Summary
The body's immunological response to burn injury has been a subject of great inquiry in recent years. Burn injury disturbs the immune system, resulting in a progressive suppression of the immune response that is thought to contribute to the development of sepsis. Dendritic cells (DCs) are potent antigen-presenting cells that possess the ability to stimulate naïve T cells.
DCs are derived from bone marrow progenitors and circulate in the blood as immature precursors prior to migration into peripheral tissues. Within different tissues, DCs differentiate and become active in the taking up and processing of antigens, and their subsequent presentation on the cell surface is linked to major histocompatibility molecules. Upon appropriate stimulation, DCs undergo further maturation and migrate to secondary lymphoid tissues, where they present antigen to T cells and induce an immune response. The purpose of this study was to determine the effects of burn injury on skin DCs in terms of percentage, HLA-DR, and Toll-like receptor-4 (TLR-4) expression. The skin DCs were isolated from burned skin and non-burned skin in the same patient at 7 days post-injury, and skin DCs were isolated from unburned healthy individuals as control. DCs from burned skin notably express low levels of HLA-DR and TLR-4 soon after cell isolation. In the post-burn period the ability of skin DCs to respond to bacterial stimuli is impaired. These changes in DC behaviour might contribute to the impaired host defences against bacteria during burn sepsis.
Keywords: SKIN, DENDRITIC, CELLS, BURN, PATIENTS
Abstract
La réponse immunitaire de l'organisme aux brûlures a attiré l'attention d'un grand nombre de chercheurs ces dernières années. Les brûlures dérangent le système immunitaire et provoquent une suppression progressive de la réponse immunitaire qui contribue, selon ce qu'on pense, au développement du sepsis. Les cellules dendritiques (CD) sont de puissantes cellules présentatrices d'antigènes qui possèdent la capacité de stimuler les cellules T naïves. Les CD proviennent des progéniteurs de la moelle osseuse et circulent dans le sang en tant que précurseurs immatures avant la migration vers les tissus périphériques. Au sein des divers tissus, les CD se différencient et deviennent actives dans la prise et la transformation des antigènes, et leur présentation successive à la surface cellulaire est liée à certaines importantes molécules d'histocompatibilité. Après la stimulation appropriée, les CD subissent une ultérieure maturation et migrent vers les tissus lymphoïdes secondaires, où ils présentent les antigènes aux cellules T et provoquent une réponse immunitaire. Le but de cette étude était de déterminer les effets de la brûlure sur les CD pour ce qui concerne le pourcentage, l'HLA-DR, et l'expression du récepteur-4 de type Toll (TLR-4). Les CD cutanées ont été isolées de la peau brûlée et non brûlée chez le même patient à 7 jours après la brûlure; des CD cutanées ont été isolées de sujets sains non brûlés comme témoins. Les CD provenant de la peau brûlée expriment des niveaux des niveaux notablement faibles de HLADR et de TLR-4 peu après l'isolement cellulaire. Dans la période après la brûlure, la capacité des CD cutanées de réagir aux stimuli bactériens est altérée. Ces changements dans le comportement des CD pourraient contribuer à l'altération des défenses de l'hôte contre les bactéries dans le cas d'une septicémie due à une brûlure.
Introduction
Sepsis is currently viewed as a complex dysregulation of the mechanism of inflammation subsequent to the host's inability to successfully contain an infection.1 Owing to their unique ability to induce a primary immune response, dendritic cells (DCs) serve as a critical and integrative link between the innate and the adaptative immune systems.
Normally, DCs reside in an immature state in peripheral tissues, and undergo maturation under a variety of exogenous stimuli, including microbial products. Their maturation or activation is followed by a number of functional and phenotypical changes, promoting their migration to lymph nodes, the secretion of cytokines, and ultimately leading to the activation of the T-cell compartment.2 Abnormalities in DC homeostasis have been implicated in various human diseases, including infections. Recently we demonstrated that burn patients with sepsis exhibited a dramatic reduction in both circulating myeloid and plasmacytoid DCs.3,4 The causes of this dysfunction and the decreased number of DCs are poorly defined and inadequately studied.
