Abstract
BACKGROUND AND PURPOSE
Anthelmintics are required for treatment and prophylaxis of nematode parasites of humans and domestic animals. Emodepside, a cyclooctadepsipeptide, is a modern anthelmintic that has a novel mode of action involving a Ca-activated K channel (SLO-1) in Caenorhabditis elegans, sometimes mediated by a latrophilin (LAT) receptor. We examined mechanisms of action of emodepside in a parasitic nematode, Ascaris suum.
EXPERIMENTAL APPROACH
RT-PCR was used to investigate expression of slo-1 and lat-1 in A. suum muscle flaps, and two-micropipette current-clamp and voltage-clamp techniques were used to record electrophysiological effects of emodepside.
KEY RESULTS
Expression of slo-1 and lat-1 were detected. Emodepside produced a slow time-dependent (20 min), 4-aminopyridine sensitive, concentration-dependent hyperpolarization and increase in voltage-activated K currents. Sodium nitroprusside increased the hyperpolarizations and K currents. N-nitro-L-arginine inhibited the hyperpolarizations and K currents. Phorbol-12-myristate-13 acetate increased the K currents, while staurosporine inhibited the hyperpolarizations and K currents. Iberiotoxin reduced these emodepside K currents. The effect of emodepside was reduced in Ca-free solutions. Emodepside had no effect on voltage-activated Ca currents.
CONCLUSIONS AND IMPLICATIONS
Asu-slo-1 and Asu-lat-1 are expressed in adult A. suum muscle flaps and emodepside produces slow activation of voltage-activated Ca-dependent SLO-1-like K channels. The effect of emodepside was enhanced by stimulation of protein kinase C and NO pathways. The data are consistent with a model in which NO, PKC and emodepside signalling pathways are separate and converge on the K channels, or in which emodepside activates NO and PKC signalling pathways to increase opening of the K channels.
Keywords: emodepside, Ascaris suum, mode of action, membrane potential, K currents, SLO-1, LAT-1, NO, PKC, 4-aminopyridine, iberiotoxin, Ca currents
Introduction
Parasitic nematode infections, classed as neglected tropical diseases, remain a problem in both human and veterinary medicine. The estimated global prevalence of parasitic nematode infections in humans is over two billion and the global prevalence of ascariasis alone is over 800 million (Hotez et al., 2007; 2008;). These parasites are debilitating to humans, causing lost work days, cognitive impairment and poor growth; they also depress agricultural production and food supply. Ascariasis is caused by Ascaris lumbricoides in humans: this parasite is nearly identical to the parasite, Ascaris suum of pigs, which is a good model of the human parasite, A. lumbricoides. In the absence of effective vaccines and adequate sanitation, anthelmintic drugs are used for treatment and prophylaxis. However, there have been reports of growing resistance to the classic anthelmintic drugs: benzimidazoles (albendazole), nicotinic agonists (levamisole/pyrantel) and macrocyclic lactones (ivermectin) (Prichard, 1990; 1994; Sangster and Gill, 1999; Sangster, 2001; Kaplan, 2002; Wolstenholme et al., 2004; Martin and Robertson, 2007; James et al., 2009). More recently, novel ‘resistance-busting’ anthelmintics (emodepside, a cyclooctadepsipeptide; monepantel, an amino-acetonitrile derivative, and derquantel, a paraherquamide derivative) have been released into the market. The need for the new anthelmintics and ways to combat resistance to the currently available anthelmintics cannot be overstated.
Sasaki et al. (1992) reported the isolation of the cyclooctadepsipeptide PF1022A from cultured Mycelia sterilia, a fungus found on the leaves of Camellia japonica. Emodepside is a semisynthetic analogue of PF1022A made by attaching two morpholine rings at the para-position of the two phenyllactic acids (Harder et al., 2005) in order to enhance pharmacokinetic properties. Both emodepside and PF1022A have been shown to be effective against benzimidazole-, levamisole- and ivermectin-resistant nematode parasite isolates (Samson-Himmelstjerna von et al., 2005), and to be effective against a wide range of nematode parasites (Schurmann et al., 2007).
Willson et al. (2003) reported that in A. suum, emodepside caused muscle relaxation and a 4-aminopyridine sensitive, Ca-dependent hyperpolarization of body wall muscle cells. The similarity of this response to that of the inhibitory neuropeptides PF1/PF2 led to the suggestion that emodepside may be acting at the neuromuscular junction to release an inhibitory neuropeptide with similar action to the neuropeptides PF1/PF2.
Two molecular effectors have been implicated in the mode of action of emodepside. The first, a latrophilin-like receptor, HC110-R, co-precipitated with PF1022A and was cloned from Haemonchus contortus (Saeger et al., 2001). Here, it is interesting to note that FMRFamide-like neuropeptides like PF2 have been tested and found to act as ligands of HC110-R (Muhlfeld et al., 2009). However, observations using latrophilin gene knockouts in Caenorhabditis elegans, found that emodepside still depressed locomotion but that, in the pharyngeal muscle, inhibitory effects were reduced (Willson et al., 2003; Guest et al., 2007). The observations suggest that a latrophilin receptor is involved in the response to emodepside in the pharyngeal feeding circuit, but that other molecular effectors are also involved in the inhibition of locomotion. The second emodepside effector was identified in mutagenesis screens in C. elegans. These delivered multiple alleles of the voltage-gated Ca-sensitive K channel gene, slo-1, such that slo-1 loss-of-function mutants were discovered to be resistant to the pleiotropic effects of emodepside on reproduction, locomotion and feeding.
Effects of emodepside on the voltage-activated currents in a parasitic nematode have not been studied. Here, we find evidence of the expression of SLO-1 K channels [Slo (BK) K channels, Alexander et al. 2009] and LAT-1 protein, and we have used current- and voltage-clamp electrophysiological techniques to investigate the mode of action of emodepside on membrane potential and voltage-activated currents in A. suum, as an example of a parasitic nematode. We find that emodepside activated K currents with SLO-1-like properties with effects being enhanced by protein kinase C (PKC) and NO signalling pathways.
