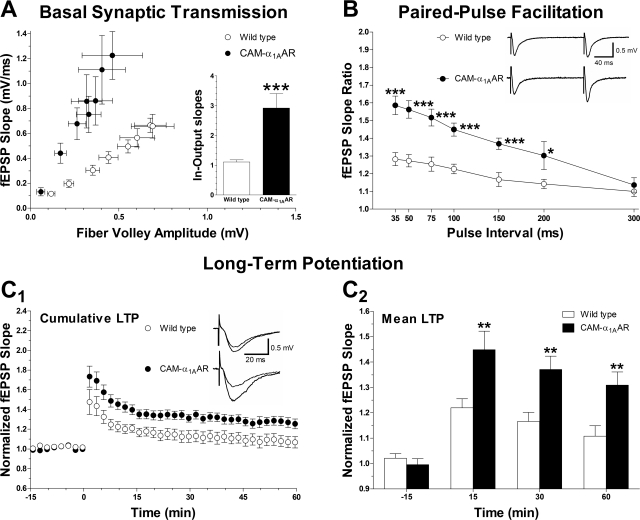
To assess whether the behavioral gains seen in CAM-α1AAR mice correlated with enhanced hippocampal plasticity, several cellular properties were investigated using electrophysiology, including basal synaptic transmission, short-term plasticity as assessed by PPF, and long-term plasticity. Basal synaptic transmission was investigated by analyzing the fEPSP slope at various stimulus intensity intervals (10–80 μA) and plotting it against fiber volley amplitude. A significant difference was observed in the average input-output slopes of basal synaptic transmission between CAM-α1AAR and WT mice (2.9 ± 0.48, n = 11 versus 1.1 ± 0.07, n = 23; p < 0.001) (Fig. 4A, inset). These findings suggest that basal synaptic transmission is enhanced in CAM-α1AAR mice compared with WT mice.
Fig. 4.
Hippocampal synaptic plasticity is enhanced with long-term α1AAR activation. A, basal synaptic transmission, as determined by the input-output relation between fiber volley amplitude and fEPSP slope, is increased in CAM-α1AAR (n = 11 slices from three animals) compared with WT mice (n = 23 slices from seven animals). The bar graph inset shows the mean input-output slopes. B, frequency facilitation (PPF) is enhanced in the CAM-α1AAR mice (n = 21 slices from three animals) compared with the WT mice (n = 23 slices from seven animals). The facilitation was plotted as a function of interpulse interval of 35, 50, 75, 100, 150, 200, and 300 ms. Superimposed representative fEPSPs were recorded at 150 ms interval. C1, long-term α1AAR activation enhances LTP in the hippocampal CA1 region, shown by cumulative data of the normalized changes in field potential slope in CAM-α1AAR mice (n = 23 slices from nine animals) and WT mice (n = 27 slices from nine animals). Superimposed representative fEPSPs were recorded 15 min before and 60 min after LTP induction. C2, multiple LTP recordings for each mouse were grouped and averaged, giving a single fEPSP slope ratio per animal at different time points before or after TBS (−15, 15, 30, and 60 min). CAM-α1AAR mice (n = 9) showed enhanced mean LTP compared with WT mice (n = 9) at each post-TBS time point. Statistically significant at *, p < 0.05; *, p < 0.01; or ***, p < 0.001.
The slope ratio of fEPSP was calculated by taking the slope of the second elicited fEPSP and dividing it by the first elicited fEPSP in PPF experiments. Interpulse intervals were then set at 35, 50, 75, 100, 150, 200, or 300 ms. A significant difference was found between CAM-α1AAR and WT mice at 35 ms (1.3 ± 0.037, n = 23 versus 1.6 ± 0.052, n = 21, p < 0.001), 50 ms (1.3 ± 0.035, n = 23 versus 1.6 ± 0.049, n = 21, p < 0.001), 75 ms (1.3 ± 0.043, n = 13 versus 1.5 ± 0.047, n = 17, p < 0.001), 100 ms (1.2 ± 0.027, n = 23 versus 1.5 ± 0.037, n = 21, p < 0.001), 150 ms (1.2 ± 0.039, n = 13 versus 1.4 ± 0.032, n = 19, p < 0.001), and 200 ms (1.1 ± 0.027, n = 10 versus 1.3 ± 0.079, n = 4, p < 0.05). These results indicate that short-term plasticity is enhanced in the CAM-α1AAR compared with WT mice.
Synaptic plasticity (particularly LTP) is thought to underlie learning and memory. We measured LTP in the apical dendrites of the hippocampal CA1 region of very old (age 22 to 24 months) CAM-α1AAR and WT mice induced by TBS (10 trains of 4 pulses at 100 Hz) of the Schaeffer collateral pathway (Fig. 4C1). These recordings showed a significant enhancement of normalized LTP in CAM-α1AAR compared with WT mice at 15 min (1.5 ± 0.073 versus 1.2 ± 0.036, p < 0.01), 30 min (1.4 ± 0.053 versus 1.2 ± 0.036, p < 0.01) and 60 min (1.3 ± 0.052 versus 1.1 ± 0.041, p < 0.01) after TBS (Fig. 4C2) (n = 9 animals for each comparison). These results demonstrate that CAM-α1AAR mice have increased LTP relative to WT mice, suggesting that long-term α1AAR stimulation enhances LTP. This finding is consistent with our observations of enhanced basal synaptic transmission and PPF in CAM-α1AAR mice compared with WT.