Since the skin is considered one of the most immunogenic organs and its immunogenic function is correlated with the high number of epidermal and dermal-resident DCs,5 we studied the burn percentage, the HLA-DR, and the Toll-like receptor-4 expression pattern of skin DCs in burn patients. For comparison, other DCs obtained from nonburned areas in the same patients (defined as nonburned), as well as DCs from the skin of healthy subjects (defined as healthy skin) were analysed. The present study provides the first evidence that skin DCs from burn patients disappear and are impaired in terms of HLADR/ TLR-4 expression.
Materials and methods
Patients
Skin biopsies were obtained from 11 burn patients (mean age, 44 ± 29 yr; range, TBSA 15-70%) and six plastic surgery patients (mean age, 51 ± 12 yr) admitted to the Burn Unit and Plastic Surgery Department, under a protocol approved by the local ethics committee of the ARNAS Civic Hospital in Palermo. At the time of sampling none of the patients presented clinical signs of sepsis, but signs of localized infection were found at the site of the injury.
Culture medium and reagents
Very low endotoxin medium RPMI 1640 (Sigma; St Louis, USA), 10 mM Hepes (Euroclone; Wetherby, Yorkshire, UK), FBS (Euroclone) penicillin, streptomycin, and 0.05 mM 2-ME (Sigma) were used as the culture medium throughout all experiments. Human recombinant GM-CSF and IL-4 were purchased from R&D systems (Wiesbaden, Germany, and Minneapolis, MN). LPS (E. coli 026: B6) was obtained from Sigma.
Antibodies
The monoclonal antibodies (MoAbs) used for staining DCs were combined to prepare a lineage cocktail with FITC labelled anti-CD3, anti-CD14, anti-CD19, anti-CD20, and anti-CD56 MoAbs with Pe-CP-labelled anti-HLA-DR MoAb (all from Beckton Dickinson, Mountain, View, CA, USA).
In addition Pe-labelled anti-CD11c and PE-labelled anti CD123 with PE-labelled IgG isotype control MoAbs were used to identify myeloid and plasmacytoid DCs respectively.
The MoAb FITC-labelled anti-TLR-4 was used (Santa Cruz Biotechnology, Santa Cruz, CA) to study DC function.
Skin sample processing and culture of total skin cells
Isolation of skin dendritic cells from skin tissue was performed as previously described. The skin was washed three times with phosphate buffer saline (PBS; Euroclone), cut in small pieces (1-10 mm), transferred to fresh PBS, and stirred at 37 °C to remove blood, debris, and intraepithelial cells. Subsequently the samples were treated with 25 U/ml collagenase (Sigma) at 37 °C overnight on a shaker.
Fresh cell suspension was either immediately analysed by fluorescence-activated cell sorter (FACS) analysis or cultured for 7 days in RPMI supplemented with 10% FBS, 100 ng/ml GM-CSF, and 200 ng/ml IL-4. On day 7, the non-adherent cell fraction was harvested, and the phenotype was analysed by flow cytometry. All cultures were set up in triplicate.
Flow cytometry analysis
Total skin cells were incubated with MoAbs for 30 min on ice and washed twice in PBS, containing 0.1% (w/v) NaN3. After staining, the cells were fixed with 1% (w/v) paraformaldehyde in PBS for 30 min at room temperature before flow cytometric analysis. Three-colour flow cytometric analysis was performed using an FACSCalibur (Becton Dickinson). At least 50,000 cells (events) were acquired for each sample. DCs were expressed as a percentage of cells within the LIN- DR+ gate. The acquired data were analysed using the CellQuest software program (Becton Dickinson). In addition, Pe-labelled anti-CD11c and Pe-labelled anti-CD123, with Pe-labelled IgG isotype control MoAbs (Becton Dickinson, Mountain View, CA, USA), were used to identify myeloid (mDCs) and plasmacytoid (pDCs) subsets, respectively.3,4
The functional status of the skin DCs was assessed on the basis of the fluorescence intensity levels of the HLADR and TLR-4 expression at rest and of stimulated cells (time 0 and after 7 days of culture in the presence of GMCSF (100 ng/ml) and IL-4 (200 ng/ml).