Methods
Collection and maintenance of worms
A. suum were collected weekly from Tyson Foods Inc. pork packing plant, Flindt Drive, Storm Lake, IA, USA and from JBS Swift and Co. pork processing plant, Marshalltown, IA, USA. The adult worms were kept in Locke's solution (NaCl 155 mM, KCl 5 mM, CaCl2 2 mM, NaHCO3 1.5 mM, glucose 5 mM) at 32°C. The solution was changed twice a day, and the worms were used within 4 days of collection.
Sequence and gene expression analysis
Database searches were performed with the BLAST Network Service (NCBI), using the tBLASTn algorithm (Altschul et al., 1997). Signal peptide predictions were carried out using the Signal P 3.0 server (Bendtsen et al., 2004). Conserved protein domains, as well as membrane-spanning regions were predicted using the SMART program (Schultz et al., 1998). Total RNA was prepared from body muscle flaps (see below) from A. suum adult worms using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's recommendations.
RNA pellets were dissolved in 100 µL of RNAsecure resuspension solution (Ambion, Austin, TX, USA) and were DNase treated with the TURBO DNA-free kit (Ambion). The RNA concentration was measured using a nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). First-strand cDNA synthesis was performed on 5 µg of total RNA using the oligo (dT) RACER primer (Invitrogen) and the superscript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Reverse transcription-PCR experiments were carried out on first strand cDNA using two rounds of nested PCR, in a final volume of 20 µL, containing 200 ng of first-strand cDNA (or 1 µL of 1/100 dilution of amplification product from the first round of PCR), 1 U of GoTaq polymerase (Promega, Charbonnieres, France), 0.25 mmol·L−1 dNTPs each and 0.3 µmol·L−1 of each primer. Primer sequences used for RT-PCR experiments are provided in Supporting Information Table S1. The reaction mixture was denatured by heating at 94°C for 5 min, followed by 33 cycles at 94°C for 45 s, 56°C for 45 s and 72°C for 45 s. A final extension step was performed at 72°C for 5 min. Amplification products were cloned in pGEM-T vector (Promega) and sequence-checked by GATC Biotech (Konstanz, Germany).
Somatic muscle preparation
The anterior portion of the worm about 4 cm away from the head was used in all recordings. About 1 cm of this part of the worm was cut-out and the resulting cylindrical worm piece was cut open along a lateral line to form a muscle flap. The gut was removed to expose the muscle cells and the muscle flap was pinned onto a 35 × 10 mm Petri-dish lined with Sylgard and containing the perfusion solution. The preparation was placed in the experimental chamber and perfused with low-potassium Ascaris perienteric fluid (APF-Ringer) solution (in mM: NaCl 23, Na acetate 110, KCl 3, CaCl2 6, MgCl2 5, glucose 11, HEPES 5, pH adjusted to 7.6 with NaOH), unless otherwise stated, at a rate of 4 mL·min−1 through a 20-gauge needle. The perfusion needle was placed directly above the muscle bag from which recordings were made. The preparation in the experimental chamber was kept at 34°C by using a Warner heating collar (DH 35) and heating the perfusate with a Warner SH-27B inline heater (Hamden, CT, USA).
Electrophysiology of somatic muscle
Two micropipette voltage- and current-clamp techniques were used in examining the electrophysiological effects of emodepside on the muscle bag of A. suum (Figure 1A). The micropipettes, made of borosilicate capillary glass with internal diameter 0.86 mm and external diameter 1.50 mm (Harvard Apparatus, Holliston, MA, USA) were pulled on a Flaming Brown micropipette puller (Sutter Instrument Co, Novato, CA, USA). The micropipettes were filled with 3 M potassium acetate to study the membrane potential and K current effects. The voltage-sensing micropipettes had resistances of 20–30 MΩ, but the tip of the current-injecting micropipettes for the current-clamp and voltage-clamp was broken to have a resistance of 3–6 MΩ. The muscle bag was impaled with both micropipettes for all recordings. An Axoclamp 2B amplifier, a 1320A Digidata interface and pCLAMP 8.2 software (Molecular Devices, Sunnyvale, CA, USA) were used in all recordings. In all current- and voltage-clamp recordings, the experiment sets were repeated on different worms to obtain the desired number of observations. In the current-clamp experiments, 40 nA hyperpolarizing pulses were injected for 500 ms at 0.30 Hz through the current-injecting micropipette and the change in membrane potential recorded with the voltage-sensing micropipette (Figure 1B).
Figure 1.
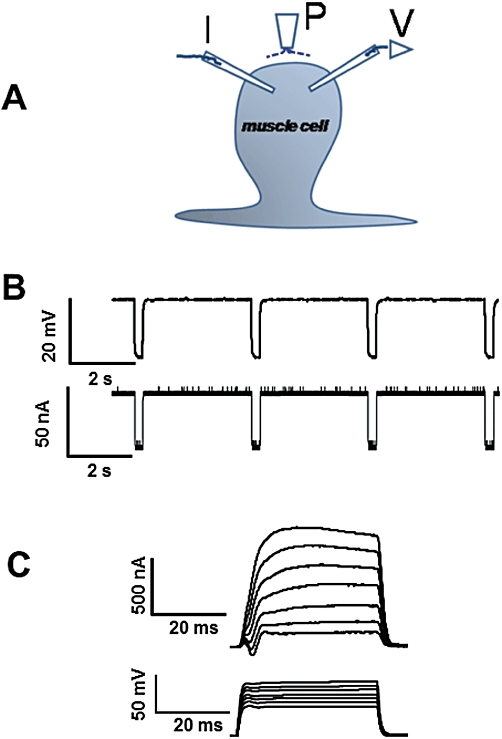
Electrophysiological techniques for recording from Ascaris suum. (A) A. suum muscle bag showing the current (I) and voltage (V) micropipettes in the bag and the perfusion needle (P). (B) Current-clamp protocol used: bottom trace shows the 40 nA currents injected for 500 ms at 0.30 Hz; top trace shows the resting membrane potential (straight line) and the membrane response to the injected current (downward deflections). (C) Voltage-clamp protocol: bottom trace shows the voltage steps from the holding potential of −35 mV to 0 mV and in steps of 5 mV to 30 mV; top trace shows the K currents generated in response to the voltage steps.
