Abstract
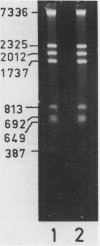
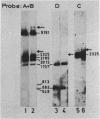
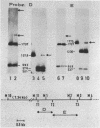
The pattern of methylation of mouse mitochondrial DNA (mtDNA) was studied using several techniques. By employing a sensitive analytical procedure it was possible to show that this DNA contains the modified base 5-methylcytosine (m5Cyt). This residue occurred exclusively at the dinucleotide sequence CpG at a frequency of 3 to 5%. The pattern of methylation was further investigated by determining the state of methylation of several MspI (HpaII) sites. Different sites were found to be methylated to a different extent, implying that methylation of mtDNA is nonrandom. Based on the known base composition and nucleotide sequence of mouse mtDNA, the dinucleotide sequence CpG was found to be underrepresented in this DNA. The features of mtDNA methylation (CpG methylation, partial methylation of specific sites and CpG underrepresentation) are also characteristic of vertebrate nuclear DNA. This resemblance may reflect functional relationship between the mitochondrial and nuclear genomes.
Full text
PDF



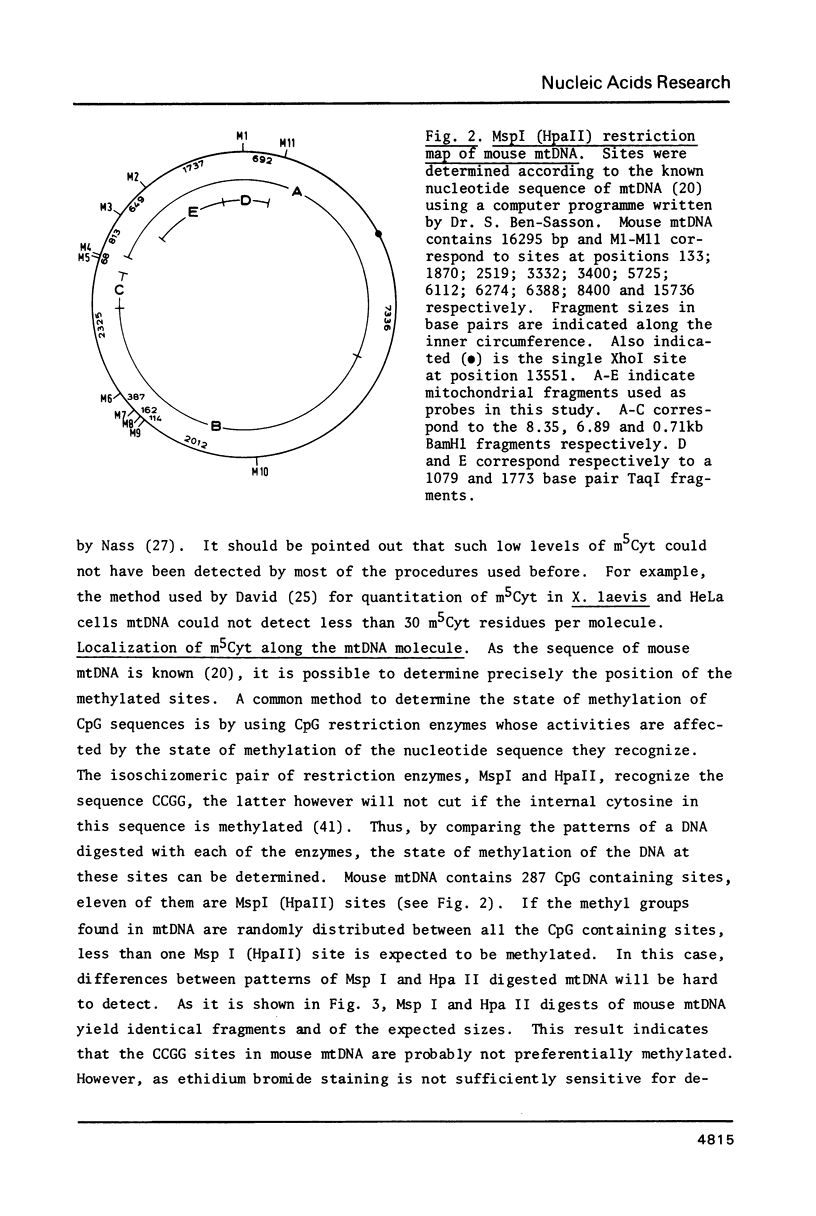





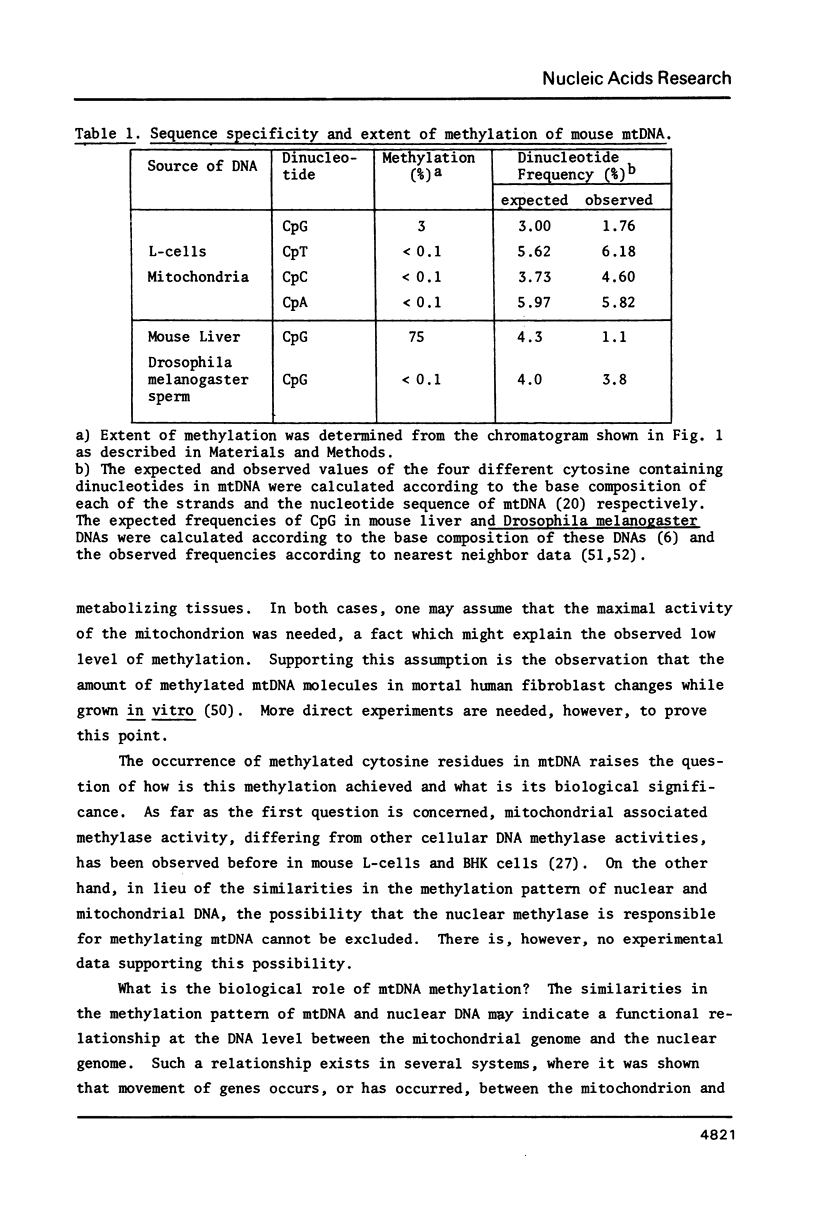



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Bonen L., Huh T. Y., Gray M. W. Can partial methylation explain the complex fragment patterns observed when plant mitochondrial DNA is cleaved with restriction endonucleases? FEBS Lett. 1980 Mar 10;111(2):340–346. doi: 10.1016/0014-5793(80)80823-4. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Grabowy C. T., Sager R. Role of methylation in the modification and restriction of chloroplast DNA in Chlamydomonas. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1390–1394. doi: 10.1073/pnas.76.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Tait A., Goddard J. M. Methylated bases in DNA from Paramecium aurelia. Biochim Biophys Acta. 1974 Nov 20;374(1):1–11. doi: 10.1016/0005-2787(74)90194-4. [DOI] [PubMed] [Google Scholar]
- DOSKOCIL J., SORMO'VA Z. THE OCCURRENCE OF 5-METHYLCYTOSINE IN BACTERIAL DEOXYRIBONUCLEIC ACIDS. Biochim Biophys Acta. 1965 Mar 15;95:513–515. [PubMed] [Google Scholar]
- Dawid I. B. 5-methylcytidylic acid: absence from mitochondrial DNA of frogs and HeLa cells. Science. 1974 Apr 5;184(4132):80–81. doi: 10.1126/science.184.4132.80. [DOI] [PubMed] [Google Scholar]
- Deumling B. Sequence arrangement of a highly methylated satellite DNA of a plant, Scilla: A tandemly repeated inverted repeat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):338–342. doi: 10.1073/pnas.78.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Blakesley R. Guide to the use of type II restriction endonucleases. Methods Enzymol. 1983;100:3–38. doi: 10.1016/0076-6879(83)00043-9. [DOI] [PubMed] [Google Scholar]