Results
The purpose of this study was to determine the effects of burn injury on skin DCs in terms of percentage, phenotype, and function of these cells. In healthy subjects DCs were 4.09 ± 2% of total skin cells.
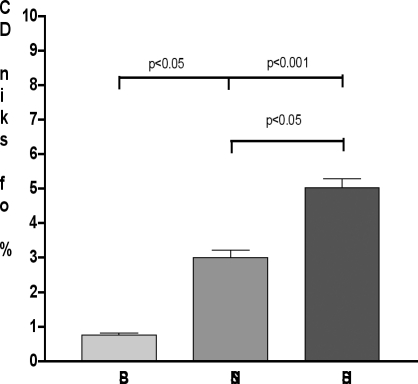
In burn patients the percentage of DCs from burned skin, i.e. skin close to the site of injury, was significantly low (0.75 ± 0.18%; p < 0.001 vs healthy subjects and 0.05 vs nonburned skin; Fig. 1), while DCs from nonburned skin (skin distant from the site of injury) were comparable to those of healthy subjects (2.99 ± 0.68%; p = non-significant). Flow cytometric analysis used to identify skin DCs obtained from healthy and burn subjects made it possible to distinguish two subsets:
Fig. 1. Percentage of skin DCs. At 7 days after burn injury, total skin cells were isolated. DCs from non-burned skin, reduced compared to those of healthy subjects (healthy subjects; p < 0.05); burned skin had significantly lower levels of DCs (p < 0.001 vs healthy subjects and 0.05 vs non-burned skin). Histograms represent the mean ± SD in each group.
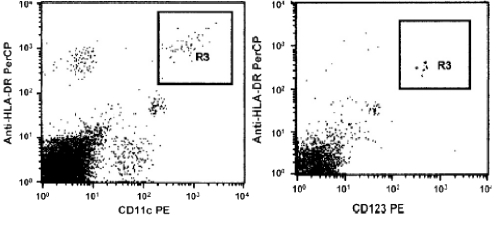
the myeloid lineage (mDC) with the expression of HLA-DR and CD11c, and the plasmacytoid lineage (pDC) with the expression of HLA-DR and CD123. Myeloid skin DCs were mainly found both in healthy subjects and in burn patients (Fig. 2), for which reason we focused our attention on this subset.
Fig. 2. Gating strategy used to identify mDCs (CD11c+) and pDCs (CD123+). The LIN- DR+ DC population was gated and used to discriminate the CD11c+ from the CD123+ DC subsets. One representative cytofluorimetric image is displayed.
To evaluate the functional state of DCs we determined the expression of MHC class II (HLA-DR) and TLR-4, both in healthy subjects and in burn patients. The skin DCs from healthy subjects had levels of HLA-DR comparable to those of DCs from non-burned skin (451 ± 11 and 441 ± 23 MFI respectively; p = non-significant), while DCs from burned skin expressed a low fluorescence intensity of HLA-DR (267.6 ± 14 MFI; p < 0.001 vs nonburned and healthy subjects). This result suggests that the burn injury could have induced a dysfunction of the DC cells.
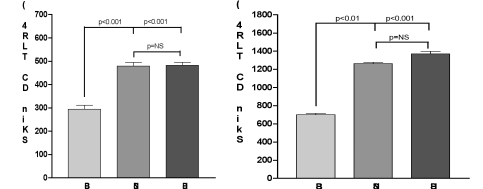
We also determined the expression of Toll-like receptor 4 (TLR-4), a key molecule that alerts the immune system to the presence of microbial infections. Total skin cells were isolated 7 days post-burn and incubated with or without LPS for 24 h. Figs. 3A and B show that DCs from burned skin expressed very low levels of TLR-4, also after LPS stimulation, in comparison with DCs from healthy subjects and from nonburned skin (p < 0.001).
Fig. 3. Expression of TLR-4 on skin DC at 7 day after burn injury. Total skin cells were isolated from burn patients and healthy subjects and stimulated in the presence or absence of LPS. After 24 h, the expression of TLR-4 on DCs was determined by means of fluorescence intensity. DCs from healthy subjects and from non-burned skin expressed higher basal levels (A) of TLR-4 than those from burned skin (p < 0.001). After LPS stimulation (B) these levels further increased, albeit to a different extent. Histograms represent the mean ± SD in each group.