Long-Term Treatment with an α1AAR-Selective Agonist Improves Cognitive Function.
To determine whether endogenous stimulation mimics the cognitive effects observed in CAM-α1AAR mice, we treated normal WT mice with the α1AAR-selective agonist cirazoline. We assessed learning and memory using the Barnes and Multi-T mazes (Fig. 5). During learning, cirazoline-treated mice solved the Barnes maze in less time (16 ± 3.4 s, n = 14) (Fig. 5A1) than control mice (49 ± 13 s, n = 11) [t(23) = 2.1, p < 0.05] while making fewer errors (2.9 ± 1.0) (Fig. 5A2) than control mice (7.1 ± 2.0) [t(23) = 1.7, p < 0.05]. Likewise, during learning, cirazoline-treated mice solved the multi-T maze in less time (72 ± 24 s, n = 10) (Fig. 5B1) than control mice (130 ± 22 s, n = 9) [t(17) = 1.8, p < 0.05]. Cirazoline-treated mice also made fewer errors (15 ± 6.2) (Fig. 5B2) than the control mice (32 ± 5.9) [t(17) = 1.9, p < 0.05]. During memory trials for the Barnes maze (Fig. 5C1), cirazoline-treated mice solved the maze in less time (24 ± 3.6 s) (Fig. 5C11, inset) than control mice (44 ± 7.3 s) [t(23) = 2.2, p < 0.05], while making fewer errors (3.0 ± 0.7) (Fig. 5C2, inset) than control (7.3 ± 1.7) [t(23) = 2.1, p < 0.05]. During multi-T maze learning trials, cirazoline-treated mice solved the maze in less time (110 ± 9.1 s) (Fig. 5D1, inset) compared with control mice (140 ± 8.3) [t(17) = 2.3, p < 0.05], while making fewer errors (25 ± 2.8) (Fig. 5D2, inset) than control mice (31 ± 1.7) [t(17) = 1.8, p < 0.05]. These results suggest that endogenous α1AAR activation by a subtype selective agonist improves cognitive function in normal mice.
Fig. 5.
Long-term treatment with an α1AAR-selective agonist improves cognitive function. Normal WT mice treated for 9 months with the α1AAR-selective agonist cirazoline (n = 11) solved the Barnes maze in less time (A1) and made fewer errors (A2) than the control WT mice (n = 14) on the last day (day 4) of learning trials. Likewise, WT mice treated with cirazoline for 2 months (n = 10) solved the multi-T maze in less time (B1) and made fewer errors (B2) than the control WT mice (n = 11) on the last day (day 5) of learning trials. During memory trials, cirazoline-treated WT mice exhibited took less time to solve (C1 and D1) and made fewer errors (C2 and D2) than the control WT mice in both the Barnes and multi-T mazes. The bar graph insets show the mean solve time and errors during memory testing. Statistically significant at *, p < 0.05 or **, p < 0.01.
α1AAR-KO Mice Display Poor Cognitive Function.
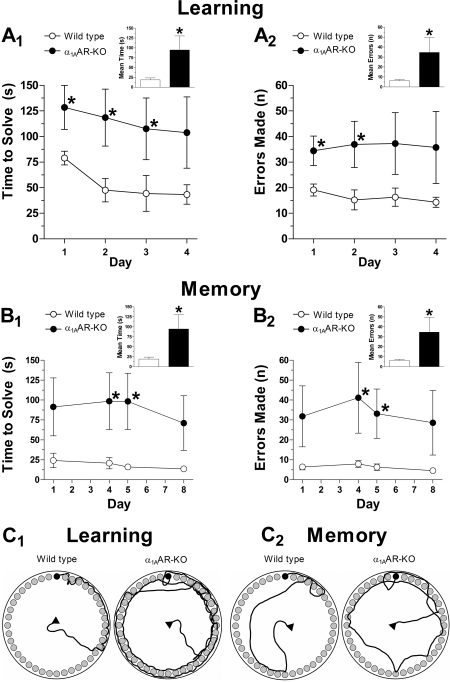
To further define the role of α1AARs in learning and memory, we next examined the effects of blocking α1AARs on cognitive function. Because α1AR antagonists can cause sedation, which would affect behavior testing, we studied α1AAR-KO mice using the Barnes maze. We chose the Barnes maze because it creates a less stressful environment than the water maze and is safer for the α1AAR-KO mice, which are prone to seizures. As shown in Fig. 6, α1AAR-KO mice (n = 12) displayed impaired learning and memory compared with WT mice (n = 10). During learning trials, α1AAR-KO mice took more time to solve the maze (120 ± 27 s) (Fig. 6A1, inset) than control mice (54 ± 9.6 s) [t(20) = 1.9, p < 0.05]. α1AAR-KO mice also made more errors (36 ± 9.8) (Fig. 6A2, inset) than control mice (16 ± 1.8) [t(20) = 1.8, p < 0.05]. During memory trials, the α1AAR-KO mice displayed a poorer recollection of the escape box's location, indicated by an increased mean solve time (95 ± 36 s) (Fig. 6B1, inset) compared with WT mice (19 ± 4.8 s) [t(20) = 1.9, p < 0.05]. α1AAR-KO mice also made more errors (35 ± 15) (Fig. 6B2, inset) than the WT mice (6.3 ± 1.0) [t(20) = 3.6, p < 0.001]. For both learning and memory trials, α1AAR-KO mice traveled a longer distance than WT mice (9.6 ± 2.5 versus 4.1 ± 0.6 m for learning, p < 0.05; 8.5 ± 3.2 versus 1.9 ± 0.26 m for memory, p < 0.05) (data not shown). These results suggest that α1AAR-KO mice have poor cognitive abilities and that the α1AAR is directly involved in affecting cognitive behavior.
