Abstract
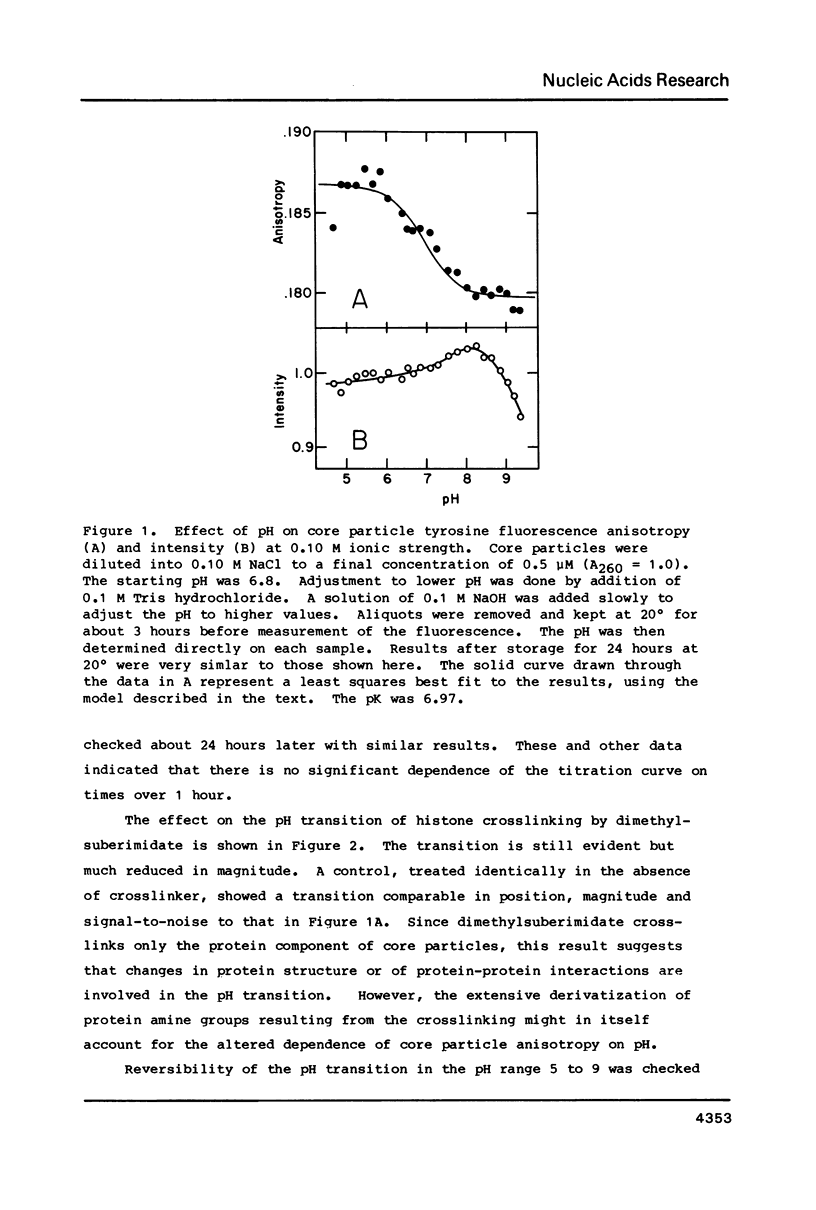
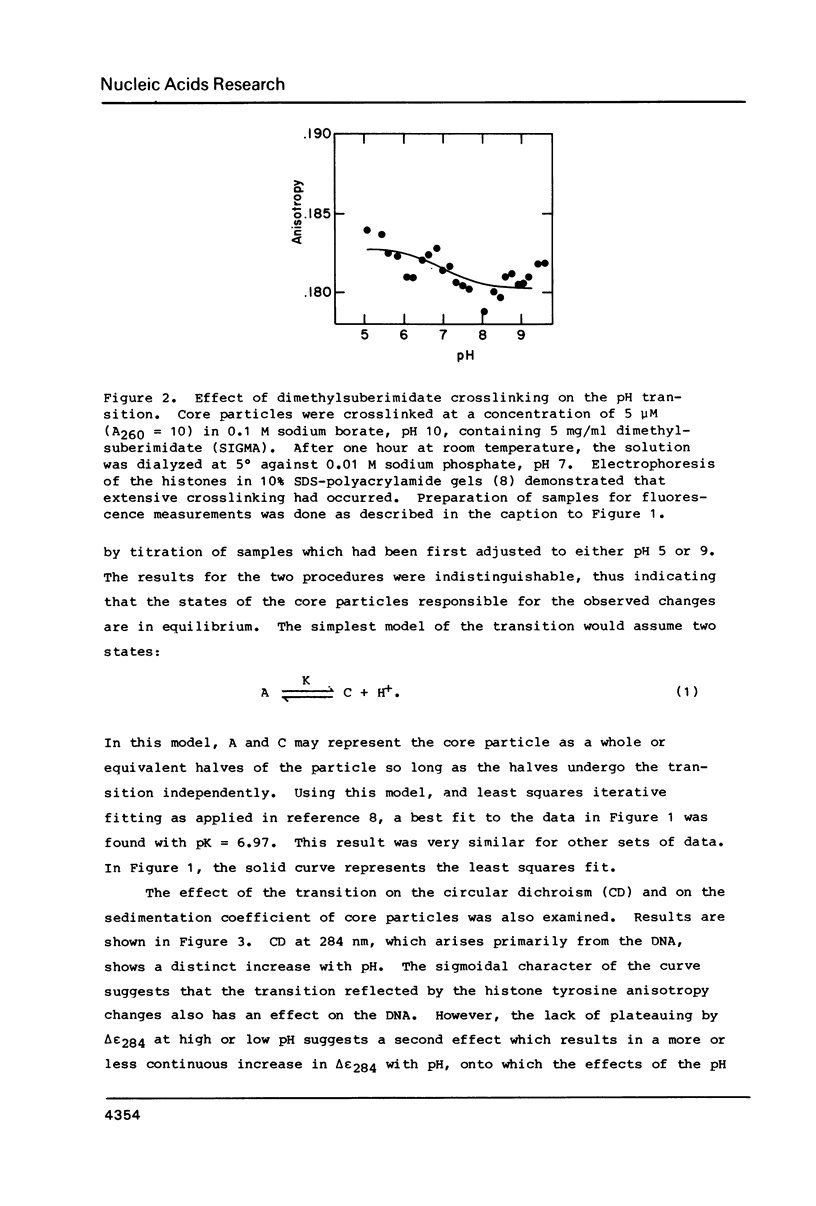
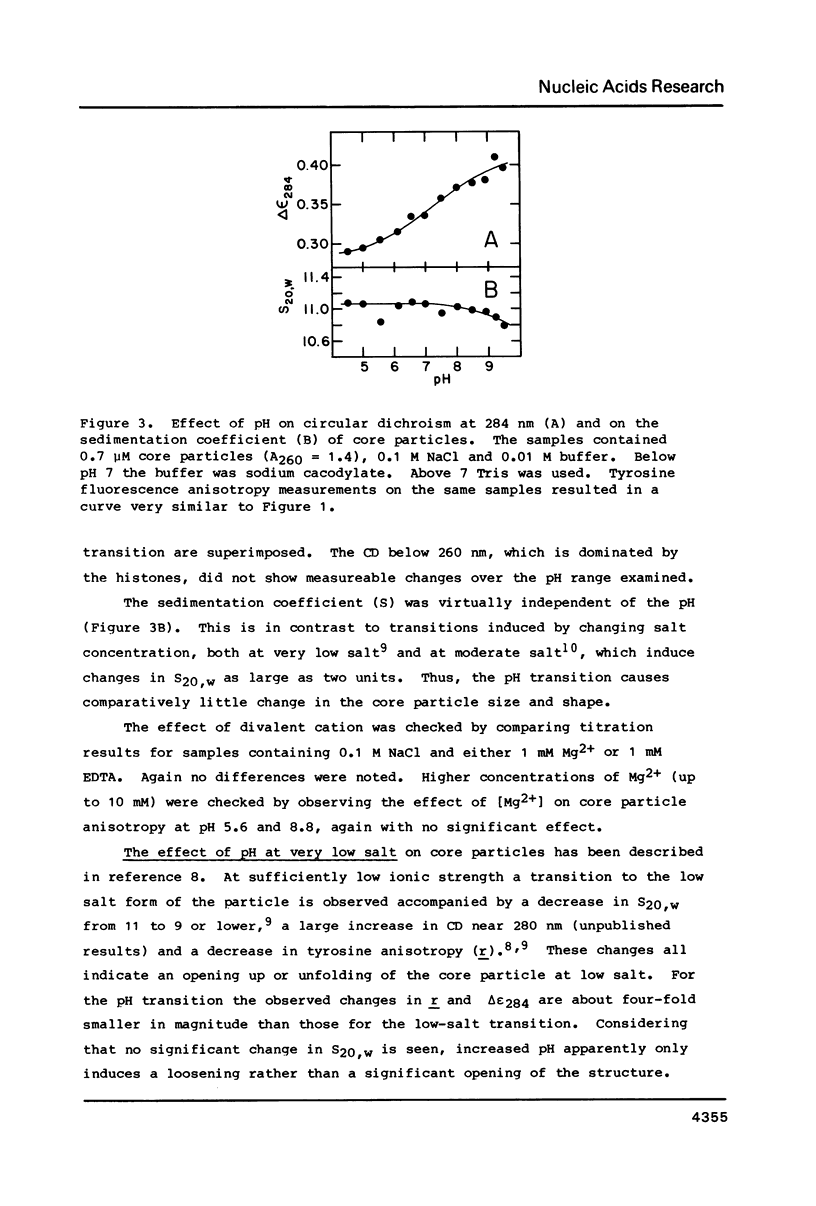
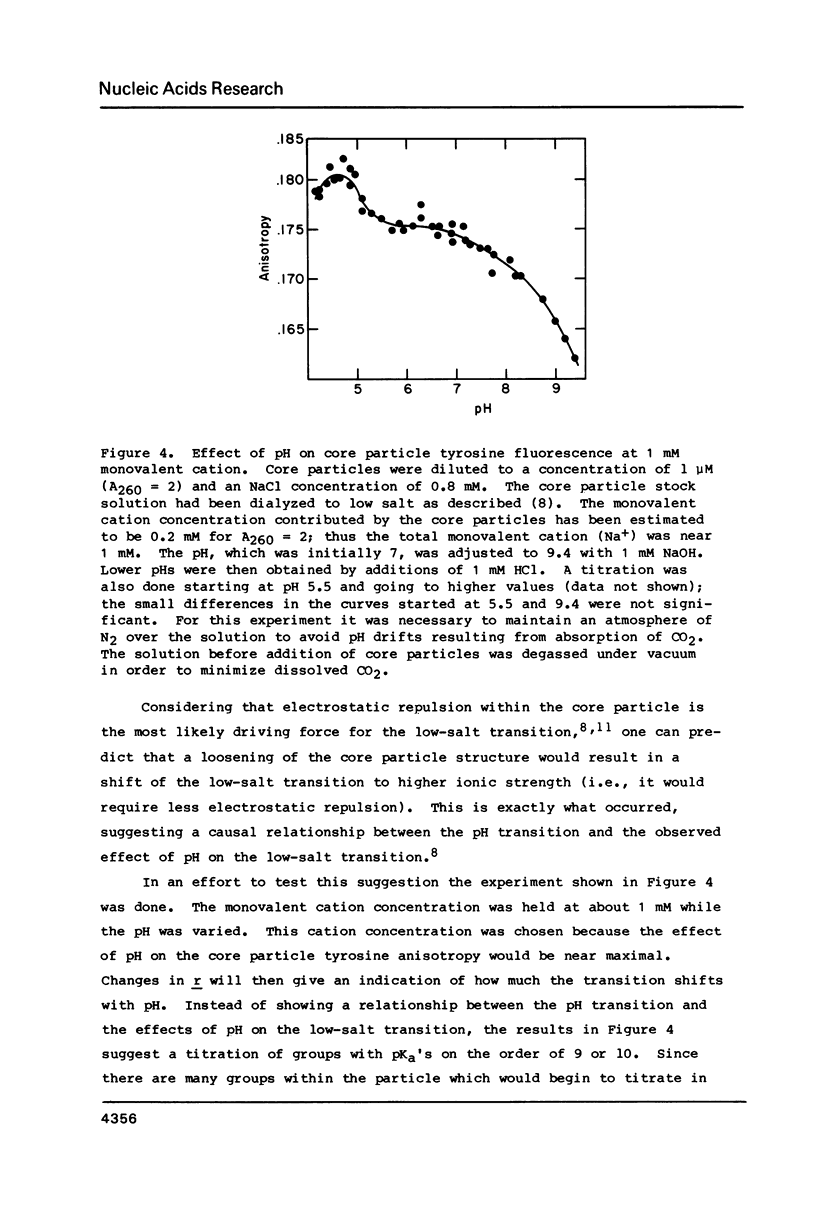
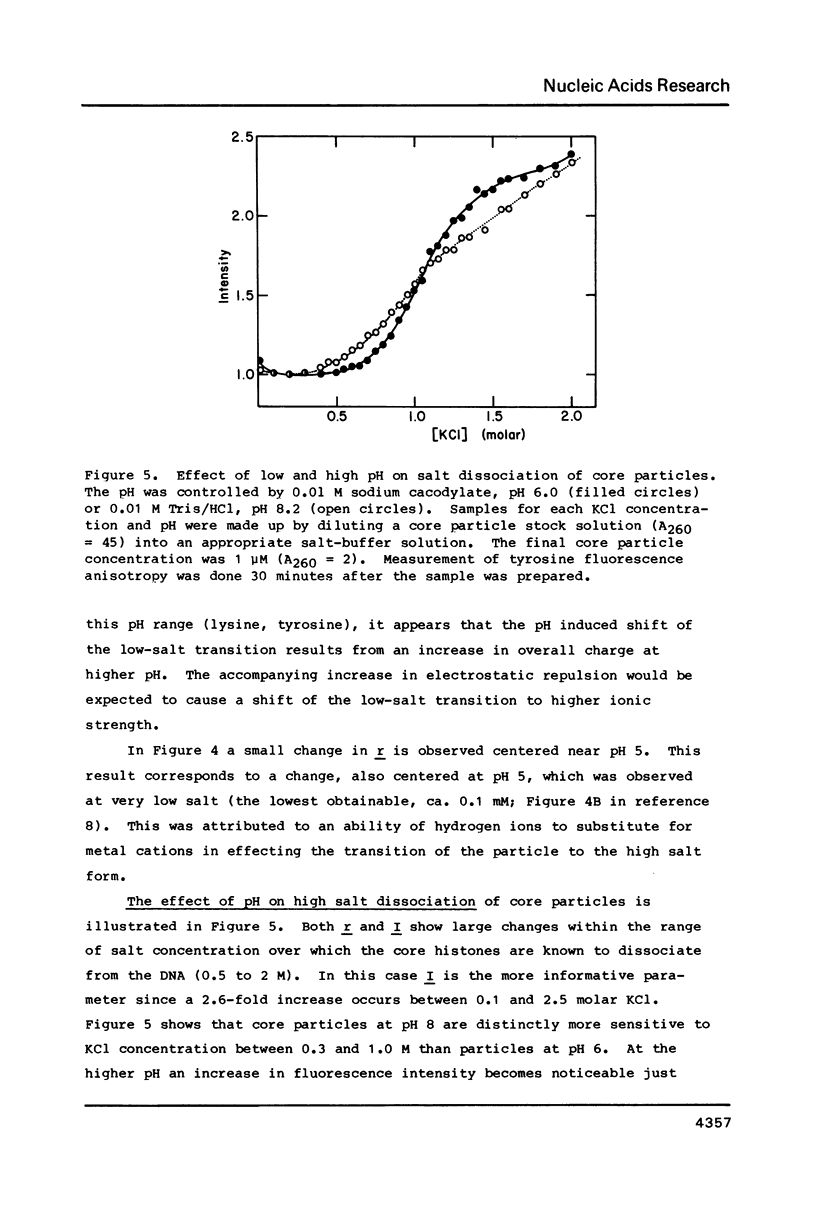
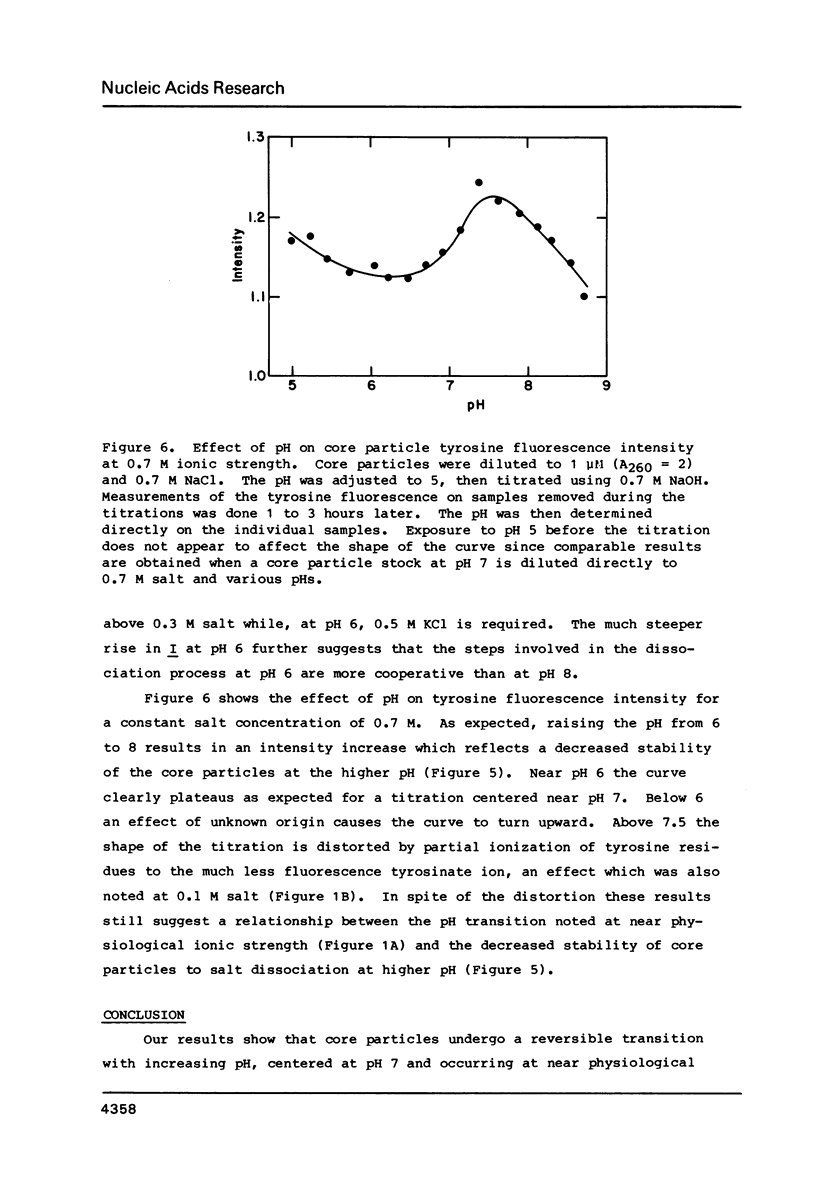
Chromatin core particles near physiological ionic strength undergo a reversible transition induced by changes in pH near neutrality. While sedimentation studies indicate no significant effect on size or shape, changes