Abstract
Late infantile neuronal ceroid lipofuscinosis (LINCL) is a progressive neurodegenerative lysosomal storage disorder caused by mutations in TPP1, the gene encoding the lysosomal protease tripeptidyl-peptidase (TPP1). LINCL primarily affects children, is fatal and there is no effective treatment. Administration of recombinant protein has proved effective in treatment of visceral manifestations of other lysosomal storage disorders but to date, only marginal improvement in survival has been obtained for neurological diseases. In this study, we have developed and optimized a large-volume intrathecal administration strategy to deliver therapeutic amounts of TPP1 to the central nervous system (CNS) of a mouse model of LINCL. To determine the efficacy of treatment, we have monitored survival as the primary endpoint and demonstrate that an acute treatment regimen (three consecutive daily doses started at 4 weeks of age) increases median lifespan of the LINCL mice from 16 (vehicle treated) to 23 weeks (enzyme treated). Consistent with the increase in life-span, we also observed significant reversal of pathology and improvement in neurological phenotype. These results provide a strong basis for both clinical investigation of large-volume/high-dose delivery of TPP1 to the brain via the cerebrospinal fluid (CSF) and extension of this approach towards other neurological lysosomal storage diseases.
Introduction
One of the most common neurodegenerative lysosomal storage diseases (LSDs) affecting children is late infantile neuronal ceroid lipofuscinosis (LINCL) (OMIM #204500) which is a recessive disorder that results from mutations in the gene TPP1 (OMIM #607998)1 that encodes the lysosomal protease tripeptidyl-peptidase I (TPP1).2 At the cellular level, LINCL is characterized by lysosomal accumulation of autofluorescent storage material, a significant portion of which consists of undigested mitochondrial ATP synthase subunit C (SCMAS).3 Disease course is characterized by seizures, and progressive neuronal death that results in loss of vision and locomotor function as well as profound retardation.4,5 Affected children die typically between 7–15 years of age6 and there is no effective treatment. A gene-targeted mouse model of LINCL with <0.2% TPP1 activity recapitulates many clinical features of the human disease7 including a lysosomal accumulation of autofluorescent storage material, progressive neuronal pathology and neurological deficits and greatly reduced lifespan.
Proof-of-principle experiments in the LINCL mouse have shown the potential of gene therapy in LINCL. Intracranial treatment with AAV-based vectors reduced lysosomal storage and improved cellular pathology.8 Importantly, early administration at multiple brain regions markedly slowed the decline in locomotor function and significantly increased survival.9,10,11 The success of these preclinical studies has helped provide justification for an ongoing phase I gene therapy trial for LINCL.12
Gene therapy approaches have also shown promise in animal models of other lysosomal storage diseases13,14,15 suggesting that they may be clinically applicable on a more general basis. However, there are important concerns and problems associated with gene therapy that currently limits its clinical utility.16,17 One alternative to gene therapy is enzyme replacement therapy (ERT) where a recombinant protein is administered to replace the deficient activity. Unlike gene therapy, ERT is a chronic treatment that must be administered throughout the life of the patient. However, it does avoid some of the serious pitfalls of gene therapy e.g., the possibility of malignant mutagenesis of transformed cells, and unlike gene therapy, ERT can readily be discontinued in the advent of adverse reactions.
Intravenous ERT has been the most widely applied strategy to date, with clinical benefits observed in non-neuronopathic forms of a number of LSDs.18,19 However, intravenous administration is largely ineffective for correcting central nervous system (CNS) symptoms because passage of proteins across the blood–brain barrier is inefficient. There are approaches under development to promote passage from the blood to the brain20,21,22 but delivery strategies that avoid the blood–brain barrier are also worth consideration.
One such strategy involves direct delivery of enzyme to the cerebrospinal fluid (CSF).23,24 Intraventricular administration of TPP1 to the LINCL mouse was achieved through a cannula implanted into the ventricle25 and this resulted in improvement in some phenotypic measurements. However, this approach was limited by the relatively small doses that could be administered and by adverse reactions to cannula implantation. Administration of enzyme into the subarachnoid space is a less invasive route for CNS delivery, and has been demonstrated to facilitate enzyme uptake into the brain parenchyma in LSD animal models.26,27,28 However, while encouraging, the amount of protein delivered and the therapeutic responses have been modest. In this study, we have developed and optimized large-volume intrathecal administration methods that allow for the delivery of physiological levels of TPP1 to the brain of LINCL mice. Acute administration studies indicate that treatment significantly increases survival, providing a strong basis for clinical investigation of delivery of TPP1 to the brain via the CSF.
Results
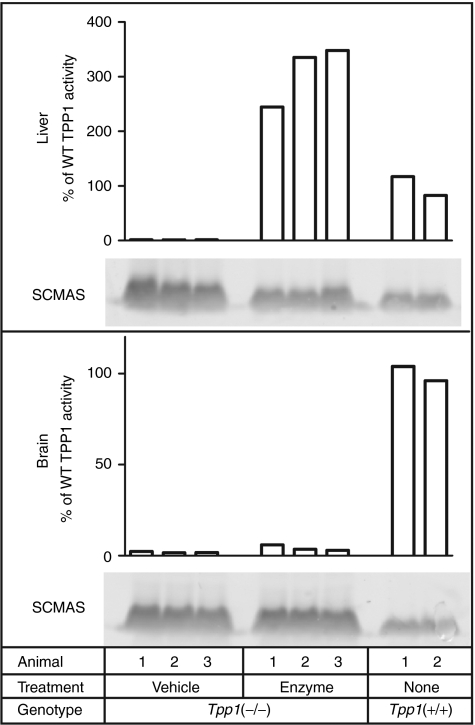
Figure 1 shows that intravenous administration of 1.2 mg recombinant human TPP1 (rhTPP1) proenzyme to adult Tpp1(−/−) mice results in substantial delivery of enzyme to the liver (approximately threefold higher TPP1 activity than wild-type). Importantly, when one takes into account the levels of endogenous mitochondrial SCMAS, intravenous administration of TPP1 reduced levels of stored SCMAS in this tissue by 72%, indicating digestion of preexisting storage material. In contrast, only small amounts of enzyme (<6% of wild-type) were detected in brain and these levels had no apparent effect on SCMAS storage.
Figure 1.
