Abstract
Tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) by the insulin receptor permits this docking protein to interact with signaling proteins that promote insulin action. Serine phosphorylation uncouples IRS-1 from the insulin receptor, thereby inhibiting its tyrosine phosphorylation and insulin signaling. For this reason, there is great interest in identifying serine/threonine kinases for which IRS-1 is a substrate. Tumor necrosis factor (TNF) inhibited insulin-promoted tyrosine phosphorylation of IRS-1 and activated the Akt/protein kinase B serine-threonine kinase, a downstream target for phosphatidylinositol 3-kinase (PI 3-kinase). The effect of TNF on insulin-promoted tyrosine phosphorylation of IRS-1 was blocked by inhibition of PI 3-kinase and the PTEN tumor suppessor, which dephosphorylates the lipids that mediate PI 3-kinase functions, whereas constitutively active Akt impaired insulin-promoted IRS-1 tyrosine phosphorylation. Conversely, TNF inhibition of IRS-1 tyrosine phosphorylation was blocked by kinase dead Akt. Inhibition of IRS-1 tyrosine phosphorylation by TNF was blocked by rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR), a downstream target of Akt. mTOR induced the serine phosphorylation of IRS-1 (Ser-636/639), and such phosphorylation was inhibited by rapamycin. These results suggest that TNF impairs insulin signaling through IRS-1 by activation of a PI 3-kinase/Akt/mTOR pathway, which is antagonized by PTEN.
Tumor necrosis factor (TNF) was first identified and characterized based on its ability to induce the regression of tumors in animals and by the cytotoxic response that it elicits in cancer cells in culture. TNF also promotes immunity, antiviral responses, inflammation, shock, and the syndrome of wasting and malnutrition known as cachexia (1, 2). Elaboration of TNF is associated with insulin resistance that accompanies endotoxemia, cancer, trauma, and obesity (3). The correlation between TNF production and insulin resistance is buttressed by the demonstration that administration of TNF to rats and humans reduces insulin sensitivity (4, 5).
The ability of TNF to induce insulin resistance in animals has been replicated in adipocytes, hepatoma cells, fibroblasts, myeloid, and muscle cells (6–11). TNF mediates its inhibitory action by targeting insulin receptor substrate-1 (IRS-1), a substrate for the insulin receptor tyrosine kinase (12). Tyrosine phosphorylation of IRS-1 by the insulin receptor promotes its interaction with cytoplasmic signaling proteins that promote insulin action (12). Treatment of adipocytes or hepatocytes with TNF induces serine phosphorylation of IRS-1, which prevents its tyrosine phosphorylation by the insulin receptor (13, 14). Thus, IRS-1 may positively or negatively affect insulin signal transduction, depending on whether it is phosphorylated on serine or tyrosine. Consequently, there is great interest in identifying kinases that serine phosphorylate IRS-1.
TNF activates phosphatidylinositol 3-kinase (PI 3-kinase) and its downstream target the Akt serine-threonine kinase, which play a role in activating NF-κB (15). The present study shows that a TNF-promoted PI 3-kinase/Akt/mTOR pathway impairs insulin-induced tyrosine phosphorylation of IRS-1. Thus, PI 3-kinase/Akt signaling is important to immunity, cell survival, and those facets of insulin action that require signaling through IRS-1.
Materials and Methods
Cell Culture and Biological Reagents.
Constitutively active Akt (CA-Akt) and kinase dead Akt (KD-Akt) were gifts from R. Roth (Stanford University School of Medicine, Palo Alto, CA). Antiphospho-Akt and Akt were from New England Biolabs. IRS-1 and mTOR/FKBP12 rapamycin-associated protein (FRAP) antibodies were from Santa Cruz Biotechnology. NIH 3T3 cells stably expressing CA-Akt (from Michael E. Greenberg, Harvard Medical School, Boston) and 293 embryonic kidney cells were grown in DMEM supplemented with 10% FBS, 100 μg/ml penicillin, 50 μg/ml streptomycin, and 1 mM glutamine. C3H 10T1/2 C18 myoblasts were cultured and differentiated into myotubes as described (16).
Transfections.
Sixty to seventy percent confluent 293 cells in 100-mm tissue culture plates were transfected with 15 μg of KD-Akt or PTEN by the calcium phosphate precipitation method. After 16 h, the cells were washed with PBS and cultured in serum-free medium for 24 h. Expression of transfected plasmids was verified by immunoblotting aliquots of cell lysates with anti-Akt or anti-PTEN. Transfection efficiency was >90%.
Immunoprecipitations and Immunoblotting.
Control and transfected cells were treated with insulin or TNF as described in the figure legends and lysed into 50 mM Hepes, (pH 7.0), 150 mM NaCl, 10% glycerol, 1 mM MgCl2, 1.2% Triton X-100, 100 mM NaF, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin while being shaken for 30 min at 4°C. Two milligrams of cell lysate was precleared with protein A/G agarose during a 1-h incubation at 4°C. Four micrograms of anti-IRS-1 or anti-mTOR/FRAP was added, and the supernatant was shaken for 3 h. After addition of Protein A/G agarose the mixture was shaken overnight at 4°C. Samples were centrifuged at 12,000 rpm for 30 s at 4°C, and the pellet was washed three times with lysis buffer, resuspended in Laemmli buffer, boiled for 5 min, and centrifuged for 30 s. Equal amounts of protein from the supernatants were fractioned by electrophoresis on 7.5% polyacryamide gels and transferred to Immobilon-P poly(vinylidene difluoride) membranes (Millipore).
Liquid Chromatography-Electron Spray Ionization-MS.
