SUMMARY
MYC contributes to the pathogenesis of a majority of human cancers, yet strategies to modulate the function of the c-Myc oncoprotein do not exist. Toward this objective, we have targeted MYC transcription by interfering with chromatin-dependent signal transduction to RNA polymerase, specifically by inhibiting the acetyl-lysine recognition domains (bromodomains) of putative co-activator proteins implicated in transcriptional initiation and elongation. Using a selective small-molecule bromodomain inhibitor, JQ1, we identify BET bromodomain proteins as regulatory factors for c-Myc. BET inhibition by JQ1 downregulates MYC transcription, followed by genome-wide downregulation of Myc-dependent target genes. In experimental models of multiple myeloma, a Myc-dependent hematologic malignancy, JQ1 produces a potent antiproliferative effect associated with cell cycle arrest and cellular senescence. Efficacy of JQ1 in three murine models of multiple myeloma establishes the therapeutic rationale for BET bromodomain inhibition in this disease and other malignancies characterized by pathologic activation of c-Myc.
INTRODUCTION
c-Myc is a master regulatory factor of cell proliferation (Dang et al., 2009). In cancer, pathologic activation of c-Myc plays a central role in disease pathogenesis, by the coordinated upregulation of a transcriptional program influencing cell division, metabolic adaptation and survival (Dang, 2009; Kim et al., 2008). Amplification of MYC is among the most common genetic alterations observed in cancer genomes (Beroukhim et al., 2010). Validation of c-Myc as a therapeutic target is supported by numerous lines of experimental evidence. Murine models of diverse malignancies have been devised by introducing genetic constructs overexpressing MYC (Harris et al., 1988; Leder et al., 1986; Stewart et al., 1984). In addition, conditional transgenic models featuring tunable transcriptional suppression have shown that even transient inactivation of MYC is capable of promoting tumor regression (Jain et al., 2002; Soucek et al., 1998; Soucek et al., 2002). Elegant studies of systemic induction of a dominant-negative Myc allele within an aggressive, KRAS-dependent murine model of lung adenocarcinoma have further suggested the putative therapeutic benefit of c-Myc inhibition (Fukazawa et al., 2010). Importantly these studies establish the feasibility of c-Myc inhibition within an acceptable therapeutic window of tolerability.
Nevertheless, a therapeutic approach to target c-Myc has remained elusive. The absence of a clear ligand-binding domain establishes a formidable obstacle toward direct inhibition, which is a challenging feature shared among many compelling transcriptional targets in cancer (Darnell, 2002). c-Myc functions as a DNA-binding transcriptional activator upon heterodimerization with another basic helix-loop-helix leucine zipper (bHLH-LZ) transcription factor, Max (Amati et al., 1993; Blackwood and Eisenman, 1991). High-resolution structures of the complex fail to identify a hydrophobic involution compatible with the positioning of an organic small molecule (Nair and Burley, 2003).
Therefore, we have targeted c-Myc transcriptional function by another means, namely the disruption of chromatin-dependent signal transduction (Schreiber and Bernstein, 2002). c-Myc transcription is associated locally and globally with increases in histone lysine side-chain acetylation, a covalent modification of chromatin regionally associated with transcriptional activation (Frank et al., 2003; Vervoorts et al., 2003). Histone acetylation templates the assembly of higher-ordered transcriptional complexes by recruiting proteins with one or more acetyl-lysine binding modules or bromodomains (Dhalluin et al., 1999; Haynes et al., 1992). Members of the bromodomain and extra-terminal (BET) subfamily of human bromodomain proteins (BRD2, BRD3 and BRD4) associate with acetylated chromatin and facilitate transcriptional activation by increasing the effective molarity of recruited transcriptional activators (Rahman et al.). Notably, BRD4 has been shown to mark select M/G1 genes in mitotic chromatin as transcriptional memory and direct post-mitotic transcription (Dey et al., 2009), via direct interaction with the positive transcription elongation factor complex b (P-TEFb) (Bisgrove et al., 2007). The discovery that c-Myc regulates promoter-proximal pause release of Pol II, also through the recruitment of P-TEFb (Rahl et al., 2010), established a rationale for targeting BET bromodomains to inhibit c-Myc-dependent transcription.
Recently, we reported the development and biochemical characterization of a first potent, selective small-molecule inhibitor of BET bromodomains, JQ1 (Figure 1A) (Filippakopoulos et al., 2010). JQ1 is a thieno-triazolo-1,4-diazepine which displaces BET bromodomains from chromatin by competitively binding to the acetyl-lysine recognition pocket. In the present study, we leverage the properties of JQ1 as a chemical probe (Frye, 2011), to interrogate the role of BET bromodomains in Myc-dependent transcription and to explore the role of BET bromodomains as cancer dependencies.
Figure 1. Integrated genomic rationale for BET bromodomains as therapeutic targets in MM.
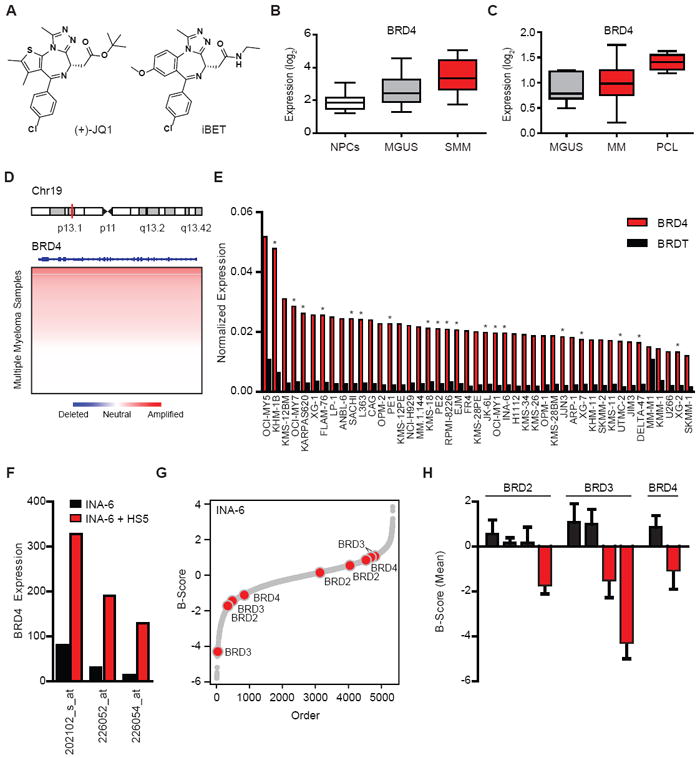
(A) Structures of the BET bromodomain inhibitors JQ1 and iBET.
(B,C) Expression levels (log2 transformed, median-centered values) for BRD4 transcripts were evaluated in oligonucleotide microarray data from normal plasma cells (NPCs) from healthy donors, individuals with MGUS or SMM patients (panel B, dataset GSE5900, (Zhan et al., 2007)); and in plasma cells from MGUS, MM and PCL patients (panel C, dataset GSE2113 (Mattioli et al., 2005)). Increased BRD4 expression is observed in SMM (or MGUS) compared to NPCs (panel B) and in PCL compared to MM (panel C) (non-parametric Kruskal-Wallis one-way analysis of variance p< 0.001 and p=0.0123, respectively; Dunn’s Multiple Comparison post-hoc tests p<0.05, in both cases).