Uptake of intravenously administrated recombinant human TPP1 (rhTPP1) and effect on subunit c of mitochondrial ATP synthase (SCMAS) storage in liver and brain. Fifteen week-old Tpp1(−/−) mice were killed 24 hours after administration of 100 µl artificial cerebrospinal fluid (CSF) (vehicle) or 12 mg/ml rhTPP1 (enzyme) and compared to age-matched wild type controls. Equal amounts of each sample (3 µg protein) were analyzed by immunoblotting. Relative intensities (mean ± SD) of SCMAS staining in each tissue for vehicle-treated (n = 3), enzyme-treated (n = 3), and wild-type controls (n = 2), respectively, were as follows: Liver- 1.00 ± 0.26, 0.53 ± 0.06, 0.35 ± 0.03; Brain- 1.00 ± 0.13, 1.03 ± 0.04, 0.37 ± 0.05. Note that this signal includes both normal mitochondrial and stored SCMAS.
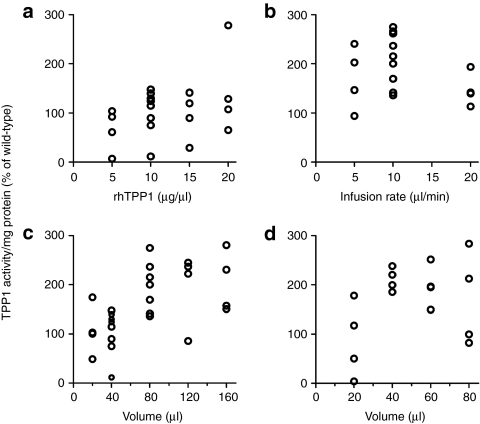
In an attempt to improve delivery to the brain, we explored intrathecal administration using variations of previously described lumbar injection procedures,29,30 including use of nonstandard, large-volume (>20 µl) infusions. All tested volumes (5–160 µl) were well tolerated, with mice recovering from anesthesia and appearing normal based on gross observation over 24 hours. After conducting a series of optimization experiments on mature (age >6 week) animals (Figure 2a–c), we developed a standardized protocol, infusing mice with 80 µl of 10 µg/µl rhTPP1 at a rate of 10 µl/minute. Additional experiments conducted on young (4–5 week old) mice indicated that at this age, delivery plateaued at 40 µl of 10 µg/µl rhTPP1 (Figure 2d). Adjusting for body weight, the dose plateaued at ~30 mg/kg for young and adult mice.
Figure 2.
Optimization of parameters for intrathecal delivery of recombinant human TPP1 (rhTPP1) to the brain. (a) Concentration and dose response using a fixed 40 µl volume with a 10 µl/minute infusion rate. (b) Effect of infusion rate using a fixed 80 µl volume of 10 µg/µl rhTPP1. (c) and (d) Volume and dose response using 10 µg/µl rhTPP1 with a 10 µl/minute infusion rate. TPP1 activity was measured 24 hours after administration to mature (>6 week old) (a,b,c) or young (4–5 week old) (d) Tpp1(−/−) mice.
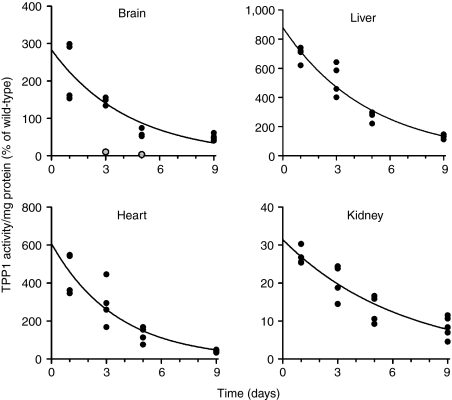
Figure 3 shows the levels of TPP1 in brain and select visceral tissues (liver, kidney, and heart) at different times following intrathecal administration. Enzyme activity measurements conducted with or without preactivation and western blotting on brain and liver indicated that the rhTPP1 was completely converted from the zymogen to the active, proteolytically processed mature form, indicating targeting to the lysosome (Supplementary Figure S1). The apparent half-life of TPP1 was between 2 and 5 days in all tissues as determined from two independent experiments (Figure 3). Extrapolation of activity curves to day 0 provides an estimate of the initial biodistribution of rhTPP1. Of the total dose administered (0.80 mg rhTPP1 proenzyme, i.e., 13.5 nmol), 0.76% was retained in brain, resulting in ~300% of wild-type activity. The largest fraction of the administered dose was found in liver (42% of total), with lesser amounts found in heart and kidney (0.25% and 0.35%, respectively). The remaining 50% of the dose was unaccounted for, presumably being taken up by other tissues or rapidly cleared.
Figure 3.
Tissue distribution and stability of recombinant human TPP1 (rhTPP1) following intrathecal administration. Animals were given a single dose of TPP1 proenzyme (80 µl of 10 µg/µl enzyme delivered at 10 µl/minute) on Day 0 and sacrificed at the indicated times. TPP1 specific activities are normalized to wild-type levels. Total endogenous wild type TPP1 levels (pmol/organ, mean ± SD, n = 3) are as follows: brain, 30 ± 1.5; liver, 626 ± 89; heart, 5.5 ± 1.4; and kidney, 144 ± 11. Enzyme half-lives in tissues were estimated fitting TPP1 specific activities to a one phase exponential decay model using GraphPad Prism 5.03. Two points in brain, indicated as gray symbols, were considered outliers and omitted from the regression analysis. Half-life best fit values in days (with 95% confidence intervals) were as follows: brain, 3.0 (2.1–5.0); liver 3.3 (2.8–4.0); heart 2.5 (1.8–3.9); kidney 4.5 (3.5–6.1). An independent experiment where eight Tpp1(−/−) animals were dosed twice a week from 4–8 weeks of age with 20 µl, 10 µg/µl rhTPP1 and then killed 4 and 11 days after the final dose yielded the following half-life estimates: brain, 4.4 (3.1–7.3); liver 4.1 (3.0–6.6); heart 4.4 (2.6–15); kidney 5.0 (3.0–15).