To examine IRS-1 phosphorylation, samples were analyzed by capillary liquid chromatography using an Applied Biosystems 140D solvent delivery system. Samples were applied directly to 300-μm i.d.-fused silica capillaries packed with Vydac C18 resin and separated with gradients of buffer A (2% acetonitrile and 98% H2O containing 0.2% isopropanol, 0.1% acetic acid, and 0.001% trifluoroacetic acid) and buffer B (95% acetonitrile and 5% H2O containing 0.2% isopropanol, 0.1% acetic acid, and 0.001% trifluoroacetic acid). Peptides were eluted with a flow rate of 7 μl/min directly into the electrospray ionization source of a Finnigan LCQ mass spectrometer. Nitrogen was used as the sheath gas, and no auxiliary gas was used. Electrospray ionization was conducted with a spray voltage of 4.8 kV, a capillary voltage of 26 V, and a capillary temperature of 200°C. Spectra were scanned over an m/z range of 200 to 2,000. All possible target ions were programmed into the computer, and product ions were trapped by using the quadrupole ion trap and further analyzed with a high-resolution scan (zoom-scan) using an isolation width of 2 m/z and collision-induced dissociation scans with a collision energy of 40.0.
Results
TNF Impairs Insulin-Promoted Tyrosine Phosphorylation of IRS-1.
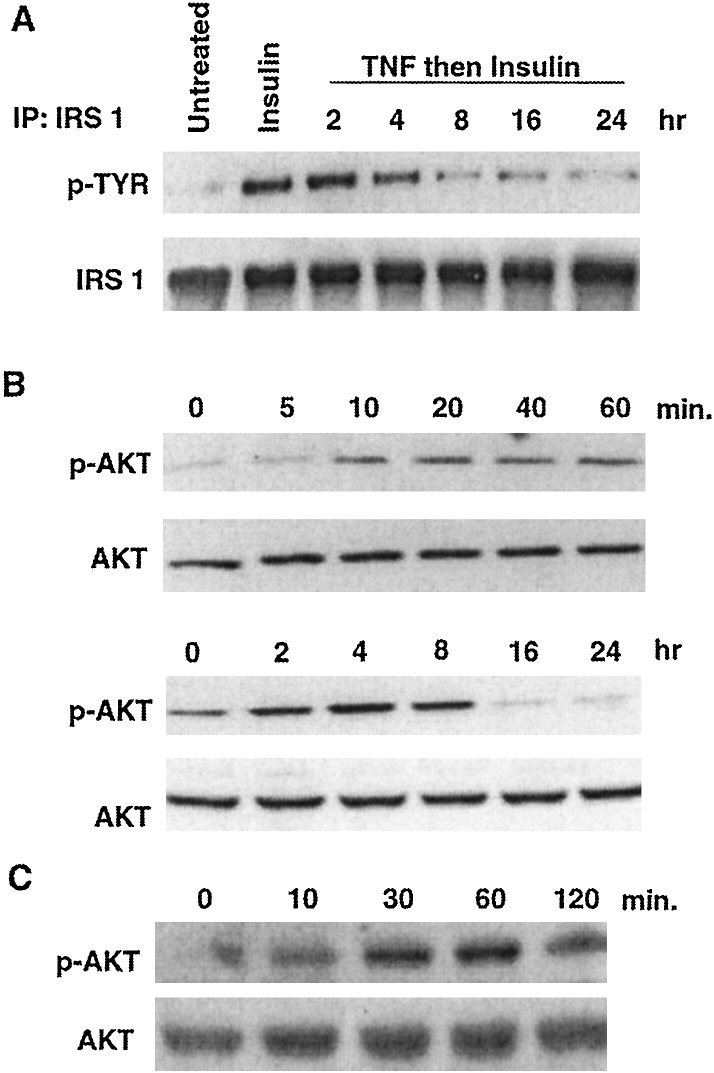
Because IRS-1 may be a substrate for Akt (17), and TNF activates PI 3-kinase/Akt signaling (15), we wondered whether the negative effect of TNF on insulin-promoted tyrosine phosphorylation of IRS-1 might be mediated by Akt. As illustrated by Fig. 1A, treatment of NIH 3T3 cells with insulin for 5 min increased IRS-1 tyrosine phosphorylation and TNF diminished this effect. Eight hours of treatment with TNF reduced insulin-promoted IRS-1 tyrosine phosphorylation by 76% relative to control.
Figure 1.
TNF inhibits tyrosine phosphorylation of IRS-1 and activates Akt. (A) Serum-starved NIH 3T3 fibroblasts were incubated with medium, insulin (10 nM, 5 min), or TNF (10 nM) for various times and then insulin. After immunoprecipitation (IP) of IRS-1 a Western blot was probed with an antibody to phosphotyrosine and then with anti-IRS-1 to demonstrate fractionation of equal amounts of protein from cell lysates. (B) Serum-starved NIH 3T3 cells were incubated with 10 nM TNF for 0–60 min (Upper) and 0–24 h (Lower). Cell lysates were fractionated on 10% polyacrylamide gels and Western blots were probed with an antibody directed against phospho-Akt (Upper), stripped, and reprobed with an anti-Akt antibody (Lower). (C) Serum-starved myotubes were incubated with 10 nM TNF for various times before fractionation of proteins on a 7% polyacrylamide gel. A Western blot was probed with anti-phospho-Akt (Upper) and then with anti-Akt (Lower).
To determine whether TNF activates Akt, NIH 3T3 cells were treated with TNF, and a Western blot was probed with an antibody to phosphorylated (active) Akt. TNF activated Akt within 10 min (Fig. 1B). Maximal activation of Akt occurred after 4 h, at which time activity was augmented 2.4-fold relative to control. After this time, Akt activity decreased and by 16 h was below the basal level. Thus, TNF reversibly activates Akt.