(D) Copy number analysis of the BRD4 locus at human chromosome 19p13.1 in primary samples from 254 MM patients. Chromosome 19p amplifications are common in MM (Figure S1).
(E) Expression levels of BRD4 (compared to BRDT) in human MM cell lines. Asterisks denote cell lines with amplification of the BRD4 locus (19p13.1).
(F) BRD4 expression (depicted on a linear scale for three different oligonucleotide microarray probes) in INA-6 MM cells cultured in vitro in the presence or absence of HS-5 bone marrow stromal cells.
(G) Silencing of BET bromodomains impairs proliferation in MM cells. Results of an arrayed lentiviral screen using a diverse shRNA library in INA-6 MM cells are presented in rank order of ascending B-Scores. The effect of shRNAs targeting BET bromodomains on INA-6 cell viability is highlighted by red circles and annotated by gene. Gray dots represent results for non-BET bromodomain shRNAs.
(H) Silencing of BET bromodomain family members in MM cells. Viability of INA-6 MM cells exposed to shRNAs directed against BRD2, BRD3 and BRD4 are reported as mean B-scores (±SD of the two normalized replicates).
Multiple myeloma (MM) represents an ideal model system for these mechanistic and translational questions given the known role of MYC in disease pathophysiology. MM is an incurable hematologic malignancy, typified by the accumulation of malignant plasma cells harboring diverse genetic lesions (Chapman et al., 2011). Dysregulation of transcription factors feature prominently in the biology of MM, including NF-κB (Keats et al., 2007), c-Maf (Hurt et al., 2004), XBP1 (Claudio et al., 2002), HSF1 (Mitsiades et al., 2002), GR (Gomi et al., 1990), IRF4 (Shaffer et al., 2008), Myb (Palumbo et al., 1989), and notably c-Myc (Dean et al., 1983). Rearrangement or translocation of MYC is among the most common somatic events in early and late stage MM (Shou et al., 2000), and transcriptional profiling identifies Myc pathway activation in more than 60% of patient-derived MM cells (Chng et al., 2011). Experimental support for the central role of c-Myc in the pathogenesis of MM is contributed by an informative, genetically-engineered murine model of MM. Lineage-specific and stochastic Activation-Induced Deaminase (AID)-dependent activation of a conditional MYC transgene in the late stages of B-cell differentiation establishes genetically-engineered mice with a plasma cell malignancy that shares clinically relevant features of MM (Chesi et al., 2008). Thus, MYC dysregulation represents a largely unifying molecular feature observed across the otherwise complex genetic landscape of MM.
In this study, we report that c-Myc transcriptional function can be modulated pharmacologically by BET bromodomain inhibition. Unexpectedly, we have discovered that MYC, itself, is transcriptionally regulated by BET bromodomains. Chromatin immunopreciptitation studies show that BRD4 is strongly enriched at immunoglobulin heavy chain (IgH) enhancers in MM cells bearing IgH rearrangement at the MYC locus. BET inhibition with JQ1 depletes enhancer-bound BRD4 and promptly inhibits MYC transcription in a dose- and time-dependent manner. In translational models of MM, JQ1 leads to depletion of the c-Myc oncoprotein and selective downregulation of the coordinated c-Myc transcriptional program, prompting cell cycle arrest and cellular senescence. These results indicate that targeting protein-protein interactions within the c-Myc transcriptional signaling network can modulate the function of c-Myc in cancer.
RESULTS
BET bromodomains as therapeutic targets in MM
We first evaluated the expression of BRD2, BRD3, BRD4 and BRDT transcripts in MM, by integrating publicly-available compendia of gene expression datasets. Among asymptomatic patients with pre-malignant disease (Zhan et al., 2007), we observed increasing expression of BRD4 in monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM) compared to normal bone marrow plasma cells (Figure 1B). In a second, independent dataset (Mattioli et al., 2005), we observed significantly higher expression of BRD4 in plasma cell leukemia (PCL) compared to MM or MGUS samples (Figure 1C). Thus, BRD4 expression correlates positively with disease progression. BRD2 and BRD3 are also expressed in MM, but expression does not clearly correlate with stage of disease (data not shown). BRDT, a testis-specific bromodomain-containing protein, is not expressed in MM.
Analysis of copy number polymorphism (CNP) data collected on 254 MM patients by the Multiple Myeloma Research Consortium (MMRC) revealed that the BRD4 locus is frequently amplified in MM patient samples (Figure 1D). The majority of patient samples exhibit broad amplification of chromosome 19p, but focal amplification at the BRD4 locus is observed (Figure S1). Among 45 established MM cell lines, expression of BRD4 was pronounced and did not correlate with amplification status (Figure 1E). Expression of BRD4 was pronounced across samples with or without BRD4 amplification (Figure 1E). Human MM cells are highly osteotropic in vivo, and interaction with bone marrow stromal cells (BMSCs) induces proliferation and contributes to drug resistance (McMillin et al., 2010). Analysis of BET bromodomain expression as influenced by MM cell binding to BMSCs (McMillin et al., 2010), revealed marked upregulation of BRD4 in the INA-6 human MM cell line upon interaction with HS5 stromal cells (Figure 1F), suggesting a plausible role for BRD4 function in MM cells within the bone marrow microenvironment.
To explore the function of BET bromodomains in MM, we examined the effect on proliferation of small hairpin RNAs (shRNAs) targeting each of the four BET proteins in comparison to shRNAs targeting 1011 kinases, phosphatases and oncogenes in a lentivirally-delivered, arrayed shRNA screen in INA-6 cells. As illustrated in Figure 1G-H, shRNA constructs targeting each of the expressed BET bromodomains are identified as reducing INA-6 proliferation as shown by normalized B-scores (Malo et al., 2006). Together, these data establish a rationale for the study of BET bromodomains, and BRD4 in particular, as tumor dependencies in MM.
BET inhibition with JQ1 arrests c-Myc transcriptional programs
To test the hypothesis that BET inhibition will specifically abrogate Myc-dependent transcription, we utilized global transcriptional profiling and unbiased gene set enrichment analysis (GSEA). We first characterized the transcriptional consequences of BET inhibition in three MM cell lines with genetically distinct activating lesions at the MYC locus (KMS11, MM.1S and OPM1) (Dib et al., 2008). Unsupervised hierarchical clustering segregated samples based on treatment assignment suggesting a common transcriptional consequence in response to JQ1 (Figure 2A). Acute JQ1 treatment did not prompt global, non-specific transcriptional silencing, but instead produced significant changes in a finite number of genes (88 down- and 25 up-regulated genes by two-fold or greater in all three MM lines).
Figure 2. Inhibition of Myc-dependent transcription by the JQ1 BET bromodomain inhibitor.