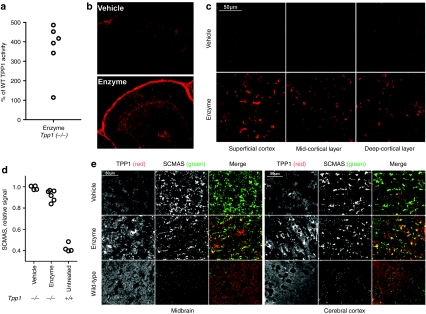
While a single intrathecal infusion could result in significant delivery of enzyme to the brain, there was still some variability in response (e.g., see gray symbols, Figure 3), presumably reflecting problems with delivery to the CSF. To minimize this variability, animals were administered three successive doses at 24 hour intervals, resulting in additive effects as well as more uniform uptake (Figure 4a), with levels of TPP1 obtained up to 500% of wild-type activity. Immunohistochemical analysis revealed intense TPP1 staining of meninges (Figure 4b and Supplementary Figure S2). TPP1 also reached a wide variety of brain regions including cerebellum, cerebral cortex, midbrain, olfactory bulb, and brain stem, with the highest levels being detected in regions proximal to the surface (Figure 4b,c,e and Supplementary Figure S2).
Figure 4.
Uptake of intrathecally administrated recombinant human TPP1 (rhTPP1) and effect on subunit c of mitochondrial ATP synthase (SCMAS) storage in brain. Fifteen week-old Tpp1(−/−) mice were administered three daily 80 µl infusions of artificial cerebrospinal fluid (CSF) (vehicle) (n = 4) or 10 µg/µl rhTPP1 (enzyme) (n = 6) and killed 24 hours after the final dose. (a) Relative TPP1 activity in enzyme treated animals. (b) TPP1 immunohistochemistry in cerebellum lobes IV/V. (c) TPP1 immunohistochemistry in different regions of the cerebral cortex. (d) SCMAS levels by quantitative western-blot analysis. Each symbol represents an average of multiple measurements (n ≥ 2) from individual animals. The difference between the vehicle and enzyme-treated group is significant using the Mann–Whitney nonparametric test (P < 0.01). (e) Double-label indirect immunofluorescence using the mouse monoclonal anti-human TPP1 antibody 8C4 (red in merge) and rabbit anti-SCMAS antibodies (green in merge). Note that the low signal for TPP1 in the wild type animal is due to poor immunoreactivity of 8C4 for murine TPP1 and that endogenous mitochondrial SCMAS is not detected under these conditions.45 Images were obtained using either a Nikon Eclipse E600 microscope with a ×20 objective (b) or a Zeiss LSM510 laser scanning confocal microscope with a 63x objective (c and e).
Analysis by quantitative western blotting indicated that short-term treatment had a small (~10%) but significant effect in reversing SCMAS storage (Figure 4d). Intrathecal administration of rhTPP1 reduced levels of stored SCMAS in liver by 74% (Supplementary Figure S3) which is very similar to the reduction observed with intravenous administration (Figure 1). The reduction in brain SCMAS was corroborated by immunohistochemical analysis (Figure 4e). Here, the inclusions containing stored SCMAS appeared smaller and less intense in the enzyme treated compared to vehicle treated Tpp1(−/−) animals. In addition, double-label immunofluorescence microscopy indicates that the rhTPP1 is delivered to lysosomes containing storage material, as shown by the considerable overlap between the TPP1 and SCMAS staining.
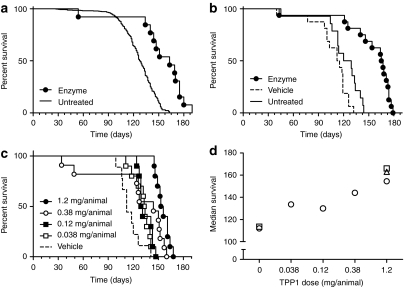
We conducted efficacy studies using 4-week old presymptomatic mice. This time-point was chosen as previous gene therapy studies indicated that early treatment is considerably more efficacious than later.9,11 Also, at 4 weeks, the blood–brain barrier has formed fully and there is down regulation of neonatal mannose 6-phosphate receptors which potentially could function to deliver peripheral enzyme to the brain.31 In an exploratory study, we treated Tpp1(−/−) mice with three daily doses of enzyme (1.2 mg total). Significant levels of TPP1 activity were achievable in the brains of 4-week old mice, ranging from three to tenfold higher than wild-type levels in 4- or 12-week-old animals (Supplementary Figure S4). Enzyme treatment increased lifespan to 163 days compared to 128 days for untreated historic controls (Figure 5a).
Figure 5.
Effect of intrathecal administration of recombinant human TPP1 (rhTPP1) on survival. Four week-old Tpp1(−/−) animals were either administered three daily infusions of 40 µl rhTPP1 at the indicated concentration, artificial cerebrospinal fluid (CSF) vehicle, or left untreated. (a) Enzyme treated (10.0 µg/µl, total dose = 1.2 mg rhTPP1/animal) (n = 13) compared to historical untreated controls (n = 601). Median survival was 163 and 128 days, respectively (P < 0.0001 by the Mantel–Cox test). (b) Vehicle treated (n = 16), enzyme treated (10.0 µg/µl, total dose = 1.2 mg rhTPP1/animal) (n = 16) and untreated (n = 14). Median survival was as follows: vehicle, 113.5 days; enzyme, 164 days; untreated, 124.5 days. Pairwise results of Mantel–Cox tests: enzyme versus vehicle or enzyme versus untreated, P < 0.0001; vehicle versus untreated, P = 0.0144). (c) Enzyme treated (10.0, 3.16, 1.00, or 0.316 µg/µl yielding total dose indicated on graph) and vehicle treated (n = 9–11 animals/group). All treated groups are significantly different from vehicle control (P < 0.05). (d) Median survival for data plotted in panels a (open triangle), b (open squares) and c (open circles).
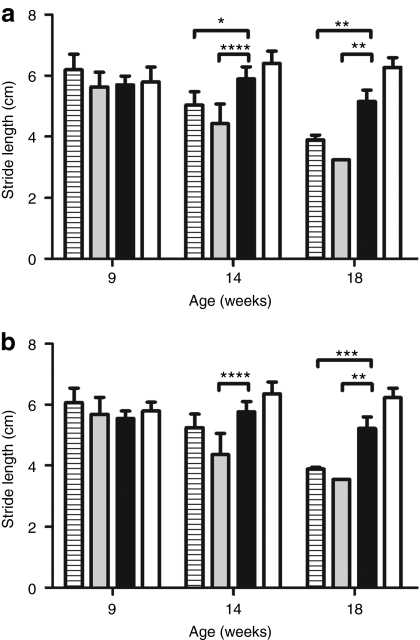
Given the encouraging nature of this result, we then analyzed matched cohorts of untreated, vehicle treated, and enzyme treated Tpp1(−/−) mice to further evaluate the effect of treatment on survival and on behavioral phenotype using minimally disruptive methods. Gait analysis was conducted at selected time points to monitor locomotor function (Supplementary Figure S5) and fore- and hind-limb stride lengths were quantified (Figure 6). At 9 weeks, all three groups of Tpp1(−/−) animals were similar to each other and resembled unaffected animals in terms of gait. At 14 weeks, a decline in stride length was observed in the vehicle and untreated animals but not in the enzyme-treated mice. At 18 weeks, the enzyme-treated mice were significantly impaired compared to unaffected controls (P < 0.001). However, enzyme treatment considerably delayed disease progression in comparison to the untreated and vehicle-treated Tpp1(−/−) mice. Consistent with these results, visual observation indicated that the ataxia and hunched appearance characteristic of the Tpp1(−/−) mice is delayed by enzyme treatment (Supplementary Videos S1–S4).