In both current- and voltage-clamp experiments, cells close to the nerve cord were used. We kept the amplifier gain high (∼100) and the phase lag low (∼0.15–0.5 ms) to limit oscillations, but adjusted these in some recordings. For activation of the K currents, the muscle bags were held at −35 mV and stepped up to 0, 5, 10, 15, 20, 25 and 30 mV (Figure 1C); the currents lasted for 40 ms. For activation of the Ca currents, the muscle bags were stepped from the holding potential of −35 mV to −25 mV and then in steps of 5 mV up to 20 mV. The Ca currents were isolated from the K currents by using 4-aminopyridine (5 mM) in the perfusion solution and equimolar amounts (1.5 M) of potassium and cesium acetates in the recording micropipettes, and not making large depolarizations (Verma et al., 2009). There was no need to block voltage-activated Na currents in isolating the Ca currents because nematodes lack voltage-activated Na currents. Calcium substitution experiments were performed by replacing calcium in the low-potassium APF-Ringer solution with equimolar cobalt.
The currents were leak-subtracted using pCLAMP 8.2 software. All drugs were initially applied in current clamp and the effects on the voltage-activated currents investigated in voltage-clamp with the drugs still perfusing. Control measurements of membrane potential and K currents were made before drug applications, then at 10 min intervals. Unless otherwise stated, all drugs used to study the mechanisms of action of emodepside were applied for 10 min before co-application for 10 min with emodepside and effects on membrane potential and K current compared with the control at 0 min. The preparation was then washed in drug-free solutions and the wash (post-emodepside) effect measured following a 20 min time period. Our subsequent account is based on recordings from more than 130 muscle cells, each from a separate A. suum preparation. The membrane potentials of the selected cells recorded from were all more negative than −25 mV with stable input conductances <4.0 µS.
Time control experiments to test the stability of effects of NO modulators, PKC modulators and iberiotoxin
We conducted separate time-control experiments, without emodepside, to determine the time-course and stability of effects of the NO and PKC modulators on membrane potential and voltage-activated K currents. We found that sodium nitroprusside (SNP; 1 mM, n = 4), staurosporine (1 µM, n = 3) and N-nitro L-arginine (NNLA; 100 µM, n = 6) had no significant effect (P > 0.05) on membrane potentials or voltage-activated K currents when tested at 10, 20 and 30 min during their application. Phorbol 12-myristate 13-acetate (PMA) produced an increase in voltage-activated K currents and a small hyperpolarization within 5 min of application, and did not show further change. We also found that iberiotoxin (10 nM) had no effect at 10, 20 and 30 min on membrane potential, but iberiotoxin (by 10 min) reduced (n = 3) voltage-activated K currents and this effect was not significantly (P > 0.05) changed at 20 and 30 min.
Data analysis
All acquired data were displayed on a Pentium IV desktop computer. Graph Pad Prism software (version 5.0, San Diego, CA, USA) and Clampfit 9.2 (Molecular Devices) were used for the analysis. We observed the biggest effect of emodepside on the late phase K currents (LK: we measured the average current during the period 30–40 ms after the step potential start) at a step potential of 0 mV. The reversal potential was calculated for voltage-activated K current experiments. To obtain the activation curves, we calculated the conductance changes from the outward K currents and the driving force, Erev–V. The activation curves were then fitted by the Boltzmann equation: G = Gmax/[1 + exp {(V50−V)/Kslope}], where G = conductance, Gmax = maximal conductance change, V50 = half-maximal voltage and Kslope = slope factor.
The current was expressed as percent control and plotted against voltage to determine how the currents changed with each voltage step. For experiments on the same preparation, one-tailed paired Student's t-tests were used to estimate statistical significance, set at P < 0.05. For comparison between effects on different preparations, an unpaired t-test was used. We show mean ± SEM values throughout the text.
To measure the emodepside-sensitive current as a proportion of the 4-aminopyridine sensitive current (at 0 mV) we first measured the control LK current, then the LK current at 10 min after application of emodepside (1 µM): the difference was the current activated by emodepside. Next, we applied 4-aminopyridine (5 mM) for 5 min and again measured the LK current: the difference between the LK current in the presence of 4-aminopyridine and the LK current at 10 min after emodepside was the 4-aminopyridine-sensitive current. The proportion was then determined and expressed as a %
Materials
Emodepside was generously provided by Achim Harder (Bayer HealthCare AG, Leverkusen, Germany). Emodepside stocks of 2 mM and 10 mM in 100% DMSO were prepared every 2 weeks. The working emodepside concentrations of 1 and 10 µM were prepared so that the final DMSO concentration was 0.1%. PF1 (SDPNFLRFamide, EZBiolab, Westfield, IN, USA) stocks of 1 mM were prepared in distilled water every week. Ryanodine, staurosporine, iberiotoxin and PMA were obtained from Tocris Biosciences (Ellisville, MO, USA); 2 mM stock ryanodine was made every week. All stock concentrations were kept at −20°C, thawed and used once. Glucose, 4-aminopyridine, SNP and NNLA were obtained from Sigma-Aldrich (St. Louis, MO USA). HEPES was obtained from Calbiochem, EMD Biosciences Inc. (La Jolla, CA, USA). All other chemicals were obtained from Fisher Scientific (Fair Lawn, NJ, USA).
Results
Slo-1 and lat-1 homologous genes from A. suum are expressed at the adult stage of A. suum
Using C. elegans slo-1 and lat-1 deduced amino-acid sequences for query, databank searches revealed homologous A. suum sequences for both genes. The alignments of C. elegans SLO-1 and LAT-1 sequences with their respective A. suum counterparts are presented in Figure 2. A. suum slo-1 cDNA sequence (GenBank accession no. ACC68842.1) encodes a putative protein of 1117 amino-acids, sharing 78% identity and 87% similarity with the Cel-SLO-1 sequence. SLO-1 sequences from both nematode species possess seven transmembrane-spanning domains (S0–S6), a P-loop, four hydrophobic intracellular segments (S7–S10) and a ‘Ca2+ bowl’ that typifies large conductance Ca-sensitive K channels (Wallner et al., 1996; Wei et al., 1996; Schreiber and Salkoff, 1997; Lim et al., 1999; Ghatta et al., 2006)
Figure 2.