- Gellissen G., Bradfield J. Y., White B. N., Wyatt G. R. Mitochondrial DNA sequences in the nuclear genome of a locust. Nature. 1983 Feb 17;301(5901):631–634. doi: 10.1038/301631a0. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Naveh-Many T., Cedar H., Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981 Aug 27;292(5826):860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Stein R., Cedar H., Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett. 1981 Feb 9;124(1):67–71. doi: 10.1016/0014-5793(81)80055-5. [DOI] [PubMed] [Google Scholar]
- Haberman A., Heywood J., Meselson M. DNA modification methylase activity of Escherichia coli restriction endonucleases K and P. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3138–3141. doi: 10.1073/pnas.69.11.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadler H. I., Dimitrijevic B., Mahalingam R. Mitochondrial DNA and nuclear DNA from normal rat liver have a common sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6495–6499. doi: 10.1073/pnas.80.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Jacobs H. T., Posakony J. W., Grula J. W., Roberts J. W., Xin J. H., Britten R. J., Davidson E. H. Mitochondrial DNA sequences in the nuclear genome of Strongylocentrotus purpuratus. J Mol Biol. 1983 Apr 25;165(4):609–632. doi: 10.1016/s0022-2836(83)80270-8. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Kühnlein U., Linn S., Arber W. Host specificity of DNA produced by Escherichia coli. XI. In vitro modification of phage fd replicative form. Proc Natl Acad Sci U S A. 1969 Jun;63(2):556–562. doi: 10.1073/pnas.63.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Biological function for 6-methyladenine residues in the DNA of Escherichia coli K12. J Mol Biol. 1974 May 15;85(2):309–322. doi: 10.1016/0022-2836(74)90366-0. [DOI] [PubMed] [Google Scholar]
- McClelland M., Ivarie R. Asymmetrical distribution of CpG in an 'average' mammalian gene. Nucleic Acids Res. 1982 Dec 11;10(23):7865–7877. doi: 10.1093/nar/10.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J., Christianson T., Levens D., Rabinowitz M., Attardi G. Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7195–7199. doi: 10.1073/pnas.79.23.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J., Gaines G. L., Attardi G. The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell. 1983 Aug;34(1):151–159. doi: 10.1016/0092-8674(83)90145-9. [DOI] [PubMed] [Google Scholar]