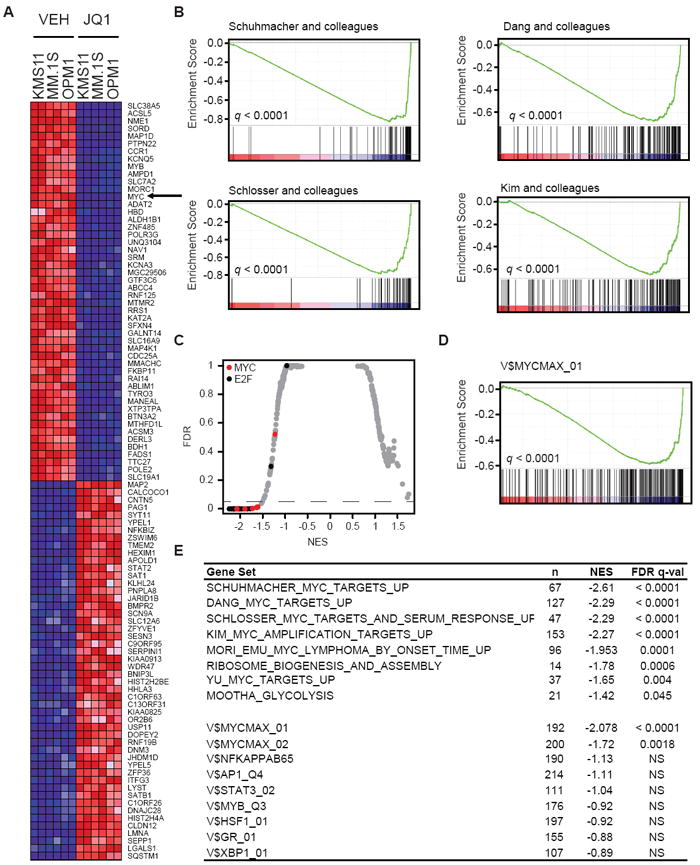
(A) Heat map representation of the top 50 down- and top 50 up-regulated genes (P < 0.001) following JQ1 treatment in MM cell lines. Data are presented row-normalized (range from -3- to +3-fold change in expression). MYC (arrow) is downregulated by JQ1 treatment.
(B) GSEA of four Myc-dependent gene sets (Kim et al., 2006; Schlosser et al., 2005; Schuhmacher et al., 2001; Zeller et al., 2003), in transcriptional profiles of MM cells treated (left) or untreated (right) with JQ1.
(C) Quantitative comparison of all transcription factor target gene sets available from the MSigDB by GSEA for reduced expression in JQ1-treated MM cell lines. Data are presented as scatterplot of false discovery rate (FDR) versus normalized enrichment score (NES) for each evaluated gene set. Colored dots indicate gene sets for MYC (red), E2F (black), or other (gray) transcription factors.
(D) GSEA showing downregulation, in JQ1-treated MM cells, of a representative set of genes with proximal promoter regions containing Myc-Max binding sites.
(E) Table of gene sets enriched among genes downregulated by JQ1 in MM cells (top group), highlighting the number of genes in each set (n), the normalized enrichment score (NES) and test of statistical significance (FDR q-value). The bottom group represents comparisons of top-ranking transcription factor target gene sets of MM master regulatory proteins, enriched among genes downregulated by JQ1 in MM cells.
To examine higher-order influences over biological networks regulated by c-Myc, we evaluated four canonical transcriptional signatures of MYC-dependent genes (Kim et al., 2006; Schlosser et al., 2005; Schuhmacher et al., 2001; Zeller et al., 2003). All four signatures were strongly correlated with downregulation of expression by JQ1 (Figure 2B). As a measure of the specificity of this effect, an open-ended enrichment analysis was performed on the entire set of transcription factor target gene signatures available from the Molecular Signatures Database (MSigDB). Gene sets defined by adjacency to Myc-binding motifs were in almost all cases significantly enriched in JQ1-suppressed genes (Figure 2C, D). In marked contrast, JQ1 treatment did not exert significant suppression of gene sets for other transcription factors linked to pathophysiology of MM, including NF-κB, AP-1, STAT3, GR and XBP-1 (Figure 2E, S2). Notably, 27 of the 28 significantly correlated gene sets are annotated as predicted targets of MYC or E2F (Figures 2C, S2). Consistent with Myc-specific inhibition, biological modules associated with Myc (e.g. ribosomal biogenesis and assembly, and glycolysis) were also anti-correlated with JQ1 treatment (Figure 2E). BET bromodomain inhibition by JQ1 confers a selective repression of transcriptional networks induced by c-Myc.
Regulation of MYC transcription by BET bromodomains
An unexpected finding was the observed, robust inhibition of MYC expression following treatment with JQ1 (Figure 2A). As MYC is commonly activated by upstream oncogenic signaling pathways, we studied the consequence of JQ1 treatment on the expression of 230 cancer-related genes in a human MM cell line (MM.1S) using a multiplexed transcript detection assay (Figure 3A). Excellent concordance was observed between replicate measurements of expressed genes (Figure S3A). Unsupervised hierarchical clustering segregated replicate data correctly into early and late treatment time-points. Surprisingly, we observed immediate, progressive and profound downregulation of MYC transcription, itself, a unique finding among all transcripts studied (p < 0.05).
Figure 3. BET inhibition suppresses MYC transcription in MM.
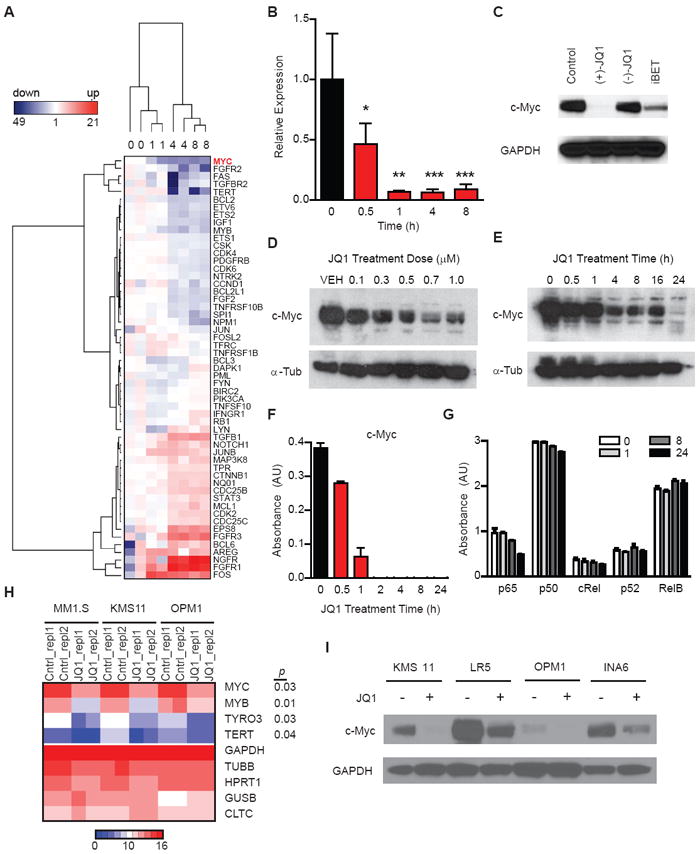
(A) Heatmap of cancer-related genes expressed in MM cells (MM.1S), treated with JQ1 (500 nM over 1, 4 and 8 h). MYC (red) is downregulated by JQ1 in a time-dependent manner, uniquely among all oncogenes studied (230 total). MYC was identified as the only statistically significant decrease in transcription at all four time-points analyzed (p < 0.05).
(B) Quantitative RT-PCR analysis for MYC levels in JQ1-treated MM.1S cells (500 nM, 0 - 8 h). Data are presented as ratio of MYC expression at each time-point compared to baseline MYC expression. Asterisks denote the level of statistical significance (* p < 0.01, ** p < 0.0002, *** p < 0.006; paired Student’s t-test each relative to t = 0 h).