To be certain that activation of Akt could be relevant to TNF signaling in a cell type important to insulin action, we tested whether TNF could induce this response in myotubes. TNF augmented Akt activity in myotubes within 10 min, and this activity was sustained for at least 2 h (Fig. 1C). Thus, activation of Akt by TNF is observed in NIH 3T3 cells, myotubes, and, as previously demonstrated (15), in 293 cells.
PI 3-Kinase Mediates TNF Inhibition of Insulin-Induced Tyrosine Phosphorylation of IRS-1.
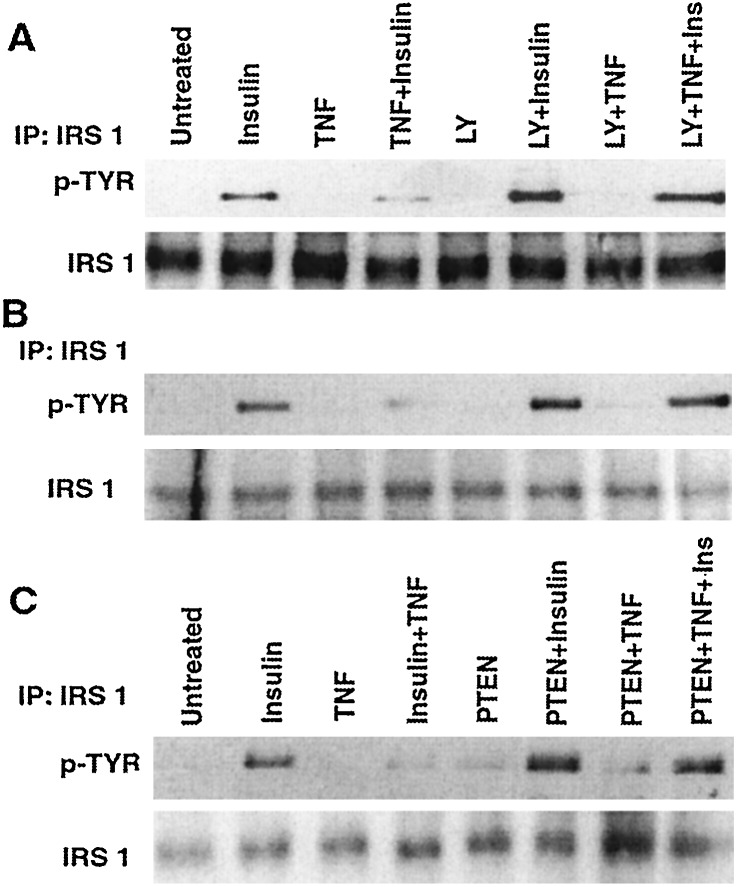
Because Akt is a downstream target for PI 3-kinase (ref. 18 and references therein), we determined whether the ability of TNF to abrogate insulin-induced tyrosine phosphorylation of IRS-1 could be blocked by inhibitors of PI 3-kinase. Treatment of NIH 3T3 cells with LY 294002, an inhibitor of PI 3-kinase, increased insulin-induced tyrosine phosphorylation of IRS-1 and attenuated the ability of TNF to inhibit this process (Fig. 2A). TNF also inhibited insulin-promoted tyrosine phosphorylation of IRS-1 in myotubes, and LY 294002 blocked this effect (Fig. 2B).
Figure 2.
Inhibition of PI 3-kinase antagonizes TNF-induced insulin resistance. (A) NIH 3T3 cells were incubated with medium or 20 μM LY 294002 for 1 h before treatment with insulin (5 min), TNF (8 h), or TNF and then insulin. After immunoprecipitation (IP) of IRS-1 from cell lysates and SDS/PAGE, a Western blot was probed with an antibody to phosphotyrosine (Upper) and then with anti-IRS-1 (Lower). (B) Myotubes were treated as described in A. After immunoprecipitation of IRS-1, a Western blot was probed with anti-phosphotyrosine (Upper) and then with anti-IRS-1 (Lower). (C) 293 cells or 293 cells transiently expressing PTEN were incubated with medium, insulin (5 min), TNF (2 h), or TNF and then insulin. After immunoprecipitation of IRS-1 and SDS/PAGE, a Western blot was probed with an antibody to phosphotyrosine (Upper) and then with anti-IRS-1 (Lower).
Because our observations implicate PI 3-kinase in TNF-induced insulin resistance, we examined whether an upstream inhibitor of PI 3-kinase signaling, PTEN (19), would have the opposite effect. To accomplish this, the insulin responsiveness of 293 cells was compared with that of 293 cells transiently expressing PTEN. PTEN increased insulin-induced tyrosine phosphorylation of IRS-1 2-fold (Fig. 2C, from left, compare lanes 2 and 6) and attenuated the capacity of TNF to impair this process (Fig. 2C). IRS-1 tyrosine phosphorylation was 3.6-fold greater in PTEN-transfected cells treated with TNF and then insulin than in cells transfected with empty vector (Fig. 2C, from left, compare lanes 4 and 8). Thus, PI 3-kinase potentiates and PTEN antagonizes TNF-induced insulin resistance.
Akt Promotes the Ability of TNF to Inhibit Insulin-Promoted Tyrosine Phosphorylation of IRS-1.