Fig. 6.
Cognitive performance in the Barnes maze is reduced in mice lacking α1AARs. During learning trials, α1AAR-KO mice (n = 12) took more time to solve the maze (A1) and made more errors (A2) compared with the WT mice (n = 10). During memory trials, α1AAR-KO mice took more time to solve the maze (B1) and made more errors (B2) compared with WT mice. Schematic drawings represent paths traveled during learning (C1) and memory (C2) trials of the WT and α1AAR-KO mice. The bar graph insets show the mean solve time and errors during learning and memory trials. Statistically significant at *, p < 0.05.
CAM-α1AAR Mice Have Normal Levels of Locomotion.
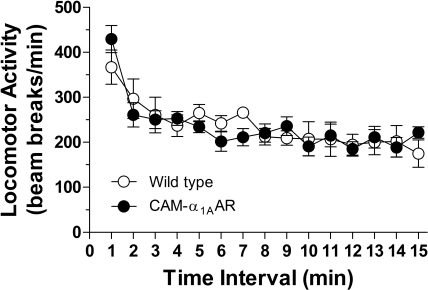
To determine whether differences on behavioral tests (e.g., Barnes, Morris water, and multi-T mazes) were due to improvements or degradation of cognitive ability or to differences in motor function, the open field locomotion test was used to assess the spontaneous locomotor activity of the CAM-α1AAR and WT mice. Total activity (or beam breaks per minute) was assessed for 15 min. As shown in Fig. 7, the CAM-α1AAR (n = 12) and WT mice (n = 7) exhibited an average total activity of 234 ± 15 and 236 ± 14 beam breaks/min, respectively, indicating no difference in motor activity between these mice. These results indicate that the differences observed in this study are not due to differences in locomotive ability.
Fig. 7.
Long-term α1AAR stimulation does not alter locomotor activity. An open field locomotion test revealed no difference in locomotor activity between the CAM-α1AAR (n = 12) and WT mice (n = 7).
Long-Term α1AAR Activation Improves Mood.
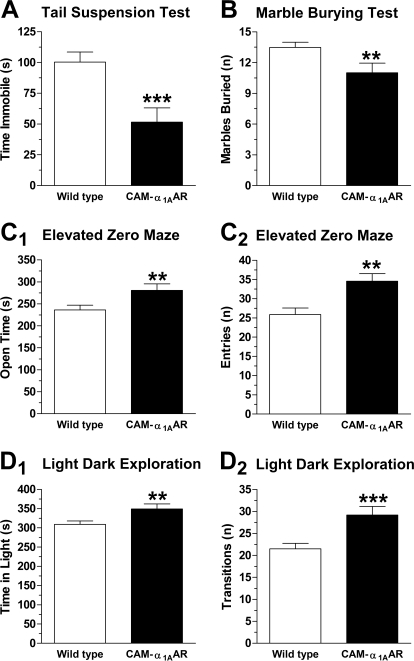
Because depression and neurogenesis may be linked, we used the tail-suspension test to compare the level of depression in the CAM-α1AAR mice with that of their WT counterparts. The time spent immobile while hanging by the tail is positively correlated with depression. As shown in Fig. 8A, CAM-α1AAR mice spent less time immobile (52 ± 12 s, n = 19) than the WT mice (100 ± 8.2 s, n = 33) [t(50) = 3.5, p < 0.001]. These results suggest that long-term α1AAR stimulation elicits antidepressant-like behavior.
Fig. 8.
Long-term α1AAR activation improves mood. Rodent tests of anxiety and depression show that α1AAR stimulation increases antidepressant-like behavior and decreases anxiety-related behaviors in mice. A, α1AAR activation elicits antidepressant-like behaviors in the tail-suspension test. CAM-α1AAR mice (n = 19) spent less time immobile than the WT mice (n = 33). B, α1AAR stimulation reduces obsessive-compulsive type anxiety in the marble-burying test. CAM-α1AAR mice (n = 21) buried fewer marbles than WT mice (n = 36). α1AAR activation decreases anxiety-like behaviors in the elevated zero maze, as CAM-α1AAR mice (n = 20) spent more time in (C1) and made more entries into (C2) the open areas than WT mice (n = 36). α1AAR stimulation also reduces anxiety-like behaviors in the light/dark exploration. CAM-α1AAR mice (n = 21) spent more time in the light side of the box (D1) and made more transitions to the dark side (D2) than WT mice (n = 34). Statistically significant at **, p < 0.01 or ***, p < 0.001.