(C) The active JQ1 enantiomer and the structurally analogous BET inhibitor, iBET (Nicodeme et al., 2010), but not the inactive (-)-JQ1 enantiomer, downregulate c-Myc expression, as determined by immunoblotting of MM.1S cells treated with compounds (500 nM) or vehicle control for 24 h.
(D-E) Immunoblotting analyses of the (D) dose- and (E) time-dependent effects of JQ1 treatment on c-Myc expression in MM.1S cells.
(F-G) Selective depletion of nuclear c-Myc following JQ1 treatment (500 nM) as measured by ELISA-based DNA binding assays for the activity of (F) c-Myc (depleted after 1-2 h) and (G) NF-κB family members (unaffected). Data represent mean ± SEM.
(H) Heatmap of clustered gene expression data from multiplexed measurement (Nanostring) of cancer-associated genes in three human MM cell lines treated with JQ1 or vehicle control. Among 230 genes studied (Figure S4), four genes (MYC, TERT, TYRO3 and MYB) exhibited statistically significant (p<0.05) downregulation. Replicate expression measurements exhibited high concordance among low and highly expressed genes (Figure S3B).
(I) Immunoblotting study of four MM lines (KMS11, LR5, OPM1, and INA-6) identifies a JQ1-induced decrease in c-Myc expression (500 nM, 24 h).
Downregulation of MYC was further confirmed by RT-PCR and immunoblot (Figure 3B, C). This effect was BET bromodomain-specific, supported by the nearly comparable activity of an analogous BET inhibitor subsequently published by Glaxo SmithKline (iBET; Figure 1A) (Nicodeme et al., 2010)), and the lack of activity of the inactive (-)-JQ1 enantiomer, which we previously characterized as structurally incapable of inhibiting BET bromodomains (Filippakopoulos et al., 2010) (Figure 3C). Inhibition of MYC transcription by JQ1 was observed to be dose- and time-dependent, with peak inhibition at sub-micromolar concentrations (Figure 3D, E). Rapid depletion of chromatin-bound c-Myc was confirmed by nuclear ELISA transcription factor binding assays (Figure 3F). In contrast, NF-κB and AP-1 chromatin binding assays failed to reveal any decrease in DNA binding within 8 hours of JQ1 treatment (Figure 3G, S4A).
To assess the breadth of these findings in MM, we expanded gene expression studies to three MM cells with distinct lesions at the MYC locus. MM.1S cells have a complex MYC rearrangement involving an IgH insertion at the breakpoint of a derivative chromosome der3t(3;8), KMS-11 cells have both MYC duplication and inversion, and OPM1 cells feature a der(8)t(1;8) (Dib et al., 2008). Among 230 genes studied, MYC was one of only four genes downregulated by treatment with JQ1, along with MYB, TYRO3 and TERT (Figure 3H and S4B). Immunoblotting analyses confirmed the JQ1 suppression of c-Myc protein expression in a further expanded panel of Myc-dependent MM cell lines (Figure 3I). Despite the intriguing potential effect on E2F transcriptional function and MYB gene expression, JQ1 did not influence E2F or MYB protein abundance through 24 hours of drug exposure (Figure S4C, D). Together, these data support the general observation that BET inhibition specifically suppresses MYC transcription across MM cells with different genetic lesions affecting the MYC locus, and with striking selectivity in comparison to other oncogenic transcriptions factors with established roles in MM pathophysiology.
BRD4 binds IgH enhancers, regulating MYC expression and function
Based on the integrated, functional genomic analysis of BET bromodomains in MM (Figure 1), we pursued further mechanistic studies of BRD4. Silencing of BRD4 using directed shRNAs validated by RT-PCR analysis (Figure 4A), elicited a marked decrease in MYC transcription (Figure 4B) accompanied by G1 cell cycle arrest in JQ1-sensitive MM cells (OPM-1; Figure 4B, S5A). We reasoned that early and sustained JQ1-induced suppression of MYC transcription may be mechanistically explained by physical interaction of BRD4 with regulatory elements influencing MYC expression. Indeed, avid binding of BRD4 to established IgH enhancers was observed by chromatin immunoprecipitation (ChIP) in MM.1S cells (Figure 4C, S6), which harbor an IgH insertion proximal to the MYC transcriptional start site (TSS). BRD4 binding was not observed at five characterized enhancer regions adjacent to the MYC gene (Pomerantz et al., 2009a; Pomerantz et al., 2009b). JQ1 treatment (500 nM) for 24 hours significantly depleted BRD4 binding to IgH enhancers and the TSS, supporting direct regulation of MYC transcription by BET bromodomains, and a model whereby BRD4 acts as a co-activator of MYC transcription potentially through long-range interactions with distal enhancer elements. Forced overexpression of c-Myc in MM cells (OPM1) by retroviral infection rescues, in part, the cell cycle arrest observed with JQ1 treatment (Figure 4D, E), arguing that MYC down-regulation by JQ1 contributes functionally to cell physiology in MM.
Figure 4. Regulation of MYC transcription by BET bromodomains.
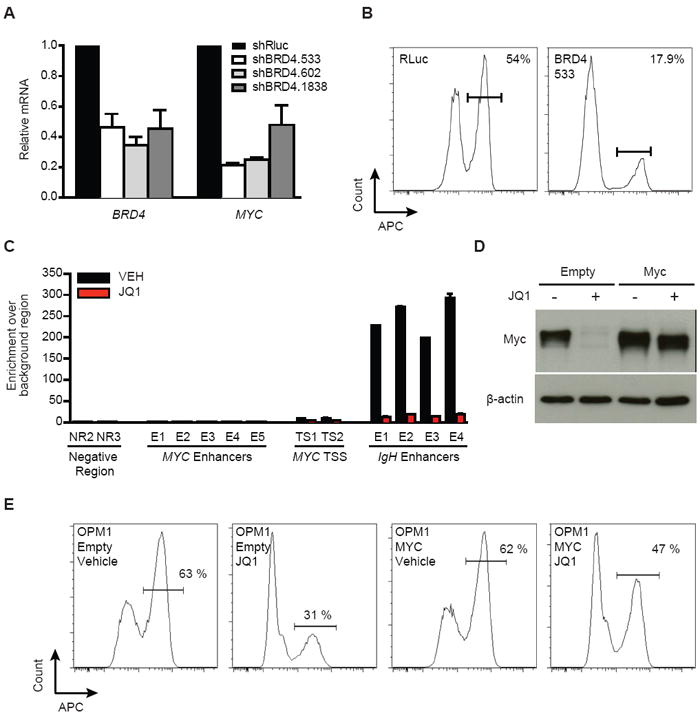
(A) BRD4 and MYC expression in OPM1 cells transduced with either shBRD4 (three different hairpins) or a Renilla control hairpin (shRluc). shRNAs were induced for five days before analysis by qRT-PCR. Data were normalized to GAPDH.
(B). Cell cycle analysis of OPM1 cells transduced with the indicated shRNA for six days. BrdU staining (APC) identifies the fraction of cells in S-phase.
(C) ChIP studiesof BRD4 (anti-BRD4; Bethyl) binding to MYC TSS or proximal enhancers in MM.1S cells. Competitive displacement of BRD4 from IgH enhancers is observed upon treatment with JQ1 (500nM for 24 h; red bars) compared to vehicle control (black bars).