- Nagley P., Clayton D. A. Transcriptional mapping of the ribosomal RNA region of mouse L-cell mitochondrial DNA. Nucleic Acids Res. 1980 Jul 11;8(13):2947–2965. doi: 10.1093/nar/8.13.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Differential methylation of mitochondrial and nuclear DNA in cultured mouse, hamster and virus-transformed hamster cells. In vivo and in vitro methylation. J Mol Biol. 1973 Oct 15;80(1):155–175. doi: 10.1016/0022-2836(73)90239-8. [DOI] [PubMed] [Google Scholar]
- Razin A., Friedman J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res Mol Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Razin A., Urieli S., Pollack Y., Gruenbaum Y., Glaser G. Studies on the biological role of dna methylation; IV. Mode of methylation of DNA in E. coli cells. Nucleic Acids Res. 1980 Apr 25;8(8):1783–1792. doi: 10.1093/nar/8.8.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L. The action of pancreatic deoxyribonuclease. II. Isomeric dinucleotides. J Biol Chem. 1955 Aug;215(2):579–583. [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Mitochondrial DNA in mortal and immortal human cells. Genome number, integrity, and methylation. J Biol Chem. 1983 Aug 10;258(15):9078–9085. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Szyf M., Gruenbaum Y., Urieli-Shoval S., Razin A. Studies on the biological role of DNA methylation: V. The pattern of E.coli DNA methylation. Nucleic Acids Res. 1982 Nov 25;10(22):7247–7259. doi: 10.1093/nar/10.22.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Kirnos M. D. Structure of animal mitochondrial DNA (base composition, pyrimidine clusters, character of methylation). Biochim Biophys Acta. 1977 Mar 18;475(2):323–336. doi: 10.1016/0005-2787(77)90023-5. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Kirnos M. D. The nucleotide composition and pyrimidine clusters in DNA from beef heart mitochondria. FEBS Lett. 1974 Feb 15;39(2):195–199. doi: 10.1016/0014-5793(74)80049-9. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. Recognition and estimation of 5-methylcytosine in nucleic acids. Biochem J. 1951 May;48(5):581–584. doi: 10.1042/bj0480581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. M., Cummings D. J. Integration of mitochondrial gene sequences within the nuclear genome during senescence in a fungus. Nature. 1983 Mar 3;302(5903):86–88. doi: 10.1038/302086a0. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]
- van den Boogaart P., Samallo J., Agsteribbe E. Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature. 1982 Jul 8;298(5870):187–189. doi: 10.1038/298187a0. [DOI] [PubMed] [Google Scholar]