Because depression and anxiety are often comorbid, we next compared levels of anxiety in CAM-α1AAR mice with their WT counterparts using a number of behavioral tests for anxiety including the marble-burying test, elevated zero maze, and light/dark exploration. In the marble-burying test, the number of marbles buried is positively correlated with increased obsessive compulsive-type anxiety. As shown in Fig. 8B, CAM-α1AAR mice buried significantly fewer marbles (11 ± 0.9, n = 21) than the WT mice (14 ± 0.5, n = 36) [t(55) = 2.5, p < 0.01]. These results suggest that CAM-α1AAR mice exhibit less obsessive compulsive-like behavior.
The elevated zero maze uses a simple paradigm of elevated walkways, two enclosed and two open, to determine anxiety levels in rodents. The amount of time spent in the open areas is positively correlated with lower anxiety. As shown in Fig. 8C1, CAM-α1AAR mice spent significantly more time in the open sections (280 ± 14 s, n = 20) than WT mice (240 ± 11 s, n = 36) [t(54) = 2.5, p < 0.01]. As illustrated in Fig. 8C2, CAM-α1AAR mice also made more entries to the open areas (35 ± 2.0) than WT mice (24 ± 1.6) [t(49) = 4.2, p < 0.001]. These results suggest that CAM-α1AAR mice exhibit less anxiety-like behavior.
In light/dark exploration, the amount of time spent in the light side and the number of transitions to the dark side are positively correlated with a reduced level of anxiety. As shown in Fig. 8D1, CAM-α1AAR mice spent significantly more time in the light area (350 ± 13 s, n = 21) compared with WT mice (310 ± 8.9 s, n = 34) [t(53) = 2.6, p < 0.01]. As shown in Fig. 8D2, CAM-α1AAR mice also made significantly more transitions to the dark side (29 ± 1.9) than WT mice (22 ± 1.3) [t(53) = 3.5, p < 0.001]. These results are consistent with our hypothesis that CAM-α1AAR mice display less anxiety-like behavior. Taken together, the results from this series of behavior tests evaluating depression and anxiety suggest that long-term α1AAR stimulation improves mood and reduces anxiety.
Body Weight Was Unaffected by Long-Term α1aAR Activation.
Weight was compared in mature adult (6–8 months), middle-aged (10–14 months), old (16–20 months), and very old (22–24 months) WT and CAM-α1AAR mice. No significant differences were observed between WT and CAM-α1AAR mice classified as mature adult (43 ± 1.1 g, n = 8, versus 42 ± 1.7 g, n = 20), middle-aged (42 ± 1.0 g, n = 58, versus 44 ± 0.9 g, n = 34), old (41 ± 1.4 g, n = 18, versus 44 ± 0.9 g, n = 34), or very old (38 ± 1.1 g, n = 20, versus 38 ± 1.4 g, n = 19). These data suggest that potential differences in caloric intake and/or metabolic rate do not account for the differences observed in this study between WT and CAM-α1AAR mice.
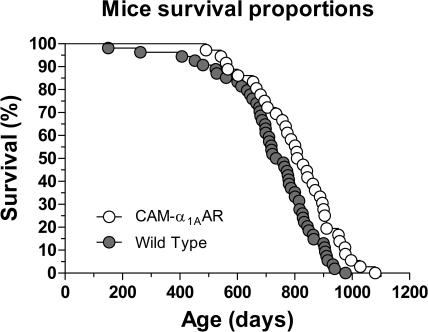
CAM-α1AAR Mice Have Increased Longevity.
Because increased neurogenesis could positively effect aging, log-rank testing was used to evaluate lifespan differences for WT and CAM-α1AAR mice. With data from both sexes, median lifespan in CAM-α1AAR mice was significantly increased by 72 days (from 747 to 819 days) or 10% relative to that of WT mice (χ2 = 7.2, p < 0.01) (Fig. 9; Table 1). Analysis of each sex showed increased longevity. The median lifespan in female CAM-α1AAR mice was increased, but not significant, by 107 days (from 711 to 818 days) or 15% relative to that of WT mice (χ2 = 0.23, p = nonsignificant). However, the median lifespan in male CAM-α1AAR mice was significantly increased, by 62 days (from 760 to 822 days) or 8% relative to that of WT mice (χ2 = 8.2, p < 0.01). As shown in Table 1, the 90th percentile age for CAM-α1AAR mice was also increased by 73 days or 8% compared with WT mice (from 909 to 982 days), suggesting that long-term α1AAR stimulation may also increase maximal lifespan. The results from this study support our hypothesis that long-term α1AAR stimulation enhances learning and memory, promotes synaptic plasticity, improves mood, and extends lifespan in this mouse strain.
Fig. 9.
Long-term α1AAR stimulation improves murine longevity. Kaplan-Meier survival plots of the CAM-α1AAR (n = 32) and WT (n = 54) mice show that α1AAR stimulation extends the average murine lifespan. p values were calculated using the Mantel-Cox log-rank test, and each symbol represents one mouse. CAM-α1AAR mice had an increased lifespan compared with the WT mice (χ2 = 7.2; df 1; p < 0.01). See also Table 1.