(D) Immunolotting of whole-cell lysates from empty MSCV vector- or Myc overexpression vector-transduced OPM1 cells after treating with JQ1 (500nM, 24h) or DMSO control.
(E) Cell cycle analysis of either empty or Myc over-expressing OPM1 cells treated with JQ1 (500nM, 24h). BrdU staining (APC) identifies the fraction of cells in S-phase.
Therapeutic implications of BET inhibition in MM
Based on this mechanistic rationale, we evaluated the therapeutic opportunity of MYC transcriptional inhibition using established translational models of MM. Antiproliferative activity of JQ1 was assessed using a panel of 25 MM cell lines or isogenic derivative lines (Figure 5A). MM cell proliferation was uniformly inhibited by JQ1 (Figure 5A), including several MM cell lines selected for resistance to FDA-approved agents (dexamethasone-resistant MM.1R and melaphalan-resistant LR5). As expected, MM cells possessing diverse genetic lesions involving MYC (Dib et al., 2008), were comparably sensitive to JQ1 (Figure 5B).
Figure 5. Anti-myeloma activity of JQ1 in vitro.
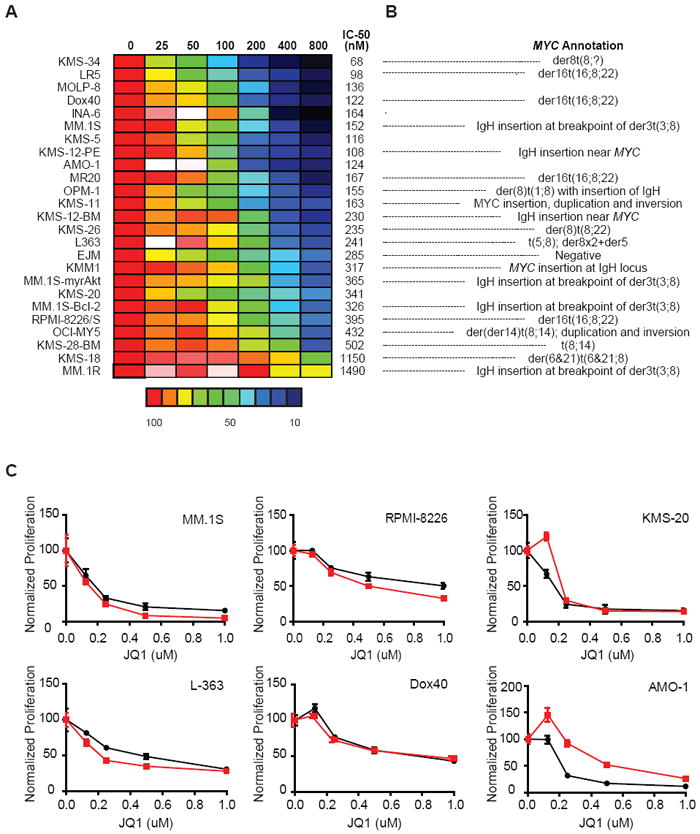
(A) A panel of MM cell lines was tested for in vitro sensitivity to JQ1 (12.5 - 800 nM, 72h), by measurement of ATP levels (Cell TiterGlo; Promega).
(B) MYC genomic status of selected MM cell lines from panel A, as annotated (Dib et al., 2008).
(C) Activity of JQ1 against MM cell lines cultured in the presence (red lines) or absence (black lines) of the HS-5 stromal cell line, assessed by CS-BLI (McMillin et al., 2010).
As interaction of MM cells with BMSCs is widely recognized to confer resistance to numerous therapeutic agents (Hideshima et al., 2007; McMillin et al., 2010), we sought to characterize the effect of BMSCs on MM cell sensitivity to BET inhibition. Using compartment specific bioluminescence imaging assays (CS-BLI), we observed that the sensitivity of MM cell lines to JQ1 is largely unchanged by the presence of HS-5 bone marrow stroma cells (Figure 5C). This pattern of broad activity in MM without evident stroma-mediated chemoresistance has been associated with efficacy of FDA-approved agents bortezomib and lenalidomide.
MM cells were then further phenotyped for Myc-specific biological effects of BET inhibition. Flow cytometry of JQ1-treated MM.1S cells revealed a pronounced decrease in the proportion of cells in S-phase, with a concomitant increase in cells arrested in G0/G1 (Figure 6A). Only a modest induction of apoptosis was observed after 48 hours of JQ1 treatment (Figure 6B), in contrast to the non-selective cytotoxic kinase inhibitor, staurosporine (Figure S5B). Transcripts previously associated with induction of cellular senescence were enriched following treatment with JQ1, by GSEA (Figure 6C). Experimentally, treatment with JQ1 resulted in pronounced cellular senescence by beta-galactosidase staining (Figure 6D). Overall, these phenotypes of arrested proliferation, G1 cell cycle arrest and cellular senescence are highly specific to anticipated effects of inhibiting cellular c-Myc function (Wu et al., 2007).
Figure 6. JQ1 induces cell cycle arrest and cellular senescence in MM cells.
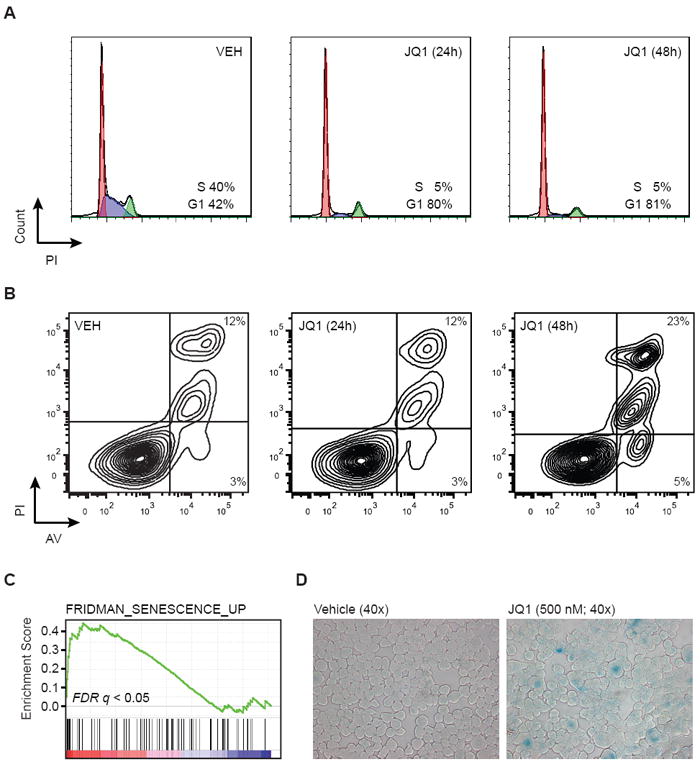
(A-B) Flow cytometric evaluation of propidium iodide (PI) staining for cell cycle analysis (panel A) and detection of Annexin V-positive apoptotic cells (panel B) in JQ1-treated MM.1S cells (0 - 48hrs, 500 nM).
(C) Enrichment of senescence-associated genes among JQ1-suppressed genes in MM.1S cells.
(D) Induction of cellular senescence in JQ1 treated MM.1S cells (500 nM, 72 h), as detected by ß-galactosidase staining.