in tyrosine fluorescence anisotropy and in circular dichroism suggest a somewhat looser structure at high pH. Further support of this suggestion is given by high salt dissociation experiments; at pH 8 core particles begin to show changes at lower salt concentration than at pH 6. The pH transition appears unaffected by the presence of Mg2+ but can be blocked by crosslinking of the histones. A possible relationship is suggested between this transition and increases in intracellular pH which correlate with enhancement in several aspects of cellular activity including DNA replication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gerson D. F., Kiefer H. Intracellular pH and the cell cycle of mitogen-stimulated murine lymphocytes. J Cell Physiol. 1983 Jan;114(1):132–136. doi: 10.1002/jcp.1041140121. [DOI] [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart P., Thoma F., Koller T. Structural changes of soluble rat liver chromatin induced by the shift in pH from 7 to 9. Eur J Cell Biol. 1981 Aug;25(1):19–27. [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Effects of pH on low-salt transition of chromatin core particles. Biochemistry. 1982 Jul 6;21(14):3327–3334. doi: 10.1021/bi00257a013. [DOI] [PubMed] [Google Scholar]
- Wilhelm M. L., Wilhelm F. X. Conformation of nucleosome core particles and chromatin in high salt concentration. Biochemistry. 1980 Sep 2;19(18):4327–4331. doi: 10.1021/bi00559a028. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Structural changes of nucleosomes in low-salt concentrations. Biochemistry. 1979 Sep 4;18(18):3960–3965. doi: 10.1021/bi00585a018. [DOI] [PubMed] [Google Scholar]
- Zama M., Olins D. E., Prescott B., Thomas G. J., Jr Nucleosome conformation: pH and organic solvent effects. Nucleic Acids Res. 1978 Oct;5(10):3881–3897. doi: 10.1093/nar/5.10.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]