TABLE 1.
Median lifespan, mean, and 95% confidence intervals (CI) in days for genotypes
The median lifespan was the point at which the fractional survival of each curve equaled 50%. Each group consisted of an equal number of male and female mice.
| Genotype | Median | Mean | S.E.M. | 95% CI | 90th Percentile | Deaths |
|---|---|---|---|---|---|---|
| days | ||||||
| CAM-α1AAR | 819 | 806 | 24 | 757–856 | 982 | 32 |
| Wild type | 747 | 724 | 22 | 679–768 | 909 | 54 |
Discussion
Although ARs are typically associated with peripheral modulation of the sympathetic nervous system, all three AR families (α1, α2, β) are highly expressed in the brain and regulate synaptic transmission. The NE system modulates cognitive function such as arousal, attention, learning, and memory, as well as behavioral responses to stress, such as depression and anxiety disorders (Sirviö and MacDonald, 1999). In humans, α1-AR ligands are currently used or are in clinical trials to treat orthostatic hypotension, seizures, alcohol dependence, cocaine addiction, and post-traumatic stress disorders. Because of a lack of highly avid antibodies, localization studies in the brain were mainly performed in the past using autoradiography, but the signal intensity was poor because of low expression of α1ARs and the lack of selective radioligands. We circumvented this problem by using mice expressing EGFP under the same α1AAR promoter as in this study and compared the expression in the brain with that of α1AAR-KO mice, where the α1AAR gene was replaced with β-galactosidase. We found that the α1AAR subtype is highly expressed in cognitive centers, such as the prefrontal cortex, entorhinal cortex, hippocampal CA1–3, and dentate gyrus. Other areas of the limbic system that also highly express the α1AAR are centers for depression and anxiety, such as the amygdala (Papay et al., 2006).
Previous studies exploring the role of α1ARs in cognitive and behavioral responses are inconsistent, with activation of α1ARs in some studies facilitating cognition and other studies showing decreased function. Some studies suggest that α1ARs inhibit memory consolidation in chicks (Gibbs and Summers, 2001) and impair spatial memory after infusion of α1AR agonists in monkeys and rats (Arnsten et al., 1999; Mao et al., 1999). In contrast, other studies suggest that α1ARs facilitated spatial and intermediate-term memory in rats (Pussinen et al., 1997; Puumala et al., 1998). These discrepancies could be due to species-specific functions of α1ARs, the use of high doses of nonselective ligands, differing modes of administration, and improper pharmacological technique. For example, in a study claiming that α1AR stimulation impairs spatial working memory in rhesus monkeys (Mao et al., 1999), low numbers of animals were used. In addition, α1AR and α2AR agonists were used at the same concentration, but they did not account for the dramatically different half-lives or receptor occupancy of these agonists. This makes any conclusions about α1AR involvement doubtful. In Arnsten et al. (1999), muscular administration of cirazoline and the short delay before behavioral testing make it difficult to ascertain cirazoline's effects on the brain. Short-term treatment with α1AR ligands may not alter behavioral functions, which may be dependent upon reaching tonic levels of elevated signaling and/or increased synaptic plasticity (Nakadate et al., 2006) or neurogenesis, a recently discovered function of the α1AAR (Gupta et al., 2009).
In our study, using a transgenic mouse model and several types of behavioral cognitive tests (Figs. 1–4), we found that long-term stimulation of the α1AAR improved learning and memory. Cognitive enhancement is not due to changes in blood flow or blood pressure; CAM-α1AAR mice have normal resting and stimulated blood pressure (data not shown). The lack of effect on blood pressure in CAM-α1AAR mice is probably due to the low receptor overexpression and/or activity in the transgenic mice because of the use of the endogenous housekeeping promoter and possible compensation on blood pressure. Knockout of the α1AAR subtype has minimal effects (8–12%) on resting blood pressure and still retains 85% of the pressor response to phenylephrine (Rokosh and Simpson, 2002). Likewise, long-term low-dose NE stimulate adaptive cardiac hypertrophy without increases in blood pressure (Jensen et al., 2011).
Numerous reports have suggested correlations between adrenergic function and age- or disease-associated changes in memory function (Szot et al., 2007). Abnormalities in α1ARs are implicated in the cognitive deficits of AD. Furthermore, polymorphisms in ARs are associated with AD susceptibility (Bullido et al., 2004). Transgenic Tg2576 mice with Alzheimer plaque pathology display increased α1AR binding (Klingner et al., 2003). In humans with AD, the expression of the α1AAR mRNA subtype is significantly reduced in specific layers of the prefrontal cortex (Szot et al., 2007), suggesting that increased activity of the α1AAR in these neurons may delay or prevent AD severity.