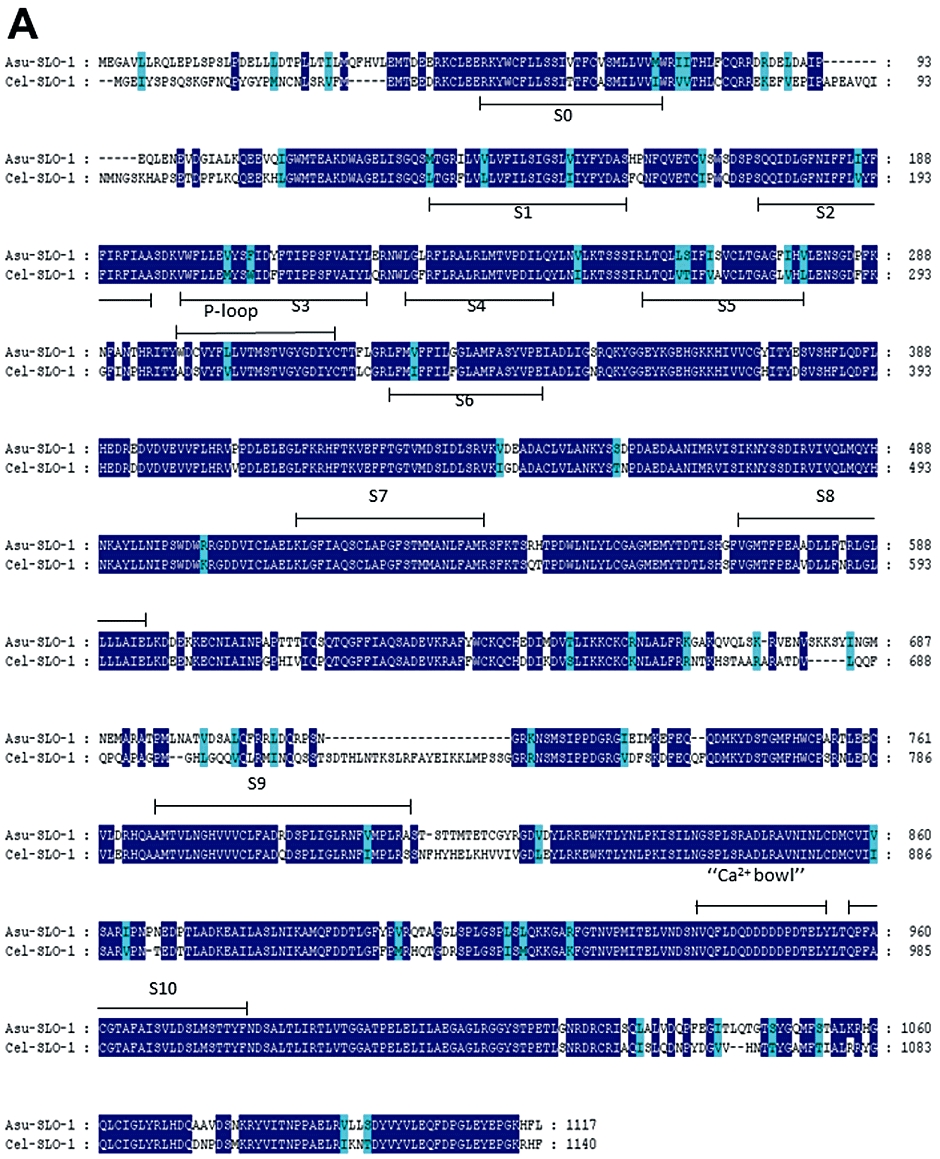
Conservation of SLO-1 and LAT-1 sequences in C. elegans and A. suum. Alignments of deduced amino-acid sequences were constructed using the MUSCLE algorithm (Edgar, 2004) and further processed using GeneDoc program (http://www.nrbsc.org/gfx/genedoc/index.html). Identical amino-acids between C. elegans and A. suum sequences are shaded in dark blue and distinct amino-acids sharing similar physico-chemical properties are shaded in light blue. Predicted signal peptide sequences are shaded in grey. The transmembrane domains (TM) are noted below the sequences. (A) Comparison of Asu-SLO-1 (GenBank accession n° ACC68842.1) and Cel-SLO-1 (GenBank accession n° NP_001024259.1) showing the 7 TM domains (S0–S6), P-loop, S7–S10 intracellular domains and ‘Ca2+ bowl’. Domain annotation corresponds to A. suum SLO-1. (B) Comparison of Asu-LAT-1 (GenBank accession n°ADY40714.1) and Cel-LAT-1 (GenBank accession n°NP_495894.1). Domain annotations correspond to A. suum LAT-1.
The cDNA sequence for A. suum-lat-1 encodes a predicted protein of 986 amino-acids (Figure 2B), which has 38% identity and 54% similarity to the Cel-LAT-1 sequence. Despite this limited conservation at the amino-acid level, the LAT-1 sequences from both nematode species share critical characteristic features: seven transmembrane regions, a galactose-binding lectin domain (PFAM Gal_Lectine: PF02140), a hormone receptor domain (SMART HormR: SM 00008) and two G-protein coupled protein domains (SMART GPS: SM00303 and PFAM 7Tm_2: PF00002).
In order to provide further evidence for the expression of slo-1- and lat-1-like genes in the muscles of A. suum adult worms, we performed a set of RT-PCR experiments using gene-specific primers (Supporting Information Table S1) designed from Asu-slo-1 and Asu-lat-1 cDNA sequences respectively. For both candidates, a single amplification product at the expected size was obtained (Supporting Information Figure S1). Sequencing reactions showed that the amplicons corresponded to Asu-slo-1 and Asu-lat-1, respectively, and confirmed their expression in the muscle flaps of adult A. suum. Evidence for the presence of these two genes encouraged electrophysiological investigation of effects of emodepside.
Emodepside has an inhibitory effect on spiking
Initially, we examined the effects of emodepside on muscle spiking under current-clamp as a way of detecting inhibitory actions of emodepside. We activated spiking by applying 1 µM ryanodine (Puttachary et al., 2010) and compared effects of emodepside with the neuropeptide PF1 because emodepside has been suggested to act by stimulating release of inhibitory neuropeptides like PF1.
