Abstract
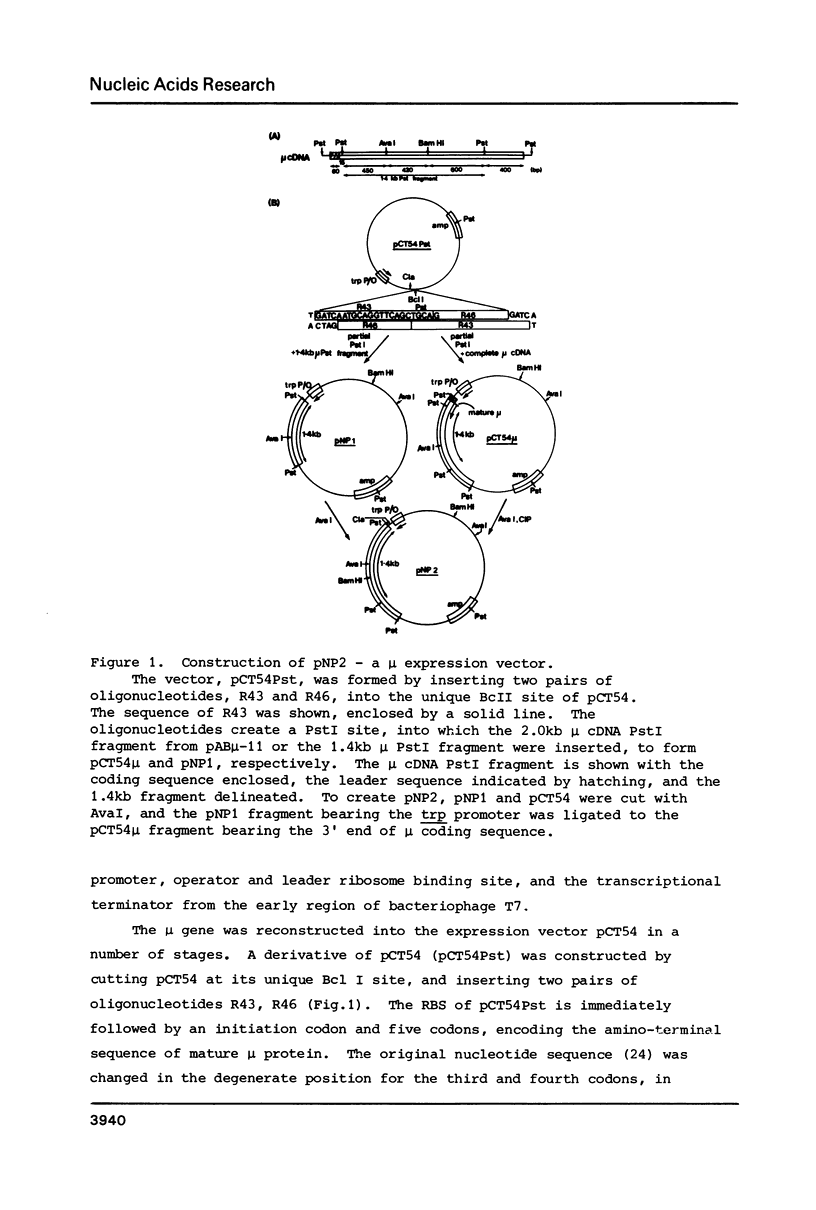
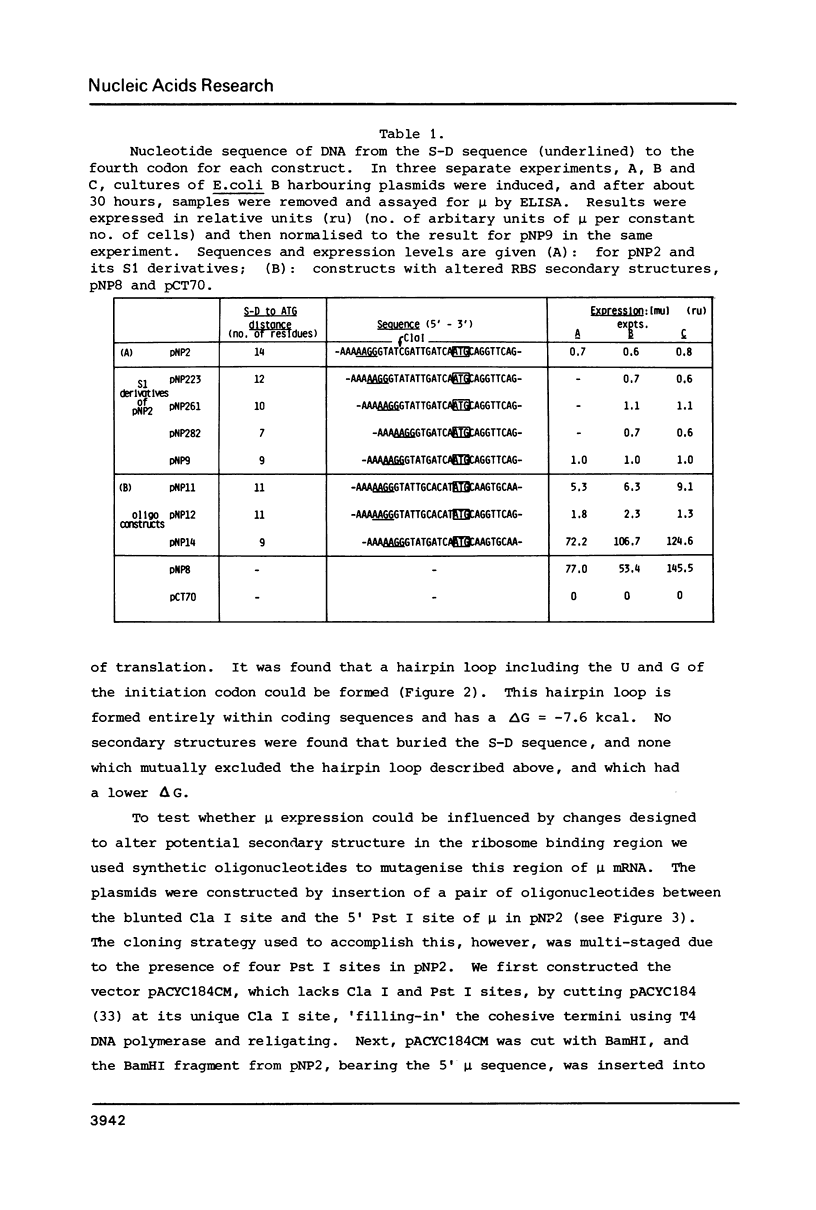
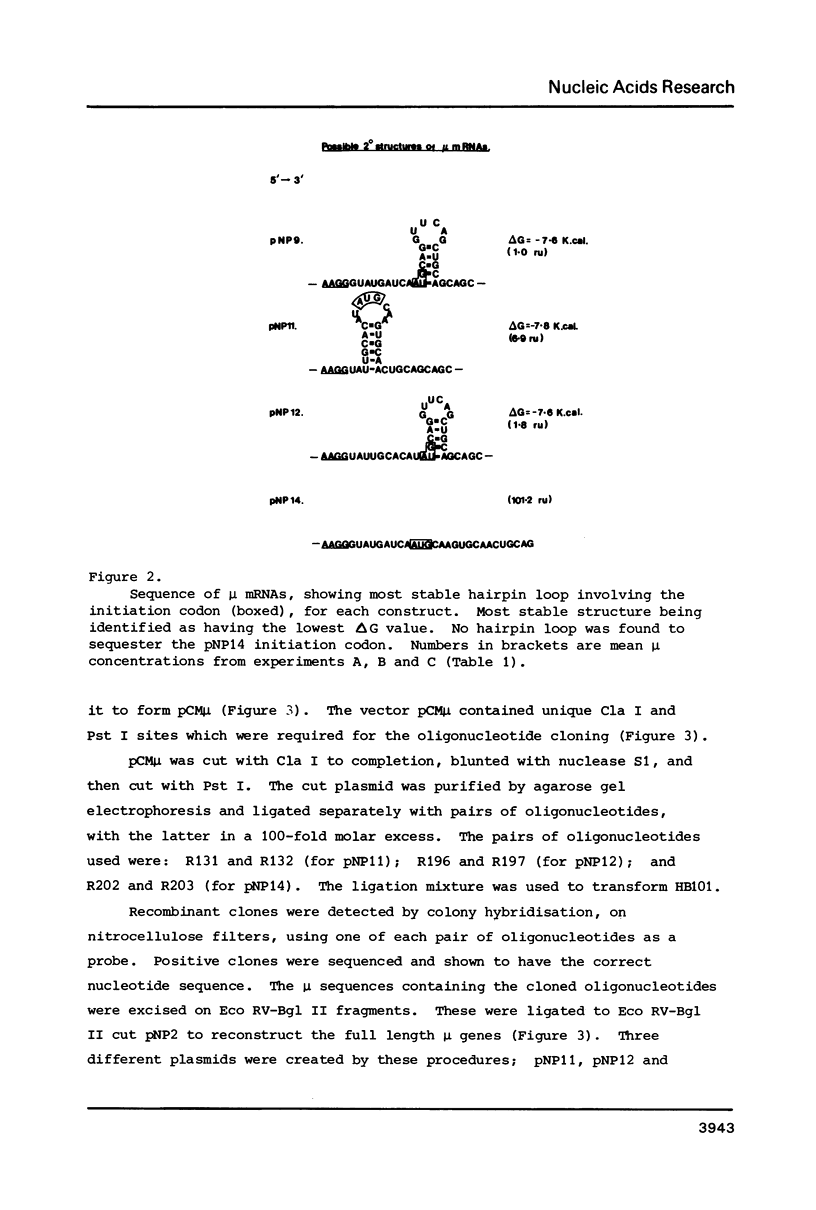
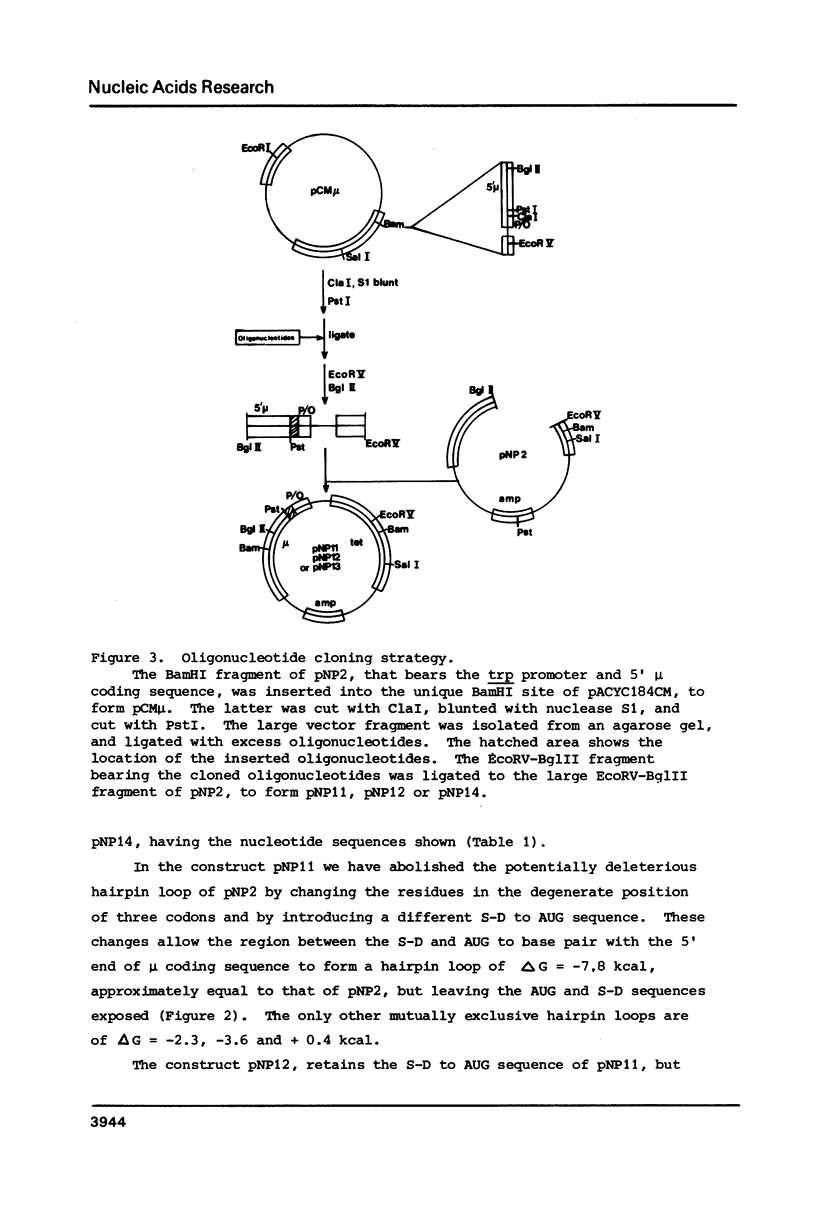
A gene for murine mu heavy chain immunoglobulin has been inserted into a bacterial expression plasmid containing the Escherichia coli trp promoter and ribosome binding site. A low level expression of mu protein was detected. Secondary structure analysis showed the presence of a hairpin loop burying the mu initiation codon. Alteration of secondary structure at this site by oligonucleotide replacement mutagenesis revealed a correlation between mu expression levels and accessibility of the ribosome binding site. Abolition of secondary structure increased mu protein expression over ninety-fold, to a level approximately equal to that of a trpE -mu fusion protein using the native trpE ribosome binding site.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos E. S., van der Doelen A. A., van Rooy N., Schuurs A. H. 3,3',5,5' - Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoassay. 1981;2(3-4):187–204. doi: 10.1080/15321818108056977. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derom C., Gheysen D., Fiers W. High-level synthesis in Escherichia coli of the SV40 small-t antigen under control of the bacteriophage lambda pL promoter. Gene. 1982 Jan;17(1):45–54. doi: 10.1016/0378-1119(82)90099-3. [DOI] [PubMed] [Google Scholar]