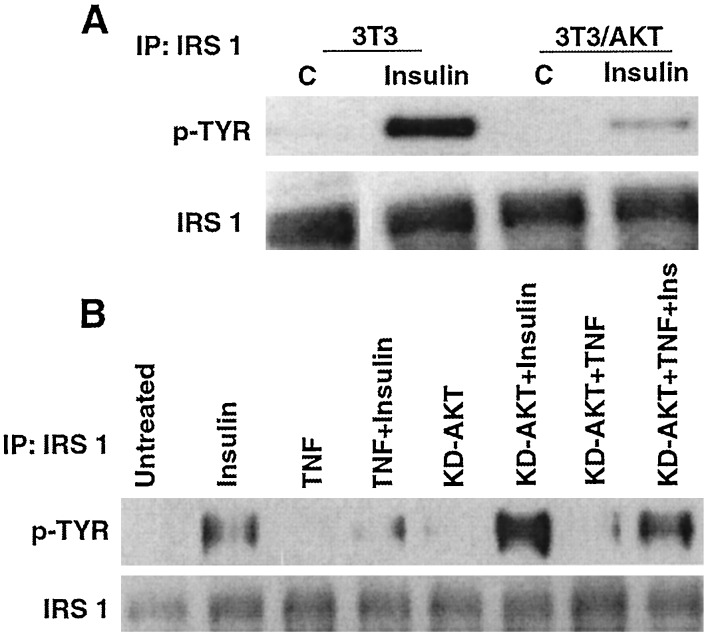
To determine whether Akt impairs insulin-induced tyrosine phosphorylation of IRS-1, this process was compared in NIH 3T3 cells and in NIH 3T3 cells stably expressing CA-Akt. Tyrosine phosphorylation of IRS-1 induced by insulin was reduced by >80% in cells expressing CA-Akt (Fig. 3A), relative to the parental line. TNF also impaired insulin-promoted tyrosine phosphorylation of IRS-1 in 293 cells (Fig. 3B). Transient expression of KD-Akt augmented insulin-induced tyrosine phosphorylation of IRS-1 3.4-fold relative to that in untransfected cells (Fig. 3B, from left, compare lanes 2 and 6). Furthermore, the ability of TNF to impair insulin action was reversed by KD-Akt (Fig. 3B, from left, compare lanes 4 and 8). Thus, TNF activates Akt, which impairs insulin action through IRS-1.
Figure 3.
Akt mediates TNF-induced insulin resistance. (A) NIH 3T3 cells and 3T3 cells stably transfected with CA-Akt were incubated with medium or insulin for 5 min. IRS-1 immunoprecipitated from cell lysates was fractionated by SDS/PAGE, and a Western blot was probed with an antibody to phosphotyrosine (Upper) and then with anti-IRS-1 (Lower). (B) 293 cells or 293 cells transiently expressing KD-Akt were incubated with medium, insulin (5 min), TNF (2 h), or TNF and then insulin. After immunoprecipitation (IP) of IRS-1 and SDS/PAGE, a Western blot was probed with an antibody to phosphotyrosine (Upper) and then with anti-IRS-1 (Lower).
mTOR Plays a Role in TNF-Induced Insulin Resistance.
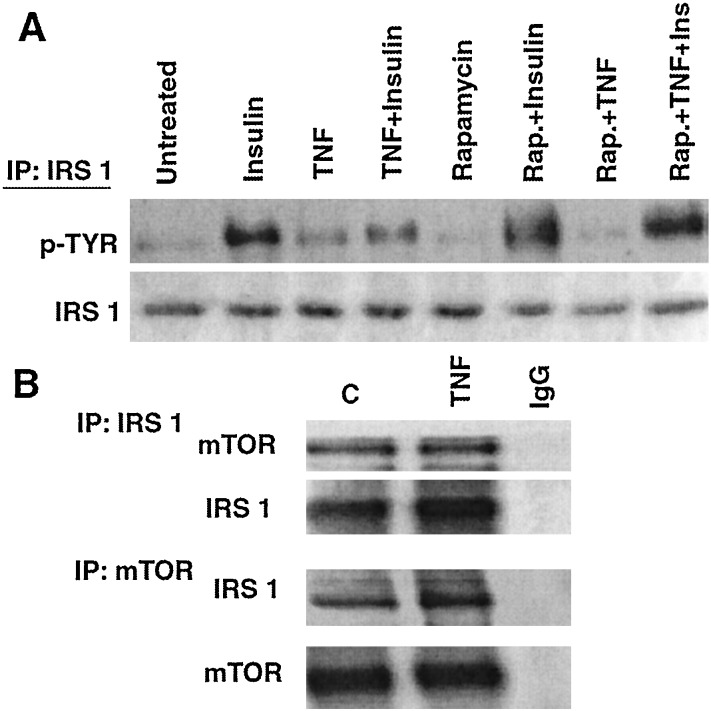
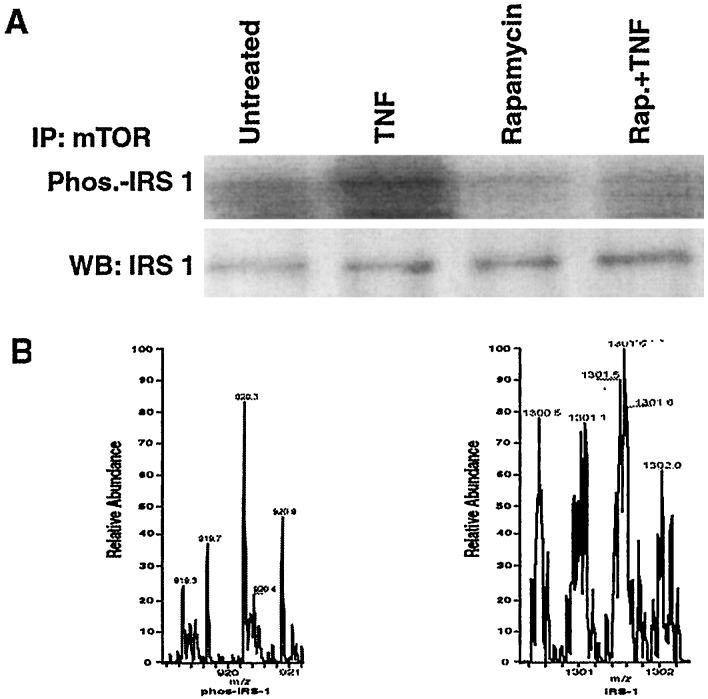
Akt may act on IRS-1 through another kinase, the mammalian target of rapamycin, mTOR/FRAP (17, 20). To determine whether TNF acts similarly, we examined whether rapamycin would block the ability of TNF to impair insulin-induced tyrosine phosphorylation of IRS-1 in myotubes and found that it did (Fig. 4A). IRS-1 and Akt in control or TNF-treated myotubes did not coimmunoprecipitate (data not shown). However, when Western blots of IRS-1 or mTOR immunoprecipitates were probed with the reciprocal antibody, it was observed that the two proteins associate constitutively (Fig. 4B).