Ryanodine (1 µM) produced a slow time-dependent increase in the spike frequency and comparison between the 20 and 35 min period (Figure 3B) shows a significant increase of 15 spikes per minute (P < 0.05, n = 7) in spike frequency. Figure 3A shows a representative trace of the inhibitory effect of emodepside on the ryanodine-induced spiking. Emodepside significantly inhibited the ryanodine increase in spike frequency between the 20 and 35 min period (Figure 3C) by 9.8 spikes·min−1 (P < 0.05, n = 5). We also tested the effect of PF1 (1 µM) on ryanodine spiking and found that it was more potent and faster acting than emodepside as PF1 decreased the ryanodine spike frequency by 46 spikes per minute in a shorter time of 10 min (P < 0.01, n = 5, Figure 3D).
Figure 3.
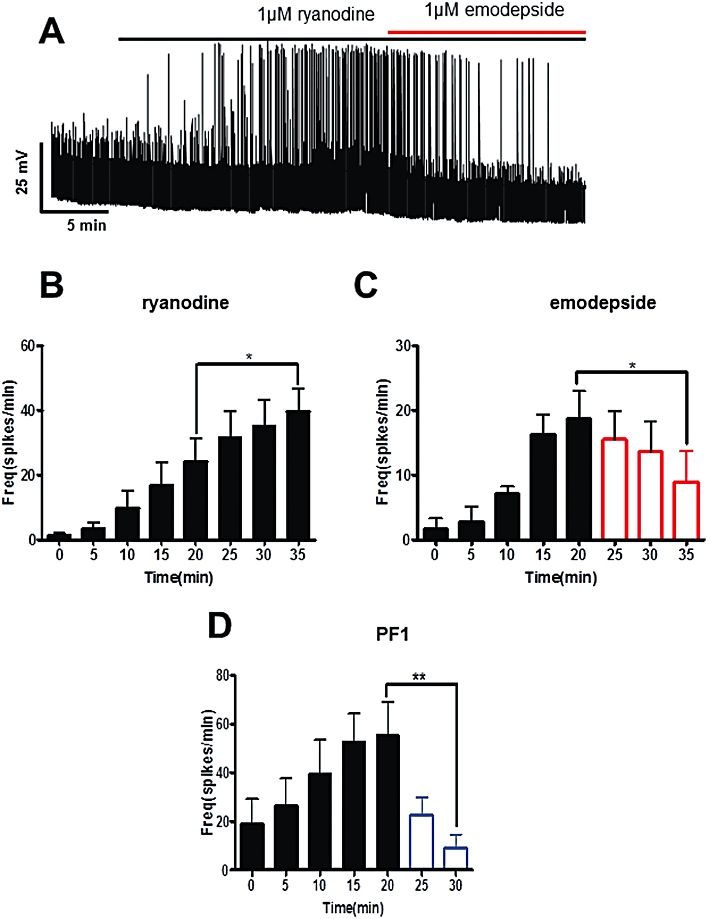
Effect of emodepside (1 µM) on membrane spiking. (A) Representative current-clamp trace showing the spiking induced by ryanodine (1 µM) and the inhibitory effect of emodepside on the spikes. (B) Bar chart (mean ± SEM) of the effects of ryanodine on the frequency of spikes. Ryanodine caused a time-dependent increase in the spike frequency; comparison was made between 20 min and 35 min of ryanodine application (P < 0.05, n = 7, paired t-test). (C) Bar chart (mean ± SEM) of the effects of emodepside on the frequency of ryanodine-induced spikes. Emodepside abolished the increase in spike frequency; comparison was made between 20 min of ryanodine application and 15 min (35 min) of emodepside + ryanodine application (P < 0.05, n = 5, paired t-test). (D) Bar chart (mean ± SEM) of the effects of PF1 (1 µM) on the ryanodine-induced spike frequency. After just 10 min application, PF1 caused a decrease in spike frequency (P < 0.01, n = 5, paired t-test).
Effect of emodepside on membrane potential and input conductance
Figure 4A shows a representative recording of the membrane potential before, during and after application of emodepside (1 µM). Perfusion of emodepside for 10 min produced a slowly developing −5.2 mV hyperpolarization, which continued to increase during drug application; the resting input conductance was 3.5 µS before drug application and was not changed significantly by emodepside. We observed that the onset of membrane hyperpolarization produced by emodepside varied between 30 s to 3 min and the mean hyperpolarization at 10 min is shown in Figure 4B. The hyperpolarization was not reversible but continued to increase gradually despite washing. At a higher concentration of 10 µM emodepside at 10 min, the hyperpolarization was greater (P < 0.001, n = 6, Figure 4B), but there was still no significant change in input conductance. Comparison of the hyperpolarization caused by 1 and 10 µM emodepside showed that the hyperpolarization was concentration-dependent; the difference, −2.6 ± 1.3 mV, was significant (P < 0.05, n = 16). The membrane potentials of some of the muscle cells showed spontaneous small depolarizing potentials which were reduced or completely abolished by the emodepside.
Figure 4.