Figure 6.
Effect of intrathecal injection on locomotor function. Four-week old Tpp1(−/−) mice were administered three daily 40 µl infusions of artificial cerebrospinal fluid (CSF) (vehicle, gray filled bars) or 10 µg/µl recombinant human TPP1 (rhTPP1) (enzyme, black filled bars) (total dose/animal = 1.2 mg proTPP1) or were left untreated (horizontal striped bars). Untreated unaffected controls (wild type and heterozygotes) are shown for reference (open bars). Fore- (a) and hind-limb (b) stride lengths were measured. Between 4 and 28 (mean, 11) usable measurements were recorded for each animal and these were averaged to provide a mean stride-length per animal. For each timepoint, between two and seven animals were analyzed except for the single surviving vehicle treated animal at 18 weeks. Plots represent the mean ± standard deviation of the mean stride-lengths for all animals in each group at each time-point. Statistical significance between indicated pairs was calculated using the Bonferroni multiple comparison post-test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
In terms of survival, vehicle treatment decreased median lifespan by 11 days (9%) compared to untreated controls (Figure 5b). In contrast, enzyme treatment markedly increased median survival, with increases of 40 days (32%) and 50 days (44%) compared to the untreated and vehicle treated controls, respectively. Finally, we conducted an experiment where five cohorts of animals were treated with vehicle or different doses of rhTPP1 (Figure 5c). Results from all three experiments are combined and presented in Figure 5d. This reveals that TPP1 treatment clearly increases survival in a dose-dependent manner.
Discussion
Effective delivery of protein therapeutics to the brain is an extremely challenging area of biomedical research.32 Given the difficulties inherent with delivery of proteins across the blood–brain barrier, we have investigated the therapeutic potential of intrathecal administration of rhTPP1 using an LINCL mouse model. Previous studies of intrathecal therapy on LSD animal models typically employed administration of the protein of interest in volumes considerably smaller than that of the CSF.27,28,33,34 Initially, we adopted a similar strategy by, for example, administering a concentrated rhTPP1 dose in 5 µl to an adult mouse where the total CSF volume is ~40 µl.35 However, this resulted in inadequate delivery based on biochemical analysis (typically <10% of wild-type activity). In the course of methods optimization, we found that the use of large volumes (up to 160 µl) resulted in dramatically improved delivery. Given that in the absence of obstruction, CSF outflow increases with increasing pressure,35 this in part may be due to replacement of existing CSF with infusate so that the brain is now bathed in a high concentration of enzyme. While unconventional, large-volume intrathecal administration of protein appears to be well tolerated in mice, and it may be applicable to humans so long as care is taken to avoid large and/or sudden pressure changes that could lead to herniation or impaired cerebral perfusion.
In this study, our approach has been to measure survival of the LINCL mouse as the primary endpoint for the evaluation of treatment efficacy and there are several justifications for this strategy. First, while the measurement of surrogates, e.g., behavioral or locomotor analyses to follow neurological decline, have allowed for the evaluation of treatments where survival was not an endpoint (e.g., refs. 8,25) life-span is one of the strongest and most unambiguous measurements of treatment efficacy. Second, the LINCL mouse is extremely sensitive to environmental stresses that can induce fatal startle seizures.7 This complicates evaluation of efficacy by behavioral phenotyping. Thus, our primary goal was to determine the effect of treatment on survival but we have investigated neurological phenotype where possible using relatively low stress methods.
Short-term treatment of 4-week old LINCL mice resulted in a dramatic increase in lifespan (6–7 weeks at the highest doses of TPP1 compared to vehicle-treated controls). Other studies have found a 1 or 2 week extension of lifespan for cerebroventricular administration of recombinant lysosomal enzymes to mouse models of Krabbe and Sandhoff diseases.33,36 However, in both cases, at least some of the survival benefit was postulated to reflect peripheral effects.37 This is not the case in our study, as there are no apparent visceral symptoms in LINCL, and also, control experiments employing peripheral administration of equivalent doses of TPP1 did not prolong survival (Supplementary Figure S6). Video recording and gait analysis indicate that the neurological phenotype of disease also improves following large volume intrathecal administration of rhTPP1.
A considerable fraction of the intrathecally administered enzyme enters the systemic circulation. In terms of visceral effects, like peripherally administered enzyme, this TPP1 rapidly reduces liver SCMAS storage. The effect on brain SCMAS storage was much less pronounced. This may reflect less efficient delivery of TPP1 to neuronal lysosomes, especially in less accessible regions of the brain. Alternatively, there may be intrinsic differences in chemical composition of storage material (e.g., components in addition to SCMAS) and/or in the aggregation state of SCMAS in different cell types (e.g., mitotic and postmitotic cells). Regardless, administration of TPP1 to the brain provides a clear benefit in ameliorating disease, prolonging survival by ~50 days. Using an upper limit of 5 days for the half-life of TPP1, the 500% of wild type levels expected to be present in brain after the third rhTPP1 dose (see Figures 3 and 4) would last 50 days before reaching a residual level of 0.5%. Given the beneficial effect of even low (3%) levels of TPP1 observed in hypomorphic mouse models,38 we cannot rule out the possibility that the survival benefit of treatment reflects prevention of further storage accumulation and thus retardation rather than reversal of disease. However, enzyme levels in individual cells vary widely, and it is possible that treatment does “set back the clock.” Further studies are required to determine the reversibility of lysosomal storage and pathological processes associated with disease progression.