Figure 4.
mTOR in TNF-induced insulin resistance. (A) Myotubes were incubated with medium or 200 nM rapamycin for 1 h before treatment with insulin (5 min), TNF (1 h), or TNF and then insulin. After immunoprecipitation (IP) of IRS-1 from cell lysates a Western blot was probed with antiphosphotyrosine (Upper) and then with anti-IRS-1 (Lower). (B) Serum-starved myotubes were incubated in the absence or presence of 10 nM TNF for 1 h before immunoprecipitation of either IRS-1 (Upper) or mTOR (Lower). A Western blot of proteins fractionated from each immunopreciptate then was hybridized with the reciprocal antibody.
To determine whether IRS-1 is phosphorylated by mTOR, myotubes were incubated in the absence or presence of rapamycin and then treated with or without TNF before immunoprecipitation of mTOR. γ32P ATP was added to the immunoprecipitates and reaction continued for 30 min at 30°C. Western blotting revealed that TNF induced phosphorylation of IRS-1 that was blocked by rapamycin (Fig. 5A). IRS-1 was also immunoprecipitated from myotubes incubated in the absence or presence of rapamycin and then treated with TNF. Immunoprecipitated IRS-1 was purified, digested with trypsin, and subjected to liquid spray ionization MS for assay of phosphorylation. From the TNF-treated sample, a predicted target ion with a m/z of 920 and charge state of m+3 was trapped and found to be phosphorylated on two serines (636 and 639), one of which (Ser-636) encompasses the Ser/Thr-Pro motif phosphorylated by mTOR (Fig. 5B Left) (21). This target ion was not recovered from cells incubated with rapamycin before TNF treatment (Fig. 5B Right). Rather a nonphosphorylated target ion with a m/z of 1,300 and a charge state of m+2 was trapped and its high-resolution scan is shown. Collision-induced fragmentation of the phosphorylated and unphosphorylated peptides yielded predicted products, which confirmed their identity (Table 1). Thus, endogenous mTOR/FRAP constitutively associates with IRS-1. TNF activates the kinase, which phosphorylates IRS-1 on specific serines.
Figure 5.
TNF induces the serine phosphorylation of IRS-1. (A) Myotubes were treated in the absence or presence of 200 nM rapamycin for 1 h, before incubation with or without TNF for 1 h. mTOR immunoprecipitates (IP) were washed three times in reaction buffer (50 mM NaCl/10 mM Hepes, pH 7.4/10 nM MnCl2/0.1 mM EGTA/1 mM DTT/50 mM β-glycerphosphate) and suspended in 100 μl of this buffer. Fifty microliters of the immune complex was incubated with 100 μM γ-32P ATP for 30 min at 30°C and reaction was terminated upon addition of 0.2 M EDTA. A Western blot (WB) was subjected to autoradiography to determine IRS-1 phosphorylation (Upper) and then probed with anti-IRS-1 (Lower). (B) IRS-1 was immunoprecipitated from myotubes incubated in the absence (Left) or presence (Right) of rapamycin and then treated with TNF for 1 h. After SDS/PAGE and Coomasie staining, the portion of the gel containing IRS-1 was excised, and phosphorylation of the protein was assayed by liquid chromatography-electron spray ionization-MS as described in Materials and Methods. The mass spectrometer was programmed to trap masses of predicted target ions containing single or multiple phosphorylations, trypsin miscleavages, and multiple charge states.
Table 1.
Sequence ions of phosphopeptides of IRS-1
| Sequence ions | Peptide m/z
|
|
|---|---|---|
| Measured | Calculated | |
| TNF | ||
| GNGDYMPMsPKsVSAPQQIINPIR | ||
| PMsPK | 621.3 | 621.2 |
| SAPQQIIN-NH3 | 835.7 | 835.4 |
| PKsVSAPQQ-H2O | 948.5 | 985.4 |
| PKsVSAPQQI-NH3 | 1099.7 | 1099.5 |
| MPMsPKsVS | 1105.9 | 1105.4 |
| MPMsPKsVSA | 1176.5 | 1176.4 |
| MPMsPKsVSAP | 1245.9 | 1245.5 |
| DYMPMsPKsVS | 1382.7 | 1383.5 |
| TNF + rapamycin | ||
| GNGDYMPMSPKSVSAPQQIINPIR | ||
| MSPKSV-NH3 | 613.0 | 613.3 |
| QQIINPI-NH4 | 790.2 | 790.4 |
| PMSKSVSAP-NH3 | 966.2 | 965.5 |
| KSVSAPQQII | 1024.8 | 1024.6 |
| SVSAQQIINPI | 1249.7 | 1248.7 |
| DYMPMSPKSVSAPQQ | 1619.8 | 1619.8 |
| MPMSPKSVSAPQQI-NH3 | 1628.6 | 1628.8 |
| PMSPSVSAPQQIINP | 1675.5 | 1675.9 |
IRS-1 was purified from myotubes treated with TNF and rapamycin or TNF alone. IRS-1 was digested with trypsin and subjected to liquid chromatography-electrospray ionization-mass spectrometry and collision-induced fragmentation. The parent ion was isolated in the ion trap. Measured fragment m/z and calculated m/z values are shown for each parent ion. Lowercase denotes phosphorylated serine. Neutral loss of ammonium, ammonia, and water from the peptide are shown as -NH4, -NH3, and -H2O.