Discussion
Dendritic cells represent the peacemakers of the immune response. They are crucial to the presentation of peptides and proteins to T and B lymphocytes and are widely recognized as the key antigen presenting cells.
Thermal injury is associated with immune dysfunction, and there is an increasing body of evidence that DCs are involved in this pathomechanism, which is associated with depression of Th1 and increased Th2 cytokine production, macrophage dysfunction, altered NK and T-cell activities, and depressed cytotoxic response.6,7,8,9
Previous studies by this laboratory reported that burn patients with sepsis exhibited a dramatic reduction in both circulating myeloid and plasmacytoid-DCs early postinjury.3,4
As DCs are important sentinels of the cutaneous immune system, we hypothesized that an alteration in the percentage and function of these cells in the skin could be responsible for immunosuppression in burn patients.
One finding of the present study was that, at day 7, DCs from burned skin showed an appreciable reduction compared to DCs from healthy and non-burned skin (p <0.001). In addition, these cells expressed low levels of HLA-DR and TLR-4 on their surface, and these levels slightly increased after LPS stimulation. Our data are not consistent with the increased proportion of activated or mature DCs seen following LPS treatment. It is known that DCs show an upregulation of MHC classII molecules very soon after LPS stimulation.10 Skin DCs are therefore not only reduced but also impaired in their function.
HLA-DR and TLR-4 play a key role in DC function, and their decreased expression, associated with a reduction in DC percentages, could have profound implications regarding the ability of the burn patients to eradicate micro organisms.
Conclusions
The data in the present study show that skin dendritic cell content decreased soon after burn injury. In addition dendritic cells expressed low levels of HLA-DR and TLR-4. This reduction in dendritic cells could contribute to the immunosuppression observed after burn injury.
Although there is an increasing body of evidence that dendritic cells are involved in the immune dysfunction associated with thermal injury, our study still requires further investigation.
References
- 1.Benjamim C.F., Lundy S.K., Lukacs N.W., Hogaboam C.M., Kunkel S.L. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105:3588–95. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J., Briere F., Caux C., et al. Immunobiology of dendritic cells. Ann. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.D'Arpa N., Accardo-Palumbo A., Amato G., et al. Decrease of circulating dendritic cells in burn patients. Ann. Burns Fire Disasters. 2007;20:199–202. [PMC free article] [PubMed] [Google Scholar]
- 4.D'Arpa N., Accardo-Palumbo A., Amato G., D'Amelio L., Pileri D., Cataldo V., Mogavero R., Lombardo C., Napoli B., Conte F. Circulating dendritic cells following burn. Burns. 2009;34:513–8. doi: 10.1016/j.burns.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Morelli A.E., Rubin J.P., Erdos G., Tkacheva O.A., Mathers A.R., Zahorchak A.F., Thomson A.W., Falo L.D., Larregina A.T. CD4+ cell responses elicited by different subsets of human skin migratory dendritic cells. J. Immunol. 2005;175:7905–15. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 6.Schwacha M.G. . Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 7.Singh H., Abdullah A., Herndon D.N. Effects of rat interleukin 2 and rat interferon gamma on the natural killer cell activity of rat spleen cells after injury. J. Burn Care Rehabil. 1992;13:617–22. doi: 10.1097/00004630-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hunt J.P., Hunter C.T., Brownstein M.R., et al. The effector Q2 component of the cytotoxic T lymphocyte response has a biphasic pattern after burn injury. J. Surg. Res. 1998;80:243–51. doi: 10.1006/jsre.1998.5488. [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan S.T., Lederer J.A., Horgan A.F., Chin D.H., Mannick J.A., Rodrick M.L. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann. Surg. 1995;222:482–92. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rescigno M., Citterio S., Théry C., Rittig M., Medaglini D., Pozzi G., Amigorena S., Ricciardi-Castagnoli P. Bacteria-induced neobiosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl Acad. Sci.USA. 1998;95:5229–34. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]