In summary, we report that large-volume intrathecal administration of rhTPP1 results in delivery of therapeutic amounts of enzyme to the brain of LINCL mice. Importantly, acute treatment significantly extends lifespan and improves neurological phenotype. Future studies using chronic dosing regimens will be necessary to further evaluate efficacy in terms of improving long term survival as well as neuropathological and behavioral manifestations of disease. While such studies will extend the proof-of-concept in an animal model, we believe that this approach may be applicable to patients. The doses used in this study are high (~30 mg/kg) but are comparable to those used clinically for treatment of Pompe disease39 so this should not be a limiting factor. In addition, complete exchange of the CSF has proven to be well-tolerated as an emergency measure for accidental intrathecal overdose,40,41 providing further support for the applicability of this approach. Thus, while unconventional, large-volume/high-dose delivery of enzyme to the CSF warrants further exploration as it has potential to overcome the long-standing barrier in developing effective treatments for LINCL and other neurodegenerative lysosomal storage diseases.
Materials and Methods
Animals. All experiments and procedures involving live animals were conducted in compliance with approved Institutional Animal Care and Use Committee protocols. Mice were maintained in a C57/BL6 strain background and genotyped as described.7
Production and purification of recombinant human TPP1. Recombinant human TPP1 proenzyme was produced in CHO cells and purified essentially as described42 except that the final gel filtration step was conducted in artificial CSF (aCSF; 148 mmol/l NaCl, 3 mmol/l KCl, 1.4 mmol/l CaCl2, 0.9 mmol/l MgCl2, 0.8 mmol/l Na2HPO4, 0.196 mmol/l NaH2PO4, pH 7.2) and precautions were taken to avoid endotoxin contamination. Protein was concentrated using a Vivaspin20 device (Sartorius Stedim Biotech, Aubagne, France) to a maximum of 20 mg/ml which approached the solubility limit. The protein and aCSF vehicle were free of endotoxin (<0.06 EU/ml) as determined by Limulus Amebocyte Lysate reagent (Charles River Laboratories, Wilmington, MA).
Intrathecal administration. Mice typically were anesthetized with isoflurane delivered through an inhalation system (VetEquip, Pleasanton, CA) or using intraperitoneal injection of avertin. Animals were shaved to expose the skin around the lumbar region and injected between vertebrae L5 and L6 using a 30 gauge needle (Becton Dickinson, Franklin Lakes, NJ) oriented towards L4 as described.43 The needle was connected to a 50 or 100 µl gas-tight Hamilton syringe by a short length of silastic tubing (0.3 mm inner diameter; Dow Corning, Midland, MI). The dose was administered at the indicated flow rate using a NE-300 syringe pump (New Era Pump Systems, Wantagh, NY).
Biochemical and histochemical analysis. Mice were deeply anesthetized using a sodium pentobarbital/sodium phenytoin mixture (Euthasol; Delmarva Laboratories, Milton, DE), killed by transcardial perfusion with saline, and tissues dissected and frozen in liquid nitrogen. In some cases, the brain left hemisphere was reserved prior to freezing, immersed in Bouin's fixative and processed for histochemical analysis (see below). Frozen tissue powders were prepared using a Bessman pulverizer (Spectrum Laboratories, Rancho Dominguez, CA), added to 50 volumes of 0.15 mol/l NaCl/0.1 % Triton X-100, and homogenized using a Polytron homogenizer (Kinematica, Bohemia, NY).
TPP1 and protein assays. Tissue homogenates were centrifuged at 14,000g and supernatants analyzed for TPP1 activity using the endpoint assay described previously.44 When indicated, purified TPP1 or samples were incubated at pH 3.5 to convert inactive proenzyme to mature, active protein.44 The absolute quantity of active enzyme was calculated using a reference standard of activated purified TPP1 analyzed in parallel. Protein concentration was measured using Advanced Protein Assay reagent (Cytoskeleton, Denver, CO).
SCMAS immunoblotting. Tissue homogenates were diluted into reducing LDS-PAGE buffer and fractionated on 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) using MES running buffer. Proteins were transferred to PVDF, the membranes blocked with phosphate-buffered saline/3% bovine serum albumin/0.2% Tween-20 and probed with affinity purified rabbit anti-SCMAS amino-terminal peptide antibodies (PAC3601/3602; Pacific Immunology, Ramona, CA). Control experiments indicated indistinguishable specificity compared to a similar reagent described previously.45 Alexafluor 488 conjugated donkey-anti-rabbit IgG (#A-21206; Invitrogen) was used as a secondary detection reagent. Signal was visualized and quantified using a Typhoon 9400 scanner and ImageQuant 5.2. (GE Healthcare, Waukesha, WI).
Immunofluorescence. Saggital cryosections (10 µm) were processed and probed essentially as described previously.38,46 TPP1 was detected using mouse monoclonal antibody 8C447 obtained courtesy of Dr Adam Golabek (Staten Island, NY) and donkey-anti-mouse Alexafluor 555 (#A-31570; Invitrogen). SCMAS was detected using reagents described above.
Behavioral and survival analysis. Animals were housed in single cages from four weeks of age onward and handled gently to avoid disturbances that can provoke fatal startle seizures.7 Gait analysis was conducted at 9, 14, and 18 weeks of age as described previously.38 Stride-length was quantified as described.48 Statistical tests were calculated using Prism 5.03 (Graph-Pad Software, San Diego, CA).