Discussion
Tyrosine phosphorylation of IRS-1 by the insulin receptor promotes association of the docking protein with effector proteins, such as PI 3-kinase (22). PI 3-kinase and its downstream target, Akt, promote insulin-induced movement of GLUT4 to the cell membrane, glucose uptake, glycolysis, glycogen synthesis, and protein synthesis (18, 23–29). PI 3-kinase/Akt signaling promotes and regulates insulin action (30). This may be because binding of PI 3-kinase to IRS-1 couples the receptor to cellular responses; however, PI 3-kinase/Akt also may induce the serine phosphoryation of IRS-1, thereby uncoupling the docking protein from the insulin receptor, and impairing the ability of the receptor to tyrosine phosphorylate IRS-1 and bind PI-3-kinase (13, 14, 31–33).
The present study shows that TNF impairs insulin-promoted tyrosine phosphorylation of IRS-1. That Akt mediates this effect is demonstrated by comparison of insulin-induced tyrosine phosphorylation of IRS-1 in NIH 3T3 cells and NIH 3T3 cells expressing CA-Akt. The former cells were responsive to insulin-induced tyrosine phosphorylation of IRS-1, whereas the latter were unresponsive. LY 294002, a PI 3-kinase inhibitor, and KD-Akt sensitize cells to insulin and attenuated TNF inhibition of insulin-promoted IRS-1 tyrosine phosphorylation. Observations with NIH 3T3 and 293 cells were recapitulated with myotubes, in which TNF induces insulin resistance (34). In myotubes, TNF also activated PI 3-kinase/Akt signaling and impaired insulin-promoted tyrosine phosphorylation of IRS-1, an effect that was blocked by LY 294002. The ability of KD-Akt to produce the same result as LY 294002 causally links PI 3-kinase/Akt signaling with the inhibitory effect of TNF on tyrosine phosphorylation of IRS-1.
One explanation for these observations was that Akt directly phosphorylates IRS-1, thereby impairing its function in insulin signal transduction. This possibility was rendered unlikely by our inability to coimmunoprecipitate Akt with IRS-1 and led us to consider the possibility that a kinase activated by Akt modifies IRS-1. Attention was drawn to mTOR/FRAP, which is activated by PI 3-kinase/Akt signaling induced by insulin or platelet-derived growth factor (35, 36) and has been implicated in autoregulation of insulin signaling (17). Our observations implicating mTOR in TNF-induced insulin resistance are: (i) its association with IRS-1; (ii) the blockade of TNF inhibition of insulin-induced IRS-1 tyrosine phosphorylation by rapamycin; (iii) IRS-1 phosphorylation in mTOR immunopreciptates from lysates of myotubes treated with TNF; (iv) TNF-promoted phosphorylation of IRS-1 on a consensus mTOR phosphorylation site; and (v) the ability of rapamycin to block such phosphorylation. Although our results do not suggest direct interaction of endogenous Akt with IRS-1, as was observed in Chinese hamster ovary–T cells transiently overexpressing Akt (37), we do not preclude the possibility that these species can transiently interact under specific conditions.
The ability of PTEN to negatively regulate insulin-induced tyrosine phosphorylation of IRS-1 further implicates a signaling pathway of which PI 3-kinase is a component in this process. PTEN is a dual-specificity protein phosphatase that also acts as a lipid phosphatase (19, 38). PTEN dephosphorylates the three position of phosphoinositides and negatively regulates cellular levels of PI 3,4,5 trisphosphate, the lipid product of PI 3-kinase. Consequently, PTEN inhibits the PI 3-kinase/Akt cell survival pathway and, through this mechanism, acts as a tumor suppressor (38). PTEN also negatively regulates insulin signaling in 3T3-L1 adipocytes (39). We have now shown that PTEN expression augments insulin-promoted tyrosine phosphorylation of IRS-1 and diminishes the ability of TNF to suppress this effect. Thus, PTEN and PI 3-kinase/Akt exert opposite effects on the ability of TNF to affect cellular responsiveness to insulin through modification of IRS-1.
TNF induces insulin resistance through multiple mechanisms. Tyrosine phosphatase inhibitors impair the ability of TNF to block insulin receptor autophosphorylation (40). Among the tyrosine phosphatases that dephosphorylate the activated insulin receptor is SHP1 (41). It is interesting that TNF promotes SHP1 association with the KDR receptor tyrosine kinase, thereby blocking its activation (42). These observations suggest that tyrosine phosphatases are used by TNF to block the function of receptor tyrosine kinases, including the insulin receptor. The present study provides characterization of a mechanism through which TNF may affect IRS-1 by showing that a PI 3-kinase/Akt/mTOR pathway, induced by TNF and antagonized by PTEN, mediates serine phosphorylation of IRS-1. Serine phosphorylation uncouples IRS-1 from the insulin receptor and blocks the ability of the receptor to tyrosine phosphorylate IRS-1. Nontyrosine-phosphorylated IRS-1 cannot bind signaling proteins such as PI 3-kinase (13, 14, 34–36). The demonstration that IRS-1 and mTOR associate and TNF can promote modification of IRS-1 on consensus mTOR phosphorylation sites therefore provides a mechanism through which TNF may impair IRS-1 function in insulin signal transduction. It remains possible that a phosphatase, such as protein phosphatase 2A, inhibited by mTOR (43) and another kinase culminate and affect the final function of the PI 3-kinase/Akt/mTOR pathway through which TNF impairs insulin-promoted tyrosine phosphorylation of IRS-1.