Synaptic plasticity is widely held as an essential component of learning, memory, and cognitive function, all of which have been shown to decline with age and neurodegenerative disorders. The hippocampus is a critical structure with respect to learning, memory, and synaptic plasticity. Within the adult hippocampus, synaptic plasticity occurs primarily in two areas: the perforant path and the Schaffer collaterals. Both areas show susceptibility to age-related declines in LTP (Landfield and Lynch, 1977). Conversely, LTD occurs more readily in aged mice, suggestive of an age-related increase in the susceptibility to depression in the synaptic strength. Moreover, hippocampal CA1 pyramidal neurons show age-related deficits in PPF (Landfield and Lynch, 1977), a form of short-term plasticity related to the amplitude of synaptic responses. Each of these alterations in synaptic plasticity (LTP, LTD, PPF) correlates to age-related deficits in cognitive performance in the murine brain (Bach et al., 1999). AD mouse models also show reductions in LTP similar to those in aged mice (Bach et al., 1999). The AD phenotype is not expressed until later in life, coinciding with considerable neuronal death. In contrast, the present study shows that aged CAM α1AAR mice possess markedly improved basal synaptic transmission, PPF, and LTP at the CA3-CA1 synapses (Fig. 4, A–C). Taken together, these results suggest that long-term α1AAR activation improves synaptic efficiency throughout senescence compared with WT mice.
Deficits in cognitive functioning seen with normal aging may be attributed to loss of neurons, as well as synaptic integrity. It is well established that young neurons near the proliferative zone in the DG have a lower threshold for LTP than mature neurons (Schmidt-Hieber et al., 2004) and that reduction of DG stem cell proliferation selectively inhibits LTP (Snyder et al., 2001), suggesting a relationship between the birth of new neurons and LTP. Neurogenesis induced by α1AAR activation may play an important role in the maintenance of LTP and cognitive functioning, providing a potential therapeutic target and defense against aging and neurodegenerative diseases such as AD.
It has been found that antidepressants, including those targeting NE, increase neurogenesis in the hippocampus (Santarelli et al., 2003). Anxiety is commonly comorbid with depression and is often relieved upon treatment with antidepressants. The length of time for antidepressant action to relieve symptoms is often several weeks, which is similar to the length of time for new cells to be created and integrated into the neural network. These factors indicate that treatment to increase NE may incidentally enhance neurogenesis, which could play a role in the attenuation of symptoms of depression and anxiety.
Selective serotonin-reuptake inhibitors (SSRIs) are often a first-line treatment for major depressive disorder but have detrimental side effects. Treatments that activate other neurotransmitter systems such as NE and dopamine are important for patients who do not respond well to SSRIs (de Montigny et al., 1999). Many antidepressants have been shown to increase neurogenesis, including antidepressants such as venlafaxine, which increases responsiveness of α1ARs (Maj and Rogóz, 1999). Our research shows that α1AAR stimulation leads to a significant reduction in depressive-like behavior in CAM-α1AAR mice compared with WT mice (Fig. 8), suggesting that treatments increasing neurogenesis or α1AAR activation may improve depression symptoms.
Because antidepressants are successfully used to treat the symptoms of obsessive-compulsive disorder (OCD), we hypothesized that stimulating the α1AAR may decrease OCD symptoms in mice. Short-term treatment with the serotonin-NE reuptake inhibitor milnacipran reduces OCD activities in mice as assessed by the marble-burying test (Sugimoto et al., 2007). Serotonin-NE reuptake inhibitors have been used successfully to treat patients who are unresponsive to SSRI treatment (Hollander et al., 2003). Our results (Fig. 8) suggest that α1AAR activation reduces anxiety in mice and may provide a better treatment for the 40% of patients who do not respond to traditional SSRI treatment for OCD.
Besides having enhanced learning, memory, and LTP and decreased anxiety and depression, CAM-α1AAR mice also live longer (Fig. 9; Table 1), suggesting that long-term α1AAR stimulation can extend lifespan. The mechanism for this effect is unknown; it may be due to the cardioprotective (Rorabaugh et al., 2005; Huang et al., 2007) and neuroprotective (Goldenstein et al., 2008) effects of the α1AAR. CAM-α1AAR mice show an increase in both median and maximal lifespan that is consistent with delayed aging, yet this does not confirm that aging is slowed in these animals. To determine whether aging is altered in these mice, a comparison of the CAM-α1AAR mouse model to other models of longevity, an assessment of telomerase activity and examination of other age-dependent changes in molecular and cellular processes in these mice are necessary.
Cognitive enhancements and antidepressive behavior seen in CAM-α1AAR mice may be due to the ability of the α1AAR to increase neurogenesis (Gupta et al., 2009), protective signals, and/or neuronal cell survival similar to the cardiomyocyte (Huang et al., 2007). Here, the signal transduction pathway involves ERK phosphorylation, which also has neuroprotective effects in the brain. It has been shown that both Akt and ERK have a role in regulating hippocampal neurogenesis, whereas reductions in ERK levels in hippocampal neurons may lead to memory deficits (Yan et al., 2007).