SUPPLEMENTARY MATERIAL Figure S1. TPP1 proenzyme is activated after delivery into brain. (a) fifteen-week old Tpp1(−/−) mice (n=2) were administered a single dose of 20 μL 7.2 μg/μL rhTPP1 and killed 48 hours afterwards. Age matched wildtype mice (n=2) were included as control. TPP1 activity in brain is measured with (open bar) or without pre-activation (grey bar) and normalized to the wildtype control. (b) fifteen-week old Tpp1(−/−) mice were administered 3 daily dose of artificial CSF (V, n=4) or 80 μL 10 μg/μL rhTPP1 (E, n=6). After 48 hours, mice were killed and TPP1 was detected in brain homogenates by western blotting using a rabbit polyclonal anti-TPP1 antibody. Samples were normalized to protein amount (7 μg per lane) and an age matched wildtype mouse was included as control (wt). Positions of in vitro activated rhTPP1 (act) and non-activated proTPP1 (pro) were also shown. Note that there is a small difference in the migration of TPP1 activated in vitro and in vivo, which probably reflects the processing of N-linked glycans within the lysosome. Figure S2. Montage image of TPP1 distribution. Fifteen week-old Tpp1(−/−) mice were administered three daily 80 μL infusions of artificial CSF (vehicle) or 10 μg/μL rhTPP1 (enzyme) and killed 24 hours after the final dose. Sagittal sections immunostained with anti-TPP1 monoclonal antibody and Alexafluor 555 conjugated donkey-anti-mouse IgG were visualized using a Nikon Eclipse 80i with a 4x objective. Figure S3. Effect of Intrathecally administrated rhTPP1 on SCMAS storage in liver. Nineteen week-old Tpp1(−/−) mice were administered three daily 80 μL infusions of artificial CSF (V1-2) or 10 μg/μL rhTPP1 (E1-3) and killed 24 hours after the final dose. Relative intensities (mean ± standard deviation) of SCMAS staining in liver for vehicle-treated (n=2), enzyme-treated (n=3), and wild-type age controls (n=2), respectively, were as follows: 1.00±0.10, 0.48±0.06, 0.30±0.01. Note that this signal includes both normal mitochondrial and stored SCMAS. Figure S4. Uptake of intrathecally administrated rhTPP1 in young mice. Four-week old Tpp1(−/−) mice (n=4) were administered three daily 40 μL infusions of 10 μg/μL rhTPP1 (Enzyme) and killed 24 hours after the final dose. TPP1 activity in brain was measured and compared to the levels in wild-type animals (WT-adult: wild-type, ∼12 weeks old; WT-young: wild-type mice, ∼4 weeks old). Figure S5. Gait analysis. Representative patterns are shown for animals with n=3-7 animals per time-point for each cohort except for the vehicle-treated group where only a single animal survived to eighteen weeks. Purple and orange represent fore- and hind-limb placement, respectively. Contrast is optimized for each individual image due to differences in inking. Figure S6. Effect of intravenous administration on survival. Four-week old Tpp1(−/−) mice were administered three daily 40 μL infusions of artificial CSF (vehicle) (n=9) or 10 μg/μL rhTPP1 (enzyme) (n=10). Survival date from untreated Tpp1(−/−) mice (Figure 5) represents a historical control. Median survival was: vehicle, 134 days; enzyme, 106 days; untreated, 124.5 days. Video S1. 15-week old Tpp1(−/−) mouse treated with enzyme as described in Figure 5b. Video S2. 15-week old Tpp1(−/−) mouse treated with vehicle as described in Figure 5b. Video S3. 19-week old Tpp1(−/−) mouse treated with enzyme as described in Figure 5b. Video S4. 19-week old untreated Tpp1(−/−) mouse. Note that no vehicle treated mice survived to this age.
Acknowledgments
This work was supported by National Institutes of Health Grant NS37918 (P.L.) and by a Batten Disease Support & Research Association Fellowship (S.X.). Some preliminary studies were supported by BioMarin Pharmaceutical. We would like to thank Drs. James H. Millonig, Loren W. Runnels and Mengqing Xiang for help with microscopy, Dr. Adam Golabek for providing anti-TPP1 antibody, and Dr. Emil Kakkis for helpful discussions. This work was conducted at CABM/UMDNJ-RWJMS in Piscataway, NJ, USA.
Supplementary Material
TPP1 proenzyme is activated after delivery into brain. (a) fifteen-week old Tpp1(−/−) mice (n=2) were administered a single dose of 20 μL 7.2 μg/μL rhTPP1 and killed 48 hours afterwards. Age matched wildtype mice (n=2) were included as control. TPP1 activity in brain is measured with (open bar) or without pre-activation (grey bar) and normalized to the wildtype control. (b) fifteen-week old Tpp1(−/−) mice were administered 3 daily dose of artificial CSF (V, n=4) or 80 μL 10 μg/μL rhTPP1 (E, n=6). After 48 hours, mice were killed and TPP1 was detected in brain homogenates by western blotting using a rabbit polyclonal anti-TPP1 antibody. Samples were normalized to protein amount (7 μg per lane) and an age matched wildtype mouse was included as control (wt). Positions of in vitro activated rhTPP1 (act) and non-activated proTPP1 (pro) were also shown. Note that there is a small difference in the migration of TPP1 activated in vitro and in vivo, which probably reflects the processing of N-linked glycans within the lysosome.
Montage image of TPP1 distribution. Fifteen week-old Tpp1(−/−) mice were administered three daily 80 μL infusions of artificial CSF (vehicle) or 10 μg/μL rhTPP1 (enzyme) and killed 24 hours after the final dose. Sagittal sections immunostained with anti-TPP1 monoclonal antibody and Alexafluor 555 conjugated donkey-anti-mouse IgG were visualized using a Nikon Eclipse 80i with a 4x objective.
Effect of Intrathecally administrated rhTPP1 on SCMAS storage in liver. Nineteen week-old Tpp1(−/−) mice were administered three daily 80 μL infusions of artificial CSF (V1-2) or 10 μg/μL rhTPP1 (E1-3) and killed 24 hours after the final dose. Relative intensities (mean ± standard deviation) of SCMAS staining in liver for vehicle-treated (n=2), enzyme-treated (n=3), and wild-type age controls (n=2), respectively, were as follows: 1.00±0.10, 0.48±0.06, 0.30±0.01. Note that this signal includes both normal mitochondrial and stored SCMAS.
Uptake of intrathecally administrated rhTPP1 in young mice. Four-week old Tpp1(−/−) mice (n=4) were administered three daily 40 μL infusions of 10 μg/μL rhTPP1 (Enzyme) and killed 24 hours after the final dose. TPP1 activity in brain was measured and compared to the levels in wild-type animals (WT-adult: wild-type, ∼12 weeks old; WT-young: wild-type mice, ∼4 weeks old).
Gait analysis. Representative patterns are shown for animals with n=3-7 animals per time-point for each cohort except for the vehicle-treated group where only a single animal survived to eighteen weeks. Purple and orange represent fore- and hind-limb placement, respectively. Contrast is optimized for each individual image due to differences in inking.
Effect of intravenous administration on survival. Four-week old Tpp1(−/−) mice were administered three daily 40 μL infusions of artificial CSF (vehicle) (n=9) or 10 μg/μL rhTPP1 (enzyme) (n=10). Survival date from untreated Tpp1(−/−) mice (Figure 5) represents a historical control. Median survival was: vehicle, 134 days; enzyme, 106 days; untreated, 124.5 days.