The relationship of our observations to the interplay between TNF and insulin action must be defined based on which cellular effects of insulin require tyrosine phosphorylation of IRS-1. Insofar as glucose transport is concerned, expression of IRS proteins in primary rat adipocytes enhances GLUT4 translocation (44) and mutation of the insulin receptor such that it cannot tyrosine phosphorylate IRS proteins abrogates insulin-induced glucose uptake (45). In addition, ablation of both the IRS-1 and IRS-2 genes in mice supports a role for these adaptors in insulin-promoted glucose uptake (46). However, IRS-1-independent signaling also may play a role in this component of insulin action. This conclusion is supported by observations showing that expression of phosphotyrosine binding or SHC and IRS-1 NPXY-binding domains, which block interaction of IRS-1 with the insulin receptor, fails to block insulin stimulation of glucose transport in adipocytes (47). Furthermore, recruitment of PI 3-kinase to the platelet-derived growth factor receptor in adipocytes (48), or recruitment of PI 3-kinase to IRS-1 proteins induced by IL-4 (49) or cell surface integrin crosslinking (50), fail to induce GLUT4 mobilization. Also, a cell-permeable analog of the PI 3-kinase lipid mediator, PIP3, did not induce glucose uptake by itself, but in the presence of insulin and wortmannin, caused GLUT4 translocation (51). These observations suggest that insulin may be able to uniquely initiate IRS-1-independent signals that collaborate with IRS-1/PI 3-kinase-dependent signals to effect GLUT4 translocation.
Although IRS-1/PI 3-kinase/Akt signaling by itself is insufficient to mediate insulin stimulation of glucose transport, this pathway plays a more direct role in the anabolic actions of insulin, such as mitogenesis and protein synthesis (31, 48, 52). In evaluating the significance of these observations to the systemic actions of TNF, it is useful to consider the integrative role that TNF plays in promoting the acute-phase response (53) to infections and invasive stimuli, such as cancers. During the acute-phase response accelerated net breakdown of skeletal muscle and fat occurs with a concomitant shift to anabolic metabolism in the liver, to support the synthesis of acute-phase reactant proteins, as well as to bone marrow and wounds, for cellular proliferation within the immune system and for healing of injured tissue (53–59). These metabolic alterations represent a reprioritization of carbon and energy utilization in the injured or septic animal for survival. TNF activation of PI 3-kinase/Akt signaling may promote the acute phase response, at least in part through activation of NF-κB (15). NF-κB up-regulates immune and cell survival genes (60) and suppresses MyoD expression in muscle (61), thereby contributing to the wasting of this tissue. As shown here, PI 3-kinase/Akt signaling also inhibits insulin signaling through IRS-1 in muscle and fat, and inhibition of such anabolic activity in these peripheral tissues would likely be of benefit to a host organism mobilizing energy reserves in support of immunity and repair.
Acknowledgments
This work was supported by National Institutes of Health Grants CA67891 and CA73023 (to D.B.D.) and DK 18849 (to J.E.D.). L.D.M. is supported by a Hematology-Oncology Training Grant from the National Institutes of Health. J.A.G. is supported by the Indiana University Diabetes Graduate Training Program.
Abbreviations
- TNF
tumor necrosis factor
- IRS-1
insulin receptor substrate-1
- PI 3-kinase
phosphatidylinositol 3-kinase
- CA-Akt
constitutively active Akt
- KD-Akt
kinase dead Akt
- FRAP
FKBP12 rapamycin-associated protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Beutler B, Bazzoni F. Blood Cells Molecules Dis. 1998;24:216–230. doi: 10.1006/bcmd.1998.0187. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B B, Natarajan K. Eur Cytokine Network. 1996;7:93–124. [PubMed] [Google Scholar]
- 3.Peraldi P, Spiegelman B. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- 4.Lang C H, Dobrescu C, Bagby G J. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 5.Van der Poll T, Romijn J A, Endert E, Borm J J, Buller H R, Sauerwein H P. Am J Physiol. 1991;261:E457–E465. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein R, Kanety H, Papa M Z, Lunenfeld B, Karasik A. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 7.Hotamisilgil G S, Murray D L, Choy L N, Spiegelman B M. Proc Natl Acad Sci USA. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotamisligil G S, Spiegelman B M. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 9.Guo D, Donner D B. J Biol Chem. 1996;271:615–618. doi: 10.1074/jbc.271.2.615. [DOI] [PubMed] [Google Scholar]
- 10.Peraldi P, Hotamisligil G S, Buurman W A, White M F, Spiegelman B M. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad F, Goldstein B J. J Cell Biochem. 1997;64:117–127. [PubMed] [Google Scholar]
- 12.Virkamaki A, Ueki K, Kahn C R. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil G S, Peraldi P, Budavari A, Ellis R, Whiete M F, Spiegelman B M. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 14.Kanety H, Feinstein R, Papa M, Hemi R, Karasik A. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 15.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. Nature (London) 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 16.Harrington M A, Gonzales G, Jones P A. Mol Cell Biol. 1988;8:4322–4327. doi: 10.1128/mcb.8.10.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, DeFea K, Roth R A. J Biol Chem. 1999;274:9351–9356. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- 18.Datta S R, Brunet A, Greenberg M E. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 19.Maehama T, Dixon J E. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 20.Sekulic A, Hudson C C, Homme J L, Yin P, Otterness D M, Karnitz L M, Abraham R T. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 21.Brunn G H, Fadden P, Haystead T A J, Lawrence J C., Jr J Biol Chem. 1997;272:32547–32550. doi: 10.1074/jbc.272.51.32547. [DOI] [PubMed] [Google Scholar]