The use of cirazoline or other types of imidazoline-like α1AAR agonists may have the best therapeutic potential. Although most imidazolines have better selectivity for the α2ARs, cirazoline is a notable exception as a strong partial agonist that has 10- to 30-fold selectivity for α1AARs over other α1AR subtypes and 100-fold over α2AR subtypes, where it displays mild antagonist properties (Ruffolo and Waddell, 1982; Minneman et al., 1994). In addition to partial agonism, the imidazoline backbone is thought to decrease the ability of imidazolines to raise blood pressure, which is a common disadvantage to using phenethylamine-type agonists, such as phenylephrine (Blue et al., 2004). Imidazoline is a nitrogen-containing heterocycle derived from imidazole, which dissipates the charged nitrogen over the ring and can therefore cross the blood-brain barrier, unlike phenylephrine (Guo et al., 1991; Davies and Wellman, 1992). Although the half-life of cirazoline in the blood has not been determined, its half-life should be longer than phenylephrine (2 h) because of its lack of breakdown by monoamine oxidase and similar to that of the other typical imidazolines (oxymetazoline = 6 h; clonidine = 12 h).
In summary, using a constitutively activated mutant α1AAR mouse model and long-term administration of an α1AAR agonist in normal mice, we demonstrated that activation of the α1AAR subtype enhances learning and memory and has antidepressant and antianxiety effects. Furthermore, long-term stimulation of the α1AAR does not seem to be physiologically damaging but has cardio- and neuroprotective effects and also enhances longevity. Therefore, α1AAR agonists may offer a potential new strategy for treating the decline in cognition and mood associated with aging and many neurological disorders.
Acknowledgments
We thank Dr. Kurt Zhang for assistance with the statistical analysis, and Karen Cisek and Elizabeth Sandquist for assistance in editing the manuscript.
This work was supported in part by the National Institutes of Health National Center for Research Resources [Grants P20-RR016741, P20-RR017699]; and the National Heart, Lung and Blood Institute [Grants R01-HL61438 (to D.M.P.) and R01-HL098279 (to P.C.S.)]; the National Science Foundation (NSF) North Dakota EPSCoR IIP Seed Award [Grant EPS-0814442] (to V.A.D.), an NSF Faculty Early Career Development Award [Grant 0347259] (to V.A.D.), an NSF Research Experience for Undergraduates Site [Grants 0639227, 0851869] (to V.A.D.), NSF Graduate Research Fellowship Program Awards (to B.L.G. and K.M.C.); respectively.
Preliminary reports of these findings were presented at the 2009 Annual Meeting of the American Society for Biochemistry and Molecular Biology (ASBMB) Northwest Regional Undergraduate Affiliate Network; 2009 Oct 30–31; Fargo, ND; the 2010 Annual Meeting of ASBMB Northwest Regional Undergraduate Affiliate Network; 2010 Oct 29–30; Moorhead, MN; the 2010 Annual Meeting of the American Society for Pharmacology and Experimental Therapeutics (ASPET); 2010 Apr 24–28, Anaheim, CA; and the 2011 Annual Meeting of ASPET; 2011 Apr 9–13; Washington, DC.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.073734.
- NE
- norepinephrine
- AR
- adrenergic receptor
- AD
- Alzheimer's disease
- KO
- knockout
- CA
- cornu ammonis
- DG
- dentate gyrus
- fEPSP
- field excitatory postsynaptic potential
- PPF
- paired-pulse facilitation
- TBS
- θ-burst stimulation
- CAM
- constitutively active mutant
- LTP
- long-term potentiation
- LTD
- long-term depression
- SSRI
- selective serotonin-reuptake inhibitor
- ERK
- extracellular signal-regulated kinase
- OCD
- obsessive-compulsive disorder
- WT
- wild type.
Authorship Contributions
Participated in research design: Doze, Papay, Goldenstein, Gupta, Collette, Nelson, Lyons, Davis, Luger, Wood, Haselton, and Perez.
Conducted experiments: Papay, Goldenstein, Collette, Nelson, Lyons, Davis, Luger, and Wood.
Contributed new reagents or tools: Simpson.
Performed data analysis: Doze, Goldenstein, Collette, Nelson, Lyons, Davis, Luger, Wood, and Perez.
Wrote or contributed to the writing of the manuscript: Doze, Goldenstein, Collette, Nelson, Lyons, Haselton, and Perez.