15-week old Tpp1(−/−) mouse treated with enzyme as described in Figure 5b.
15-week old Tpp1(−/−) mouse treated with vehicle as described in Figure 5b.
19-week old Tpp1(−/−) mouse treated with enzyme as described in Figure 5b.
19-week old untreated Tpp1(−/−) mouse. Note that no vehicle treated mice survived to this age.
REFERENCES
- Sleat DE, Donnelly RJ, Lackland H, Liu CG, Sohar I, Pullarkat RK.et al. (1997Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis Science 2771802–1805. [DOI] [PubMed] [Google Scholar]
- Vines DJ., and, Warburton MJ. Classical late infantile neuronal ceroid lipofuscinosis fibroblasts are deficient in lysosomal tripeptidyl peptidase I. FEBS Lett. 1999;443:131–135. doi: 10.1016/s0014-5793(98)01683-4. [DOI] [PubMed] [Google Scholar]
- Palmer DN, Fearnley IM, Walker JE, Hall NA, Lake BD, Wolfe LS.et al. (1992Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease) Am J Med Genet 42561–567. [DOI] [PubMed] [Google Scholar]
- Steinfeld R, Heim P, von Gregory H, Meyer K, Ullrich K, Goebel HH.et al. (2002Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations Am J Med Genet 112347–354. [DOI] [PubMed] [Google Scholar]
- Worgall S, Kekatpure MV, Heier L, Ballon D, Dyke JP, Shungu D.et al. (2007Neurological deterioration in late infantile neuronal ceroid lipofuscinosis Neurology 69521–535. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Gin RM, Sohar I, Wisniewski K, Sklower-Brooks S, Pullarkat RK.et al. (1999Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder Am J Hum Genet 641511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Wiseman JA, El-Banna M, Kim KH, Mao Q, Price S.et al. (2004A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration J Neurosci 249117–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini MA, Dodge JC, Bu J, Yang W, Zhao Q, Sondhi D.et al. (2006Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis J Neurosci 261334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Salazar MA, Roskelley EM, Bu J, Hodges BL, Yew N, Dodge JC.et al. (2007Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease Mol Ther 151782–1788. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM.et al. (2007Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector Mol Ther 15481–491. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Peterson DA, Edelstein AM, del Fierro K, Hackett NR., and, Crystal RG. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp Neurol. 2008;213:18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N.et al. (2008Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA Hum Gene Ther 19463–474. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Zhi Rao H, Passini MA, Curtis M, Vanier MT, Zaka M.et al. (2005AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy Mol Ther 11734–744. [DOI] [PubMed] [Google Scholar]
- Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, Wenger DA.et al. (2005AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2 Mol Ther 12422–430. [DOI] [PubMed] [Google Scholar]
- Karolewski BA., and, Wolfe JH. Genetic correction of the fetal brain increases the lifespan of mice with the severe multisystemic disease mucopolysaccharidosis type VII. Mol Ther. 2006;14:14–24. doi: 10.1016/j.ymthe.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Connelly JP., and, Pruett SM. A look to future directions in gene therapy research for monogenic diseases. PLoS Genet. 2006;2:e133. doi: 10.1371/journal.pgen.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley SL., and, Sands MS. Promising CNS-directed enzyme replacement therapy for lysosomal storage diseases. Exp Neurol. 2009;218:5–8. doi: 10.1016/j.expneurol.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- Muenzer J, Gucsavas-Calikoglu M, McCandless SE, Schuetz TJ., and, Kimura A. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol Genet Metab. 2007;90:329–337. doi: 10.1016/j.ymgme.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Levy B, Galvin N, Tan Y., and, Sly WS. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., and, Pardridge WM. Delivery of beta-galactosidase to mouse brain via the blood-brain barrier transferrin receptor. J Pharmacol Exp Ther. 2005;313:1075–1081. doi: 10.1124/jpet.104.082974. [DOI] [PubMed] [Google Scholar]
- Spencer BJ., and, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci USA. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PI. Novel treatments and future perspectives: outcomes of intrathecal drug delivery. Int J Clin Pharmacol Ther. 2009;47 Suppl 1:S124–S127. [PubMed] [Google Scholar]
- Hemsley KM., and, Hopwood JJ. Delivery of recombinant proteins via the cerebrospinal fluid as a therapy option for neurodegenerative lysosomal storage diseases. Int J Clin Pharmacol Ther. 2009;47 Suppl 1:S118–S123. doi: 10.5414/cpp47118. [DOI] [PubMed] [Google Scholar]
- Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA.et al. (2008Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis Mol Ther 16649–656. [DOI] [PubMed] [Google Scholar]
- Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P.et al. (2004Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I Mol Genet Metab 83163–174. [DOI] [PubMed] [Google Scholar]
- Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M.et al. (2007Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid Mol Genet Metab 9161–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley KM, King B., and, Hopwood JJ. Injection of recombinant human sulfamidase into the CSF via the cerebellomedullary cistern in MPS IIIA mice. Mol Genet Metab. 2007;90:313–328. doi: 10.1016/j.ymgme.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hylden JL., and, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Kovács KJ., and, Larson AA. Chronic daily intrathecal injections of a large volume of fluid increase mast cells in the thalamus of mice. Brain Res. 2005;1056:76–84. doi: 10.1016/j.brainres.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS., and, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer AG., and, Gaillard PJ. Strategies to improve drug delivery across the blood-brain barrier. Clin Pharmacokinet. 2007;46:553–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- Lee WC, Tsoi YK, Troendle FJ, DeLucia MW, Ahmed Z, Dicky CA.et al. (2007Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy FASEB J 212520–2527. [DOI] [PubMed] [Google Scholar]