- Donch J., Chung Y. S., Greenberg J. Locus for radiation resistance in Escherichia coli strain B-r. Genetics. 1969 Feb;61(2):363–370. doi: 10.1093/genetics/61.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Effect of RNAase III, cleavage on translation of bacteriophage T7 messenger RNAs. J Mol Biol. 1975 Dec 15;99(3):487–499. doi: 10.1016/s0022-2836(75)80140-9. [DOI] [PubMed] [Google Scholar]
- Duvall E. J., Williams D. M., Lovett P. S., Rudolph C., Vasantha N., Guyer M. Chloramphenicol-inducible gene expression in Bacillus subtilis. Gene. 1983 Oct;24(2-3):171–177. doi: 10.1016/0378-1119(83)90077-x. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Angal S., Doel M. T., Harris T. J., Jenkins B., Lilley G., Lowe P. A. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3671–3675. doi: 10.1073/pnas.80.12.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage J. S., Tacon W. C., Catlin G. H., Jenkins B., Porter A. G., Carey N. H. Influenza antigenic determinants are expressed from haemagglutinin genes cloned in Escherichia coli. Nature. 1980 Jan 10;283(5743):171–174. doi: 10.1038/283171a0. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Sullivan P., Cunningham C., Hader P., Kofoid E. C., Neilson T. Effect of bases contiguous to AUG on translation initiation. J Biol Chem. 1982 Jul 25;257(14):8228–8232. [PubMed] [Google Scholar]
- Gheysen D., Iserentant D., Derom C., Fiers W. Systematic alteration of the nucleotide sequence preceding the translation initiation codon and the effects on bacterial expression of the cloned SV40 small-t antigen gene. Gene. 1982 Jan;17(1):55–63. doi: 10.1016/0378-1119(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gottesman M., Oppenheim A., Court D. Retroregulation: control of gene expression from sites distal to the gene. Cell. 1982 Jul;29(3):727–728. doi: 10.1016/0092-8674(82)90434-2. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Kohr W. J., Keck R., Harkins R. N. Characterization of intact and trypsin-digested biosynthetic human growth hormone by high-pressure liquid chromatography. Anal Biochem. 1982 May 15;122(2):348–359. doi: 10.1016/0003-2697(82)90294-9. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Adelman J., Bock S. C., Franke A. E., Houck C. M., Najarian R. C., Seeburg P. H., Wion K. L. The sequence of human serum albumin cDNA and its expression in E. coli. Nucleic Acids Res. 1981 Nov 25;9(22):6103–6114. doi: 10.1093/nar/9.22.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Pirtle I. L., Takeishi K., Inouye M. Messenger ribonucleic acid of the lipoprotein of the Escherichia coli outer membrane. II. The complete nucleotide sequence. J Biol Chem. 1980 Jan 10;255(1):210–216. [PubMed] [Google Scholar]
- Nichols B. P., van Cleemput M., Yanofsky C. Nucleotide sequence of Escherichia coli trpE. Anthranilate synthetase component I contains no tryptophan residues. J Mol Biol. 1981 Feb 15;146(1):45–54. doi: 10.1016/0022-2836(81)90365-x. [DOI] [PubMed] [Google Scholar]
- Nussinov R., Tinoco I., Jr Sequential folding of a messenger RNA molecule. J Mol Biol. 1981 Sep 25;151(3):519–533. doi: 10.1016/0022-2836(81)90008-5. [DOI] [PubMed] [Google Scholar]
- Patel T. P., Millican T. A., Bose C. C., Titmas R. C., Mock G. A., Eaton M. A. Improvements to solid phase phosphotriester synthesis of deoxyoligonucleotides. Nucleic Acids Res. 1982 Sep 25;10(18):5605–5620. doi: 10.1093/nar/10.18.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Shafferman A. Conditional expression of the vesicular stomatitis virus glycoprotein gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6670–6674. doi: 10.1073/pnas.78.11.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard H. M., Yelverton E., Goeddel D. V. Increased synthesis in E. coli of fibroblast and leukocyte interferons through alterations in ribosome binding sites. DNA. 1982;1(2):125–131. doi: 10.1089/dna.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Steege D. A. 5'-Terminal nucleotide sequence of Escherichia coli lactose repressor mRNA: features of translational initiation and reinitiation sites. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4163–4167. doi: 10.1073/pnas.74.10.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton N., Boseley P. G., Porter A. G. Increased expression of a cloned gene by local mutagenesis of its promoter and ribosome binding site. Nucleic Acids Res. 1983 Sep 10;11(17):5837–5854. doi: 10.1093/nar/11.17.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Hui A., Comstock L. J., Wong E., Vasser M. Portable Shine-Dalgarno regions: a system for a systematic study of defined alterations of nucleotide sequences within E. coli ribosome binding sites. DNA. 1983;2(3):231–235. doi: 10.1089/dna.1983.2.231. [DOI] [PubMed] [Google Scholar]