References
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrié P. (2004) Blockade of CRF1 or V1b receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 9:278–286 [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. (1999) α1-noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry 45:26–31 [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. (1999) Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA 96:5280–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue DR, Daniels DV, Gever JR, Jett MF, O'Yang C, Tang HM, Williams TJ, Ford AP. (2004) Pharmacological characteristics of Ro 115–1240, a selective α1A/1L-adrenoceptor partial agonist: a potential therapy for stress urinary incontinence. BJU Int 93:162–170 [DOI] [PubMed] [Google Scholar]
- Bullido MJ, Ramos MC, Ruiz-Gómez A, Tutor AS, Sastre I, Frank A, Coria F, Gil P, Mayor F, Jr, Valdivieso F. (2004) Polymorphism in genes involved in adrenergic signaling associated with Alzheimer's. Neurobiol Aging 25:853–859 [DOI] [PubMed] [Google Scholar]
- Davies BT, Wellman PJ. (1992) Effects on ingestive behavior in rats of the α1-adrenoceptor agonist cirazoline. Eur J Pharmacol 210:11–16 [DOI] [PubMed] [Google Scholar]
- de Montigny C, Silverstone PH, Debonnel G, Blier P, Bakish D. (1999) Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol 19:401–406 [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doze VA, Handel EM, Jensen KA, Darsie B, Luger EJ, Haselton JR, Talbot JN, Rorabaugh BR. (2009) α1A- and α1B-adrenergic receptors differentially modulate antidepressant-like behavior in the mouse. Brain Res 1285:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. (2001) Stimulation of α1-adrenoceptors inhibits memory consolidation in the chick. Eur J Neurosci 14:1369–1376 [DOI] [PubMed] [Google Scholar]
- Goldenstein B, Jurgens C, Knudson C, Lichter J, Carr P, Perez D, Doze V. (2008) α1 adrenergic receptor regulation of seizures and neurodegeneration (Abstract). FASEB J 22:748.12 [Google Scholar]
- Guo TZ, Tinklenberg J, Oliker R, Maze M. (1991) Central α1-adrenoceptor stimulation functionally antagonizes the hypnotic response to dexmedetomidine, an alpha 2-adrenoceptor agonist. Anesthesiology 75:252–256 [DOI] [PubMed] [Google Scholar]
- Gupta MK, Papay RS, Jurgens CW, Gaivin RJ, Shi T, Doze VA, Perez DM. (2009) α1A adrenergic receptors regulate neurogenesis and gliogenesis. Mol Pharmacol 76:314–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Friedberg J, Wasserman S, Allen A, Birnbaum M, Koran LM. (2003) Venlafaxine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 64:546–550 [DOI] [PubMed] [Google Scholar]
- Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O'Connell TD. (2007) An α1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation 115:763–772 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jensen BC, O'Connell TD, Simpson PC. (2011) Alpha-1adrenergic receptors: Targets for agonist drugs to treat heart failure. J Mol Cell Cardiol doi:10.1016/j.yjmcc.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner M, Apelt J, Kumar A, Sorger D, Sabri O, Steinbach J, Scheunemann M, Schliebs R. (2003) Alterations in cholinergic and non-cholinergic neurotransmitter receptor densities in transgenic Tg2576 mouse brain with beta-amyloid plaque pathology. Int J Dev Neurosci 21:357–369 [DOI] [PubMed] [Google Scholar]
- Landfield PW, Lynch G. (1977) Impaired monosynaptic potentiation in in vitro hippocampal slices from aged, memory-deficient rats. J Gerontol 32:523–533 [DOI] [PubMed] [Google Scholar]
- Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, Kukull WA. (2004) Survival after initial diagnosis of Alzheimer disease. Ann Intern Med 140:501–509 [DOI] [PubMed] [Google Scholar]
- Libert S, Cohen D, Guarente L. (2008) Neurogenesis directed by Sirt1. Nat Cell Biol 10:373–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Rogóz Z. (1999) Pharmacological effects of venlafaxine, a new antidepressant, given repeatedly, on the α1-adrenergic, dopamine and serotonin systems. J Neural Transm 106:197–211 [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. (1999) Local infusion of an α1-adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry 46:1259–1265 [DOI] [PubMed] [Google Scholar]
- Minneman KP, Theroux TL, Hollinger S, Han C, Esbenshade TA. (1994) Selectivity of agonists for cloned α1-adrenergic receptor subtypes. Mol Pharmacol 46:929–936 [PubMed] [Google Scholar]
- Nakadate K, Matsukawa M, Okado N. (2006) Identification of adrenoceptor subtype-mediated changes in the density of synapses in the rat visual cortex. Neuroscience 138:37–46 [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. (2008) Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28:6239–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA, Perez DM. (2006) Localization of the mouse α1A-adrenergic receptor (AR) in the brain: α1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol 497:209–222 [DOI] [PubMed] [Google Scholar]
- Pussinen R, Nieminen S, Koivisto E, Haapalinna A, Riekkinen P, Sr, Sirvio J. (1997) Enhancement of intermediate-term memory by an α1 agonist or a partial agonist at the glycine site of the NMDA receptor. Neurobiol Learn Mem 67:69–74 [DOI] [PubMed] [Google Scholar]
- Puumala T, Greijus S, Narinen K, Haapalinna A, Riekkinen P, Sr, Sirviö J. (1998) Stimulation of α1-adrenergic receptors facilitates spatial learning in rats. Eur Neuropsychopharmacol 8:17–26 [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. (2002) Knockout of the α1A/C-adrenergic receptor subtype: the α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA 99:9474–9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, Perez DM. (2005) α1A- but not α1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res 65:436–445 [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Waddell JE. (1982) Receptor interactions of imidazolines. IX. Cirazoline is an alpha-1 adrenergic agonist and an alpha-2 adrenergic antagonist. J Pharmacol Exp Ther 222:29–36 [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809 [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187 [DOI] [PubMed] [Google Scholar]
- Sirviö J, MacDonald E. (1999) Central α1-adrenoceptors: their role in the modulation of attention and memory formation. Pharmacol Ther 83:49–65 [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. (2001) Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85:2423–2431 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Tagawa N, Kobayashi Y, Hotta Y, Yamada J. (2007) Effects of the serotonin and noradrenaline reuptake inhibitor (SNRI) milnacipran on marble burying behavior in mice. Biol Pharm Bull 30:2399–2401 [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. (2007) Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience 146:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. (2007) Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology 53:487–495 [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. (2009) Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]