We next extended the study of JQ1 in MM cells to primary MM samples. JQ1 exposure led to a significant reduction in cell viability among the majority of CD138+ patient-derived MM samples tested (Figure 7A). In primary cells isolated from a patient with relapsed/refractory MM, JQ1 treatment ex vivo conferred a time-dependent suppression of c-Myc expression (Figure 7B). In contrast, JQ1 treatment of phytohemaglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) suppressed PHA-induced proliferation, but did not adversely influence cell viability, indicating that the anti-MM effect of JQ1 is not accompanied by a non-specific, toxic effect on all hematopoietic cells (Figure S7A). To model the therapeutic effect of JQ1 in vivo, we evaluated anti-MM efficacy in multiple orthotopic models of advanced disease. First, JQ1 was studied using an established, bioluminescent MM model (MM.1S-luc), which recapitulates the clinical sequelae, anatomic distribution of MM lesions, and hallmark bone pathophysiology observed in MM patients (Mitsiades et al., 2004). Tumor-bearing mice were treated with JQ1 administered by intraperitoneal injection (50 mg/kg daily) or vehicle control. JQ1 treatment significantly decreased the burden of disease measured by serial, whole-body, non-invasive bioluminescence imaging (Figure 7C-D). Importantly, treatment with JQ1 resulted in a significant prolongation in overall survival compared to vehicle-treated animals (Figure 7E). In a second, plasmacytoma xenograft which more accurately models extramedullary disease, JQ1 also exhibited a significant disease-modifying response (Figure S7B). Finally, the effect of JQ1 was explored in an aggressive, genetically-engineered model of Myc-dependent MM (Chesi et al., 2008). To date, two animals with advanced disease have completed 14 days of JQ1 treatment (25 mg/kg daily, adjusted to tolerability). Both animals reveal objective evidence of response by measurement of monotypic serum immunoglobulins, including a complete response (CR) in the second animal (Figure 7F, S7C). In this orthotopic model, only the FDA-approved agent, bortezomib, has previously prompted a CR (M.C. and P.L.B., unpublished data). These results establish in vivo proof-of-concept supporting the investigational study of BET bromodomain inhibitors in MM.
Figure 7. Translational implications of BET bromodomain inhibition in MM.
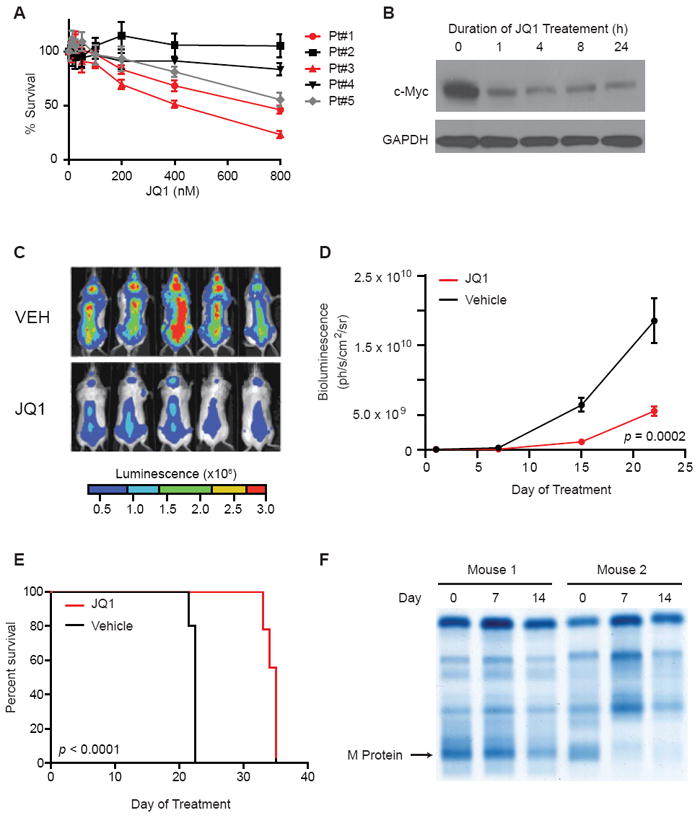
(A) JQ1 arrests the proliferation of primary, patient-derived CD138+ MM cells (Cell TiterGlo; Promega).
(B) c-Myc immunoblot shows JQ1-induced downregulation in short-term culture of primary, patient-derived MM cells (500 nM, duration as indicated).
(C) Representative whole-body bioluminescence images of SCID-beige mice orthotopically xenografted after intravenous injection with MM.1S-luc+ cells and treated with JQ1 (50 mg/kg IP daily) or vehicle control.
(D) Tumor burden of SCID-beige mice orthotopically xenografted after intravenous injection with MM.1S-luc+ cells. Upon detection of MM lesions diffusely engrafted in the skeleton, mice were randomly assigned to receive JQ1 (50 mg/kg IP daily) or vehicle control. Data are presented as mean +/- SEM (n = 10/group).
(E) Survival curves (Kaplan-Meier) of mice with orthotopic diffuse MM lesions show prolongation of overall survival with JQ1 treatment compared to vehicle control (log-rank test, p < 0.0001).
(F) Serum protein electrophoresis to detect monoclonal, tumor-derived, immunoglobulin (M-protein) in two MM-bearing Vk*myc mice before or after 7 and 14 days of JQ1 treatment. JQ1 treatment induced partial and complete responses, respectively, in mouse 1 and mouse 2.
DISCUSSION
Despite the centrality of Myc in the pathogenesis of cancer, conventional approaches toward direct Myc inhibition have not proven successful. To date, efforts to target c-Myc have identified only a small number of molecules with low biochemical potency and limited biological characterization (Bidwell et al., 2009; Hammoudeh et al., 2009; Jeong et al., 2010), underscoring both the challenge of targeting c-Myc as well as the enduring need for chemical probes of c-Myc transcriptional function. Considering chromatin as a platform for signal transduction (Schreiber and Bernstein, 2002), we have undertaken to inhibit Myc transcription and function through displacement of chromatin-bound, co-activator proteins using competitive small molecules. Using a first-in-class, small-molecule bromodomain inhibitor developed by our laboratories, JQ1, we validate BET bromodomains as determinants of c-Myc transcription and as therapeutic targets in MM, an ideal model system for the mechanistic and translational study of Myc pathway inhibitors.
Most importantly, we illustrate the feasibility of selectively downregulating transcription of MYC, itself, via the molecular action of a selective, small molecule. The ensuing suppression of c-Myc protein levels, depletion of chromatin-bound c-Myc and concomitant downregulation of the Myc-dependent transcriptional network, lead to growth-inhibitory effects sharing the specificity of phenotypes associated with prior genetic models of Myc inhibition. This is a notable observation, which distinguishes the transcriptional consequences of BET inhibition from other, non-selective transcriptional inhibitors, such as actinomycin D, alpha-amanitin and flavopiridol.
A compelling finding is the observed, direct interaction of BRD4 with IgH enhancers in MM cells possessing IgH rearrangement into the MYC locus, and the depletion of BRD4 binding by JQ1. This suggests BET inhibition as a putative strategy for targeting other structural rearrangements in cancer involving IgH or other strong enhancers, and has potential implications for the modulation of immunoglobulin gene expression in autoimmune diseases. An unexpected finding was the pronounced and concordant suppression of multiple E2F-dependent transcriptional signatures. In this instance, E2F1 protein and transcript levels were not affected by BET inhibition, suggesting either an unrecognized function of BET bromodomains in E2F transcriptional complexes or a dominant effect of Myc downregulation causing cell cycle arrest in G1 leading to silencing of E2F. These observations are also compatible with the known role of Myc and E2F1 as transcriptional collaborators in cell cycle progression and tumor cell survival (Matsumura et al., 2003; Trimarchi and Lees, 2002).