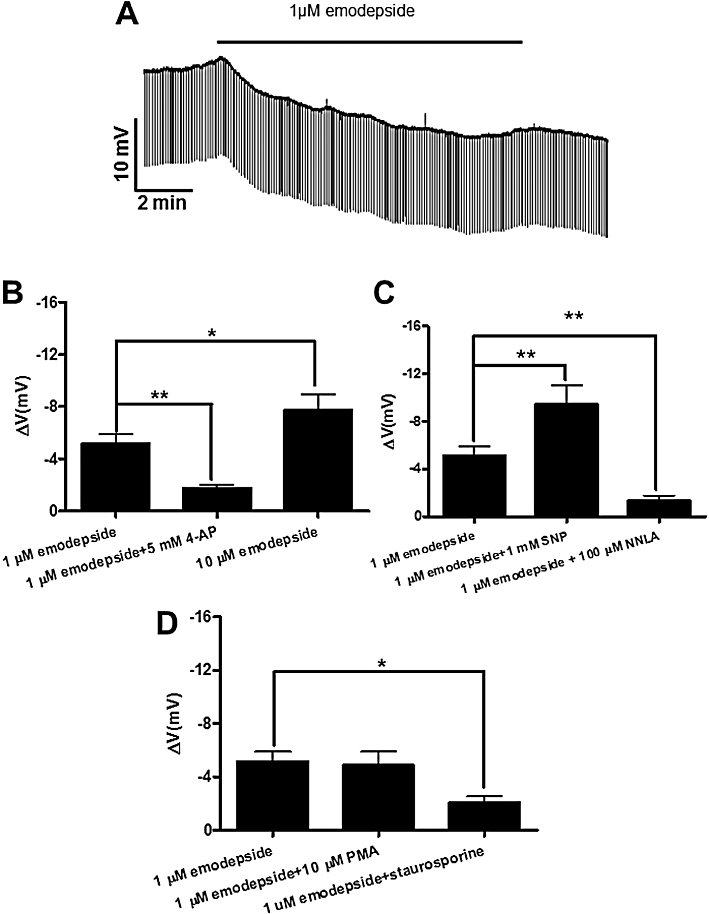
Effect of emodepside (1 µM) on membrane potential. (A) Representative current-clamp trace showing the membrane potential before, during and after 10 min application of 1 µM emodepside. (B) Bar chart (mean ± SEM) of the effects of emodepside on the membrane potential. Comparison was made between membrane potential before and during emodepside application. Emodepside caused a significant membrane hyperpolarization (P < 0.001, n = 10, paired t-test) which was reduced in the presence of 4-aminopyridine (5 mM) (P < 0.01, n = 8, unpaired t-test). Emodepside (10 µM) caused an increased hyperpolarization in comparison to 1 µM emodepside (P < 0.05, unpaired t-test). (C) Bar chart (mean ± SEM) of the effects of NO on emodepside-induced hyperpolarization. In the presence of 1 mM SNP, emodepside (1 µM) caused an increased hyperpolarization (P < 0.01, n = 4, paired t-test). NNLA (100 µM) decreased the hyperpolarization caused by emodepside (P < 0.01, unpaired t-test). (D) Bar chart (mean ± SEM) of the effects of protein kinase modulators on emodepside-induced hyperpolarization. PMA (10 µM) had no significant effects on the hyperpolarization caused by emodepside. However, staurosporine (1 µM) decreased the hyperpolarization caused by emodepside (P < 0.05, unpaired t-test).
To test if the membrane hyperpolarization caused by emodepside was mediated by opening of K channels, we used the K channel blocker, 4-aminopyridine (5 mM), in the perfusion solution throughout the experiment. In the presence of 4-aminopyridine, the mean resting membrane potential was unchanged but the hyperpolarizing effect of emodepside at 10 min was significantly reduced (Figure 4B).
Emodepside effect on membrane potential: role of NO and PKC
The slow hyperpolarizing effect of emodepside and sensitivity to 4-aminopyridine has similarities to the effect of the neuropeptide PF1 in A. suum and the effects of PF1 are mediated by NO synthase (Bowman et al., 1995; 2002; Verma et al., 2009). We therefore tested the effects of NNLA, an inhibitor of inducible NO synthase (iNOS) and SNP, an NO-donor on the actions of emodepside.
In the presence of 100 µM NNLA, the emodepside hyperpolarization was significantly reduced to −1.3 ± 0.44 mV (P < 0.01, n = 6, Figure 4C). In contrast, in the presence of 1 mM SNP, emodepside caused a significantly increased hyperpolarization of −9.5 ± 1.6 mV (P < 0.01, n = 4, Figure 4C). Both of these observations suggested a role for NO in the mediation of the emodepside effects. In time control experiments, NNLA and SNP had no significant effect by themselves on the membrane potential.
We further investigated the role of PKC in mediating the effects of emodepside as emodepside has been suggested to bind to latrophilin-like receptors in C. elegans which can signal through Gq proteins and PKC in other preparations. Staurosporine (1 µM), a broad-spectrum protein kinase inhibitor, significantly reduced the hyperpolarization produced by emodepside (1 µM) to −2.1 ± 0.48 mV (P < 0.05, n = 4, Figure 4D). Staurosporine, by itself, had no significant effects on the membrane potential. The PKC activator, PMA (10 µM), produced a small but significant hyperpolarization (−2.1 ± 0.3 mV, P < 0.05, n = 5). However, in the presence of the 10 µM PMA, 1 µM emodepside produced an additional membrane hyperpolarization of −4.9 ± 1.0 mV (P < 0.01, n = 5, Figure 4D). The effects of PMA and staurosporine are consistent with a role for PKC in the emodepside signalling pathway.
Effect of emodepside (1 µM) on voltage-activated K currents
We investigated the effects of emodepside (1 µM) on the voltage-activated K currents. Given the slow onset of emodepside effects on spiking and membrane potential, we investigated the time-dependent effects of emodepside on the K currents (Figure 5). In control recordings, we tested and observed no significant change in the voltage-activated K currents at 10, 20, 30 and 40 min. We applied emodepside for 30 min and examined the effect on the K currents at 10 min intervals. Figure 5A shows representative K current traces of the control and the effects of emodepside at 10, 20 and 30 min, where it produced time-dependent increases in the currents. The maximum increase in the late phase K currents (LK) was observed at the 0 mV voltage step. At the higher voltage steps, emodepside produced smaller changes with no effect at +30 mV. In the sample plot of Figure 5B, emodepside increased LK at 0 mV to 130%, 146% and 167% at 10 min, 20 min and 30 min respectively. A similar trend was observed in all recordings (0 mV, Figure 5C, n = 4), where emodepside significantly increased the currents at 10 min, 20 min and 30 min. We tested the effects of emodepside on the K current activation curves and were only able to see small changes in the kinetic parameters V50 and Gmax throughout the application of emodepside.
Figure 5.