- 22.White M F, Yenush L. Curr Top Microbiol Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 23.Calera M R, Martinez C, Liu H, Jack A K, Birnbaum M J, Pilch P F. J Biol Chem. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- 24.Foran P B, Fletcher L M, Oatey P B, Mohammed N, Dolly J O, Tavare J M. J Biol Chem. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- 25.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 26.Hill M M, Clark S F, Tucker D F, Birnbaum M J, James D E, Macaulay S L. Mol Cell Biol. 1999;19:7771–7781. doi: 10.1128/mcb.19.11.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Somwar R, Bilan P J, Liu Z, Jin J, Woodgett J R, Klip A. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 29.Cong L N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Mol Endocrinol. 1997;11:1881–1990. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 30.Egawa K, Sharma P M, Nakashima N, Huang Y, Huver E, Boss G R, Olefsky J M. J Biol Chem. 1999;274:14306–14314. doi: 10.1074/jbc.274.20.14306. [DOI] [PubMed] [Google Scholar]
- 31.Tanti J-F, Gremeaux T, van Obberghen E, Le Marchand-Brustel Y. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 32.DeFea K, Roth R A. Biochemistry. 1997;6:12939–12947. doi: 10.1021/bi971157f. [DOI] [PubMed] [Google Scholar]
- 33.Staubs P A, Nelson J G, Reichart D R, Olefsky J M. J Biol Chem. 1998;273:25139–25147. doi: 10.1074/jbc.273.39.25139. [DOI] [PubMed] [Google Scholar]
- 34.Del Aguila L F, Claffey K P, Kirwan J P. Am J Physiol. 1999;276:E849–E855. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 35.Scott P H, Brunn G J, Koh A D, Roth R A, Lawrence J C., Jr Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnett P E, Barrow R K, Cohen N A, Snyder S H, Sabatini D M. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paz K, Liu Y-F, Shorer H, Hemi R, LeRoith D, Quan M, Kanety H, Seger R, Zick Y. J Biol Chem. 1999;274:28816–28822. doi: 10.1074/jbc.274.40.28816. [DOI] [PubMed] [Google Scholar]
- 38.Maehama T, Dixon J E. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima N, Sharma P M, Imamura T, Bookstein R, Olefsky J M. J Biol Chem. 2000;275:12889–12895. doi: 10.1074/jbc.275.17.12889. [DOI] [PubMed] [Google Scholar]
- 40.Kroder G, Bossenmaier B, Kellerer M, Capp E, Stoyanov B, Muhlhofer A, Berti L, Horikoshi H, Ullrich A, Haring H. J Clin Invest. 1996;97:1471–1477. doi: 10.1172/JCI118569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellerer M, Lammers R, Haring H-U. Exp Clin Endocrinol Diabetes. 1999;107:97–106. doi: 10.1055/s-0029-1212082. [DOI] [PubMed] [Google Scholar]
- 42.Guo D-Q, Wu L-W, Dunbar J D, Ozes O N, Mayo L D, Kessler K M, Gustin J A, Baerwald M R, Jaffe E A, Warren R S, Donner D B. J Biol Chem. 2000;275:11216–11221. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- 43.Peterson K T, Besai B N, Hardwick J S, Schreiber S L. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quon M J, Butte A J, Zarnowski M J, Sesti G, Cushman S W, Taylor S I. J Biol Chem. 1994;274:27920–27924. [PubMed] [Google Scholar]
- 45.White M F, Livingston J N, Backer J M, Lauris V, Dull T J, Ullrich A, Kahn C R. Cell. 1988;54:641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- 46.Bruning J C, Winnay J, Bonner-Weir S, Taylor S I, Accili D, Kahn C R. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 47.Sharma P M, Egawa K, Gustafson T A, Martin J L, Olefsksy J M. Mol Cell Biol. 1997;17:7386–7397. doi: 10.1128/mcb.17.12.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nave B T, Haigh R J, Hayward A C, Siddle K, Sheperd P R. Biochem J. 1996;318:55–60. doi: 10.1042/bj3180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isakoff S J, Taha C, Rose E, Marcusohn J, Klip A, Skolnik E Y. Proc Natl Acad Sci USA. 1995;92:10247–10251. doi: 10.1073/pnas.92.22.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guilherme A, Czech M P. J Biol Chem. 1998;273:33119–33122. doi: 10.1074/jbc.273.50.33119. [DOI] [PubMed] [Google Scholar]
- 51.Jiang T, Sweeney G, Rudolf M T, Kip A, Traynor-Kaplan A, Tsien R Y. J Biol Chem. 1998;273:11017–11024. doi: 10.1074/jbc.273.18.11017. [DOI] [PubMed] [Google Scholar]
- 52.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kushner I. Ann NY Acad Sci. 1986;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg A F, Baracos V, Rodeman P, Waxman L, Dinarello C A. Fed Proc Fed Am Soc Exp Biol. 1985;43:1301–1306. [PubMed] [Google Scholar]
- 55.Millward D J. Biochem Soc Trans. 1986;13:1023–1026. doi: 10.1042/bst0131023. [DOI] [PubMed] [Google Scholar]
- 56.Beisel W R. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- 57.Pepys M B, Baltz M L. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 58.Rosenblatt S, Clowes G H A, George B C, Hirsch E, Lindberg B. Arch Surg (Chicago) 1983;118:175–176. doi: 10.1001/archsurg.1983.01390020023004. [DOI] [PubMed] [Google Scholar]
- 59.Warren R S, Donner D B, Starnes H F, Jr, Brennan M F. Proc Natl Acad Sci USA. 1987;84:8619–8622. doi: 10.1073/pnas.84.23.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C-Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 61.Guttridge D C, Mayo M W, Madrid L V, Wang C-Y, Baldwin A S., Jr Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]