Insights provided by our study identify rational strategies for combination therapeutic approaches warranting exploration in MM. MYC activation is commonly accompanied by anti-apoptotic signaling in human cancer. In MM, constitutive or microenvironment-inducible activation of anti-apoptotic Bcl-2 proteins has been reported (Harada et al., 1998; Legartova et al., 2009). Thus, Myc pathway inhibition by JQ1 may demonstrate synergism with targeted pro-apoptotic agents (e.g. ABT-737) (Oltersdorf et al., 2005; Trudel et al., 2007). Additionally, the selective effect of JQ1 on Myc and E2F1 transcriptional programs provides an opportunity to combine BET inhibitors with pathway-directed antagonists of the NF-κB, STAT3, XBP1 or HSF1 transcriptional programs.
Direct inhibition of MYC remains a central challenge in the discipline of ligand discovery. Inhibition of MYC expression and function, demonstrated herein, presents an immediate opportunity to study and translate the concept of MYC inhibition more broadly in human cancer. During the course of this research, a collaborative effort with the laboratories of Christopher Vakoc and Scott Lowe revealed BRD4 as a tumor dependency in acute myeloid leukemia. Consistent with our observations described here in MM, leukemia cells similarly require BRD4 to sustain MYC expression to enforce aberrant self-renewal (Zuber et al.). Collectively, these findings highlight a broad role for BRD4 in maintaining MYC expression in diverse hematopoietic malignancies and suggest the utility of drug-like BET bromodomain inhibitors as novel therapeutic agents in these diseases.
EXPERIMENTAL PROCEDURES
Gene expression analysis
MM cells treated with JQ1 (500 nM, 24 h) were processed for transcriptional profiling using Affymetrix Human Gene 1.0 ST microarrays. Expression of individual genes was assessed in the context of dose- and time-ranging experiments by real-time quantitative polymerase chain reaction, multiplexed direct detection (Nanostring) and immunoblotting using antibodies as described in Extended Experimental Procedures.
Chromatin immunoprecipitation
ChIP was performed on MM.1S cells cultured in the presence or absence of JQ1 (500 nM, 24 hours). Specific antibodies, detailed methods, primer sequences for MYC and IgH enhancers, as well as the MYC TSS, are described in Extended Experimental Procedures.
In vitro and in vivo MM studies
The impact of JQ1 on cell viability, proliferation and cell cycle was assessed in human MM cells as documented in Extended Experimental Procedures. In vivo efficacy studies were performed with protocols approved by Institutional Animal Care and Use Committees at the DFCI or Mayo Clinic Arizona. JQ1 was administered by intraperitoneal injection into SCID-beige mice with MM lesions established after subcutaneous or intravenous injections; and in non-immunocompromised tumor-bearing Vk*myc mice. Tumor burden in these models was quantified by caliper measurement, whole-body bioluminescence imaging and serum protein electrophoresis, respectively, as detailed in Extended Experimental Procedures.
Supplementary Material
Acknowledgments
We are grateful to S. Lowe for sharing unpublished information; A. Roccaro and A. Azab for technical support; E. Fox for microarray data; J. Daley and S. Lazo-Kallanian for flow cytometry; the MMRF, MMRC and Broad Institute for establishing the MM Genomics Portal (http://www.broadinstitute.org/mmgp/). This research was supported by NIH-K08CA128972 (JEB), NIH-R01CA050947 (CSM), NIH-R01HG002668 (RAY) and NIH-R01CA46455 (RAY); the Chambers Medical Foundation (PGR, CSM), the Stepanian Fund for Myeloma Research (PGR, CSM) and the Richard J. Corman Foundation (PGR, CSM); an American Cancer Society Postdoctoral Fellowship, 120272-PF-11-042-01-DMC (PBR); the Burroughs-Wellcome Fund, the Smith Family Award, the Damon-Runyon Cancer Research Foundation and the MMRF (to JEB, CSM).
Footnotes
AUTHOR CONTRIBUTIONS
JEB and CSM designed the study, analyzed data and prepared the manuscript. JED, HMJ, and EK assayed MM drug sensitivity. GCI and JED assessed the effects of JQ1 on Myc expression. JQ performed scaling synthesis and purification of JQ1. PGR and KCA provided primary MM samples. PBR and TG conducted ChIP experiments, and PBR and RAY contributed to their interpretation. RMP, TPH and MRM performed RNA expression analysis. IMG and KCA provided support and interpreted cellular data. ACS and WCH designed and performed shRNA screens. MEL analyzed expression array data. JS and CRV performed Myc rescue experiments. ALK supervised in vivo efficacy and biostatistical studies. MC and PLB performed in vivo GEMM studies. JEB and CSM supervised the research. All authors edited the manuscript.
ACCESSION NUMBERS
Oligonucleotide microarray data have been deposited in GEO under the accession number GSE31365.