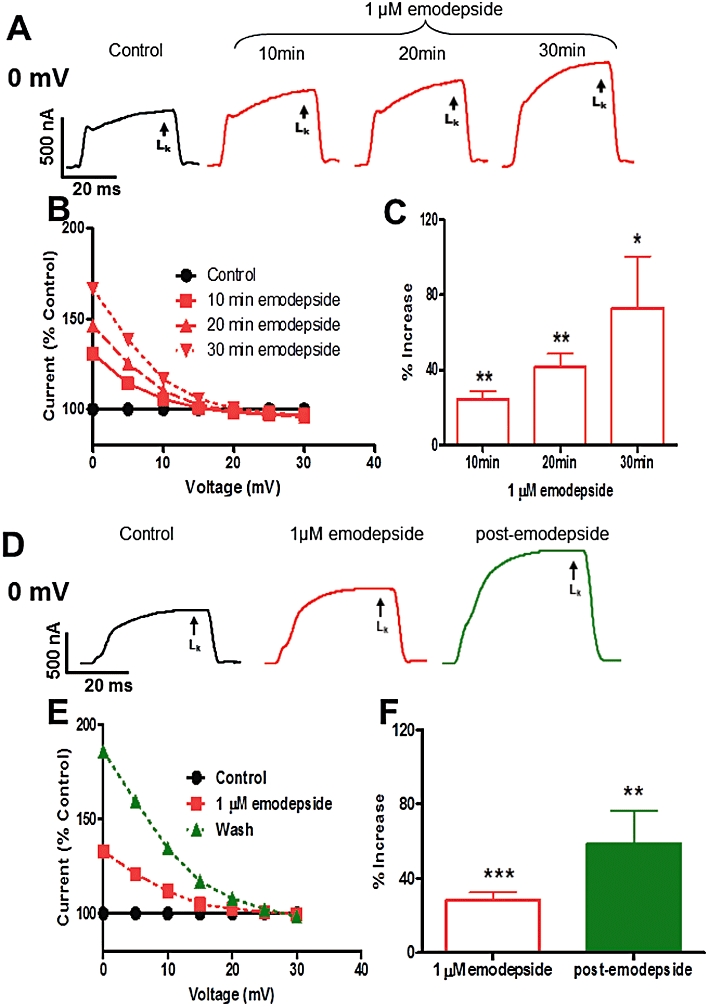
Effect of emodepside (1 µM) on voltage-activated LK currents. (A) Representative voltage-clamp traces of control K current and the time-dependent effects of emodepside on the K currents, all at 0 mV. (B) Sample plot of current (% control) against voltage showing the voltage-dependence of the K currents increased by emodepside at different time points. (C) Bar chart (mean ± SEM) of the effects of emodepside on LK currents. Comparison was made between the control 0 mV step current at 30–40 ms and the corresponding current increased by emodepside at 10, 20 and 30 min. Emodepside increased LK currents at 10 min (P < 0.01, n = 4, paired t-test), 20 min (P < 0.01, n = 4, paired t-test) and 30 min (P < 0.05, n = 4, paired t-test). (D) Representative voltage-clamp traces of control LK current, the effects of emodepside on the LK current and the LK currents after 20 min wash (post-emodepside). (E) Sample current (% control) versus voltage plot showing how the LK currents change with voltage. Emodepside increased the current after 10 min and the increase was sustained at the post-emodepside period. (F) Bar chart (mean ± SEM) of the effects of emodepside on the LK currents. After 10 min perfusion, emodepside increased the LK currents (P < 0.001, n = 9) and the increase was sustained during the post-emodepside period (P < 0.01, n = 9).
When we applied emodepside for 10 min and then washed for 20 min (post-emodepside period), we observed a continued increase in the K current following the onset of washing (Figure 5D–F). In the sample plot in Figure 5E, LK increased to 133% after 10 min exposure to emodepside and to 186% following a further period of 20 min washing. We observed a similar effect in all 9 recordings, as shown in Figure 5F.
Effect of emodepside (10 µM) on voltage-activated K currents
We increased the concentration of emodepside to 10 µM in order to resolve effects on the kinetic parameters of the K current activation curves. Figure 6A shows representative traces and the average effect from 6 recordings, of emodepside (10 µM) on the LK is shown in Figure 6B. The activation curve, Figure 6C, shows that 10 µM emodepside shifted V50 by 2.7 mV in the hyperpolarizing direction and by 6.4 mV during the post-emodepside period; there was no change in the Gmax. Similar hyperpolarizing shifts without changes in Gmax were observed in all 6 experiments: 2.9 ± 0.3 mV (P < 0.05) in 10 µM emodepside and 6.6 ± 1.0 mV (P < 0.01) in the post-emodepside period. In control experiments we found that the conductance-voltage plots showed little or no change in V50 throughout a 40 min recording period: specifically, at 20 min it was 0.28 ± 0.10 mV, n = 6; at 30 min it was 0.50 ± 0.20 mV, n = 6. The shape of the conductance activation curve with an increase in V50, can explain why the emodepside K current is greater at 0 mV with little change near +30 mV: emodepside is not altering the maximum opening of channels but their voltage-sensitivity.
Figure 6.
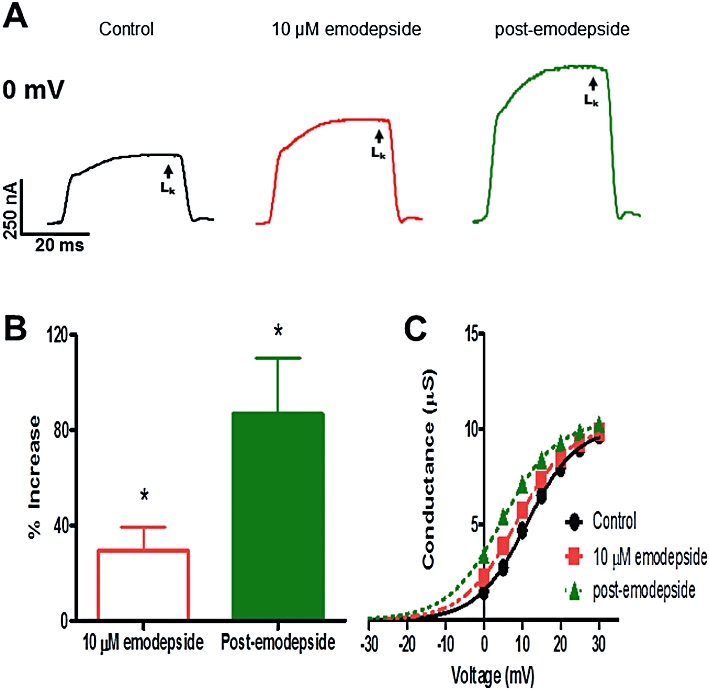
Effect of emodepside (10 µM) on the voltage-activated LK currents. (A) Representative traces of the control LK current, the effects of emodepside on the current and the post-emodepside LK current all at 0 mV. (B) Bar chart (mean ± SEM) of % increase in LK currents produced by emodepside (P < 0.05, n = 6, paired t-test) and the continued increase at the post-emodepside period (P < 0.05, n = 6, paired t-test). (C) Sample activation curve of the LK currents; conductance-voltage plots were fitted to the Boltzmann equation. Emodepside shifted the V50 in the hyperpolarization direction and the shift was sustained during the post-emodepside period. Essentially no change in Gmax was observed.