- Vite CH, Wang P, Patel RT, Walton RM, Walkley SU, Sellers RS.et al. (2011Biodistribution and pharmacodynamics of recombinant human alpha-l-iduronidase (rhIDU) in mucopolysaccharidosis type I-affected cats following multiple intrathecal administrations Mol Genet Metab 103268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA., 3rd, , Klinge PM, Brinker T, Stopa EG., and, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji D, Akeboshi H, Matsuoka K, Yasuoka H, Miyasaki E, Kasahara Y.et al. (2011Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis Ann Neurol 69691–701. [DOI] [PubMed] [Google Scholar]
- Lee WC, Courtenay A, Troendle FJ, Stallings-Mann ML, Dickey CA, DeLucia MW.et al. (2005Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy FASEB J 191549–1551. [DOI] [PubMed] [Google Scholar]
- Sleat DE, El-Banna M, Sohar I, Kim KH, Dobrenis K, Walkley SU.et al. (2008Residual levels of tripeptidyl-peptidase I activity dramatically ameliorate disease in late-infantile neuronal ceroid lipofuscinosis Mol Genet Metab 94222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL.et al. (2007Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease Neurology 6899–109. [DOI] [PubMed] [Google Scholar]
- Malbora B, Ozyurek E, Kocum AI., and, Ozbek N. Delayed recognition of intrathecal methotrexate overdose. J Pediatr Hematol Oncol. 2009;31:352–354. doi: 10.1097/MPH.0b013e3181914709. [DOI] [PubMed] [Google Scholar]
- Jakobson AM, Kreuger A, Mortimer O, Henningsson S, Seidel H., and, Moe PJ. Cerebrospinal fluid exchange after intrathecal methotrexate overdose. A report of two cases. Acta Paediatr. 1992;81:359–361. doi: 10.1111/j.1651-2227.1992.tb12244.x. [DOI] [PubMed] [Google Scholar]
- Lin L., and, Lobel P. Production and characterization of recombinant human CLN2 protein for enzyme-replacement therapy in late infantile neuronal ceroid lipofuscinosis. Biochem J. 2001;357 Pt 1:49–55. doi: 10.1042/0264-6021:3570049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WP, Xu XJ., and, Hao JX. Chronic lumbar catheterization of the spinal subarachnoid space in mice. J Neurosci Methods. 2004;133:65–69. doi: 10.1016/j.jneumeth.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Sohar I, Lin L., and, Lobel P. Enzyme-based diagnosis of classical late infantile neuronal ceroid lipofuscinosis: comparison of tripeptidyl peptidase I and pepstatin-insensitive protease assays. Clin Chem. 2000;46:1005–1008. [PubMed] [Google Scholar]
- Ryazantsev S, Yu WH, Zhao HZ, Neufeld EF., and, Ohmi K. Lysosomal accumulation of SCMAS (subunit c of mitochondrial ATP synthase) in neurons of the mouse model of mucopolysaccharidosis III B. Mol Genet Metab. 2007;90:393–401. doi: 10.1016/j.ymgme.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Sleat DE, Jadot M., and, Lobel P. Glial fibrillary acidic protein is elevated in the lysosomal storage disease classical late-infantile neuronal ceroid lipofuscinosis, but is not a component of the storage material. Biochem J. 2010;428:355–362. doi: 10.1042/BJ20100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuizon S, DiMaiuta K, Walus M, Jenkins EC, Jr, Kuizon M, Kida E.et al. (2010A critical tryptophan and Ca2+ in activation and catalysis of TPPI, the enzyme deficient in classic late-infantile neuronal ceroid lipofuscinosis PLoS ONE 5e11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Labattu B., and, Tison F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods. 2002;113:123–130. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TPP1 proenzyme is activated after delivery into brain. (a) fifteen-week old Tpp1(−/−) mice (n=2) were administered a single dose of 20 μL 7.2 μg/μL rhTPP1 and killed 48 hours afterwards. Age matched wildtype mice (n=2) were included as control. TPP1 activity in brain is measured with (open bar) or without pre-activation (grey bar) and normalized to the wildtype control. (b) fifteen-week old Tpp1(−/−) mice were administered 3 daily dose of artificial CSF (V, n=4) or 80 μL 10 μg/μL rhTPP1 (E, n=6). After 48 hours, mice were killed and TPP1 was detected in brain homogenates by western blotting using a rabbit polyclonal anti-TPP1 antibody. Samples were normalized to protein amount (7 μg per lane) and an age matched wildtype mouse was included as control (wt). Positions of in vitro activated rhTPP1 (act) and non-activated proTPP1 (pro) were also shown. Note that there is a small difference in the migration of TPP1 activated in vitro and in vivo, which probably reflects the processing of N-linked glycans within the lysosome.
Montage image of TPP1 distribution. Fifteen week-old Tpp1(−/−) mice were administered three daily 80 μL infusions of artificial CSF (vehicle) or 10 μg/μL rhTPP1 (enzyme) and killed 24 hours after the final dose. Sagittal sections immunostained with anti-TPP1 monoclonal antibody and Alexafluor 555 conjugated donkey-anti-mouse IgG were visualized using a Nikon Eclipse 80i with a 4x objective.
Effect of Intrathecally administrated rhTPP1 on SCMAS storage in liver. Nineteen week-old Tpp1(−/−) mice were administered three daily 80 μL infusions of artificial CSF (V1-2) or 10 μg/μL rhTPP1 (E1-3) and killed 24 hours after the final dose. Relative intensities (mean ± standard deviation) of SCMAS staining in liver for vehicle-treated (n=2), enzyme-treated (n=3), and wild-type age controls (n=2), respectively, were as follows: 1.00±0.10, 0.48±0.06, 0.30±0.01. Note that this signal includes both normal mitochondrial and stored SCMAS.
Uptake of intrathecally administrated rhTPP1 in young mice. Four-week old Tpp1(−/−) mice (n=4) were administered three daily 40 μL infusions of 10 μg/μL rhTPP1 (Enzyme) and killed 24 hours after the final dose. TPP1 activity in brain was measured and compared to the levels in wild-type animals (WT-adult: wild-type, ∼12 weeks old; WT-young: wild-type mice, ∼4 weeks old).
Gait analysis. Representative patterns are shown for animals with n=3-7 animals per time-point for each cohort except for the vehicle-treated group where only a single animal survived to eighteen weeks. Purple and orange represent fore- and hind-limb placement, respectively. Contrast is optimized for each individual image due to differences in inking.
Effect of intravenous administration on survival. Four-week old Tpp1(−/−) mice were administered three daily 40 μL infusions of artificial CSF (vehicle) (n=9) or 10 μg/μL rhTPP1 (enzyme) (n=10). Survival date from untreated Tpp1(−/−) mice (Figure 5) represents a historical control. Median survival was: vehicle, 134 days; enzyme, 106 days; untreated, 124.5 days.
15-week old Tpp1(−/−) mouse treated with enzyme as described in Figure 5b.
15-week old Tpp1(−/−) mouse treated with vehicle as described in Figure 5b.
19-week old Tpp1(−/−) mouse treated with enzyme as described in Figure 5b.
19-week old untreated Tpp1(−/−) mouse. Note that no vehicle treated mice survived to this age.