Supplemental information includes Extended Experimental Procedures and seven figures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amati B, Brooks MW, Levy N, Littlewood TD, Evan GI, Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993;72:233–245. doi: 10.1016/0092-8674(93)90663-b. [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135:2–10. doi: 10.1016/j.jconrel.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13690–13695. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann GJ, Adli M, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesi M, Robbiani DF, Sebag M, Chng WJ, Affer M, Tiedemann R, Valdez R, Palmer SE, Haas SS, Stewart AK, et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell. 2008;13:167–180. doi: 10.1016/j.ccr.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng WJ, Huang GF, Chung TH, Ng SB, Gonzalez-Paz N, Troska-Price T, Mulligan G, Chesi M, Bergsagel PL, Fonseca R. Clinical and biological implications of MYC activation: a common difference between MGUS and newly diagnosed multiple myeloma. Leukemia. 2011;25:1026–1035. doi: 10.1038/leu.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio JO, Masih-Khan E, Tang H, Goncalves J, Voralia M, Li ZH, Nadeem V, Cukerman E, Francisco-Pabalan O, Liew CC, et al. A molecular compendium of genes expressed in multiple myeloma. Blood. 2002;100:2175–2186. doi: 10.1182/blood-2002-01-0008. [DOI] [PubMed] [Google Scholar]
- Dang CV. MYC, microRNAs and glutamine addiction in cancers. Cell Cycle. 2009;8:3243–3245. doi: 10.4161/cc.8.20.9522. [DOI] [PubMed] [Google Scholar]
- Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- Dean M, Kent RB, Sonenshein GE. Transcriptional activation of immunoglobulin alpha heavy-chain genes by translocation of the c-myc oncogene. Nature. 1983;305:443–446. doi: 10.1038/305443a0. [DOI] [PubMed] [Google Scholar]
- Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20:4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- Dib A, Gabrea A, Glebov OK, Bergsagel PL, Kuehl WM. Characterization of MYC translocations in multiple myeloma cell lines. J Natl Cancer Inst Monogr. 2008:25–31. doi: 10.1093/jncimonographs/lgn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 2003;4:575–580. doi: 10.1038/sj.embor.embor861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye SV. The art of the chemical probe. Nat Chem Biol. 2011;6:159–161. doi: 10.1038/nchembio.296. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Maeda Y, Matsuoka J, Yamatsuji T, Shigemitsu K, Morita I, Faiola F, Durbin ML, Soucek L, Naomoto Y. Inhibition of Myc effectively targets KRAS mutation-positive lung cancer expressing high levels of Myc. Anticancer Res. 2010;30:4193–4200. [PubMed] [Google Scholar]
- Gomi M, Moriwaki K, Katagiri S, Kurata Y, Thompson EB. Glucocorticoid effects on myeloma cells in culture: correlation of growth inhibition with induction of glucocorticoid receptor messenger RNA. Cancer Res. 1990;50:1873–1878. [PubMed] [Google Scholar]
- Hammoudeh DI, Follis AV, Prochownik EV, Metallo SJ. Multiple independent binding sites for small-molecule inhibitors on the oncoprotein c-Myc. J Am Chem Soc. 2009;131:7390–7401. doi: 10.1021/ja900616b. [DOI] [PubMed] [Google Scholar]
- Harada N, Hata H, Yoshida M, Soniki T, Nagasaki A, Kuribayashi N, Kimura T, Matsuzaki H, Mitsuya H. Expression of Bcl-2 family of proteins in fresh myeloma cells. Leukemia. 1998;12:1817–1820. doi: 10.1038/sj.leu.2401168. [DOI] [PubMed] [Google Scholar]
- Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167:353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SR, Dollard C, Winston F, Beck S, Trowsdale J, Dawid IB. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucleic Acids Res. 1992;20:2603. doi: 10.1093/nar/20.10.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5:191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- Jeong KC, Ahn KO, Yang CH. Small-molecule inhibitors of c-Myc transcriptional factor suppress proliferation and induce apoptosis of promyelocytic leukemia cell via cell cycle arrest. Mol Biosyst. 2010;6:1503–1509. doi: 10.1039/c002534h. [DOI] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Girard L, Giacomini CP, Wang P, Hernandez-Boussard T, Tibshirani R, Minna JD, Pollack JR. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene. 2006;25:130–138. doi: 10.1038/sj.onc.1208997. [DOI] [PubMed] [Google Scholar]
- Leder A, Pattengale PK, Kuo A, Stewart TA, Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986;45:485–495. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Legartova S, Krejci J, Harnicarova A, Hajek R, Kozubek S, Bartova E. Nuclear topography of the 1q21 genomic region and Mcl-1 protein levels associated with pathophysiology of multiple myeloma. Neoplasma. 2009;56:404–413. doi: 10.4149/neo_2009_05_404. [DOI] [PubMed] [Google Scholar]
- Malo N, Hanley JA, Cerquozzi S, Pelletier J, Nadon R. Statistical practice in high-throughput screening data analysis. Nat Biotechnol. 2006;24:167–175. doi: 10.1038/nbt1186. [DOI] [PubMed] [Google Scholar]
- Matsumura I, Tanaka H, Kanakura Y. E2F1 and c-Myc in cell growth and death. Cell Cycle. 2003;2:333–338. [PubMed] [Google Scholar]
- Mattioli M, Agnelli L, Fabris S, Baldini L, Morabito F, Bicciato S, Verdelli D, Intini D, Nobili L, Cro L, et al. Gene expression profiling of plasma cell dyscrasias reveals molecular patterns associated with distinct IGH translocations in multiple myeloma. Oncogene. 2005;24:2461–2473. doi: 10.1038/sj.onc.1208447. [DOI] [PubMed] [Google Scholar]
- McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D, Joseph M, Libermann TA, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SK, Burley SK. X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell. 2003;112:193–205. doi: 10.1016/s0092-8674(02)01284-9. [DOI] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Palumbo AP, Pileri A, Dianzani U, Massaia M, Boccadoro M, Calabretta B. Altered expression of growth-regulated protooncogenes in human malignant plasma cells. Cancer Res. 1989;49:4701–4704. [PubMed] [Google Scholar]
- Pomerantz MM, Ahmadiyeh N, Jia L, Herman P, Verzi MP, Doddapaneni H, Beckwith CA, Chan JA, Hills A, Davis M, et al. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet. 2009a;41:882–884. doi: 10.1038/ng.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz MM, Beckwith CA, Regan MM, Wyman SK, Petrovics G, Chen Y, Hawksworth DJ, Schumacher FR, Mucci L, Penney KL, et al. Evaluation of the 8q24 prostate cancer risk locus and MYC expression. Cancer Res. 2009b;69:5568–5574. doi: 10.1158/0008-5472.CAN-09-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, Howley PM. The Brd4 Extraterminal Domain Confers Transcription Activation Independent of pTEFb by Recruiting Multiple Proteins, Including NSD3. Mol Cell Biol. 2011;31:2641–2652. doi: 10.1128/MCB.01341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser I, Holzel M, Hoffmann R, Burtscher H, Kohlhuber F, Schuhmacher M, Chapman R, Weidle UH, Eick D. Dissection of transcriptional programmes in response to serum and c-Myc in a human B-cell line. Oncogene. 2005;24:520–524. doi: 10.1038/sj.onc.1208198. [DOI] [PubMed] [Google Scholar]
- Schreiber SL, Bernstein BE. Signaling network model of chromatin. Cell. 2002;111:771–778. doi: 10.1016/s0092-8674(02)01196-0. [DOI] [PubMed] [Google Scholar]
- Schuhmacher M, Kohlhuber F, Holzel M, Kaiser C, Burtscher H, Jarsch M, Bornkamm GW, Laux G, Polack A, Weidle UH, et al. The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res. 2001;29:397–406. doi: 10.1093/nar/29.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y, Martelli ML, Gabrea A, Qi Y, Brents LA, Roschke A, Dewald G, Kirsch IR, Bergsagel PL, Kuehl WM. Diverse karyotypic abnormalities of the c-myc locus associated with c-myc dysregulation and tumor progression in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:228–233. doi: 10.1073/pnas.97.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Helmer-Citterich M, Sacco A, Jucker R, Cesareni G, Nasi S. Design and properties of a Myc derivative that efficiently homodimerizes. Oncogene. 1998;17:2463–2472. doi: 10.1038/sj.onc.1202199. [DOI] [PubMed] [Google Scholar]
- Soucek L, Jucker R, Panacchia L, Ricordy R, Tato F, Nasi S. Omomyc, a potential Myc dominant negative, enhances Myc-induced apoptosis. Cancer Res. 2002;62:3507–3510. [PubMed] [Google Scholar]
- Stewart TA, Pattengale PK, Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984;38:627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, Reece D, Paterson J, Wang D, Wen XY. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res. 2007;13:621–629. doi: 10.1158/1078-0432.CCR-06-1526. [DOI] [PubMed] [Google Scholar]
- Vervoorts J, Luscher-Firzlaff JM, Rottmann S, Lilischkis R, Walsemann G, Dohmann K, Austen M, Luscher B. Stimulation of c-MYC transcriptional activity and acetylation by recruitment of the cofactor CBP. EMBO Rep. 2003;4:484–490. doi: 10.1038/sj.embor.embor821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13028–13033. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller KI, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4:R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F, Barlogie B, Arzoumanian V, Huang Y, Williams DR, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Zangari M, et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109:1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