Ca is required for effects of emodepside on K currents
We replaced extracellular Ca with equimolar Co to determine the Ca requirement for the action of emodepside on K currents. In the absence of Ca, the effect of emodepside (1 µM) was reduced and we observed only a 12.0 ± 5.0% increase in LK at 10 min and a 33.0 ± 17.0% increase post-emodepside. The increases were not significantly different from the control LK (P > 0.05, n = 10). These observations show that Ca was required for effects of emodepside on the LK current and because Ia– and IK–like K currents are present in A. suum under Ca-free conditions (Martin et al., 1992) the experiments suggest that emodepside does not act directly on Ia– or IK–like K currents.
Emodepside effects on K currents: role of NO and PKC
We investigated the role of NO on the effects of emodepside (1 µM) on the K currents using SNP as an NO donor and NNLA as an iNOS inhibitor. SNP (1 mM) by itself did not cause a significant change in the LK currents even after 30 min (P > 0.05, n = 4). Figure 7A shows representative K current traces with control, effects of SNP and emodepside + SNP and post-emodepside LK currents. In six preparations, emodepside, in the presence of SNP, increased the LK currents at 10 min (P < 0.05, n = 5, Figure 7D) and a further increase during the post-emodepside period. The activation curve, Figure 7C, shows that in the presence of SNP, emodepside shifted the V50 in the hyperpolarization direction by 2.9 mV and during the post-emodepside period, the shift was increased to 4.6 mV. The mean shift in the V50 at 10 min was 2.4 mV (P < 0.05, n = 6) and 6.2 mV (P < 0.01, n = 6) post-emodepside.
Figure 7.
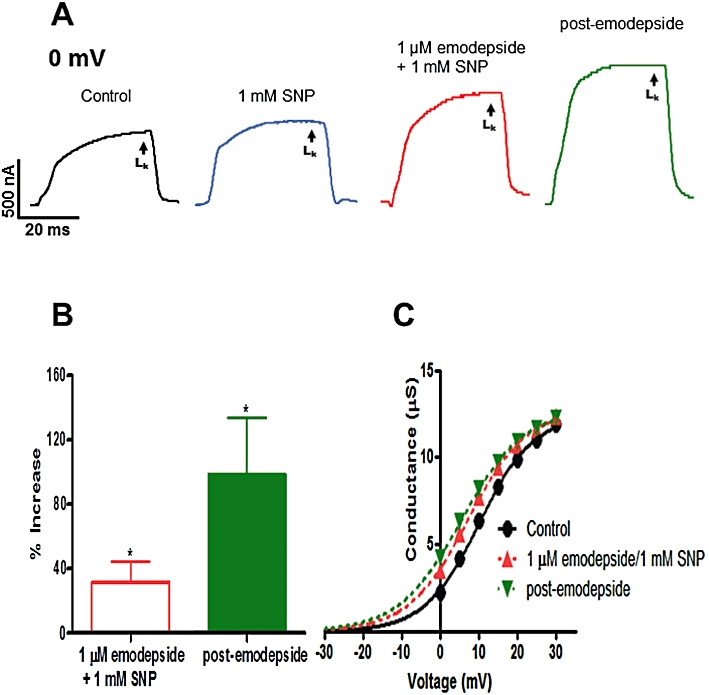
Effect of emodepside (1 µM) and SNP (1 mM) on the voltage-activated LK currents. (A) Representative traces of control LK current, the effects of emodepside + SNP on the LK current and the post-emodepside LK current, all at 0 mV. (B) Bar chart (mean ± SEM) of % increase in LK current produced by emodepside in the presence of SNP (P < 0.05, n = 5, paired t-test) and the continued increase in the post-emodepside period (P < 0.05, n = 4, paired t-test). (C) Sample activation curve of the LK currents; conductance-voltage plots were fitted to the Boltzmann equation. In the presence of SNP, emodepside shifted the V50 in the hyperpolarization direction. Essentially no change in Gmax was observed.
NNLA (100 µM) had no significant effect on K currents or membrane potential in control experiments (P > 0.05, n = 3) but this compound did inhibit the effect of emodepside (1 µM) on K currents. In the presence of NNLA, there was little effect (1.9 ± 1.0% increase, P > 0.05, n = 6) of emodepside on the LK currents at 10 min and the effect at the 20 min post-emodepside period was reduced to 26.0 ± 11.0% (P < 0.05, n = 5). Thus NNLA reduced the effects of emodepside on the K currents. The effects of both SNP and NNLA together indicated a role for NO during the action of emodepside.
We investigated the role of PKC in emodepside effects on the K currents using PMA as a PKC activator and staurosporine as a kinase inhibitor. PMA (10 µM), by itself produced a small stable but significant increase in the K currents and a small hyperpolarization. Figure 8A shows representative traces of the control LK current, the effects of PMA alone, of emodepside + PMA and of the post-emodepside period on the LK current. The mean data from all 5 recordings is summarised in Figure 8B. In the representative activation curve in Figure 8C, PMA shifted the V50 in the hyperpolarization direction, whilst emodepside + PMA caused a further hyperpolarizing shift in the V50 and during the post-emodepside period, there was a further additional hyperpolarizing shift. On average, PMA shifted V50 by 1.9 mV (P < 0.05, n = 5) and emodepside + PMA shifted it by 2.8 mV (P < 0.01, n = 5). There was no change in Gmax. From these observations, it is clear that PMA reproduces some of the effects of emodepside.
Figure 8.
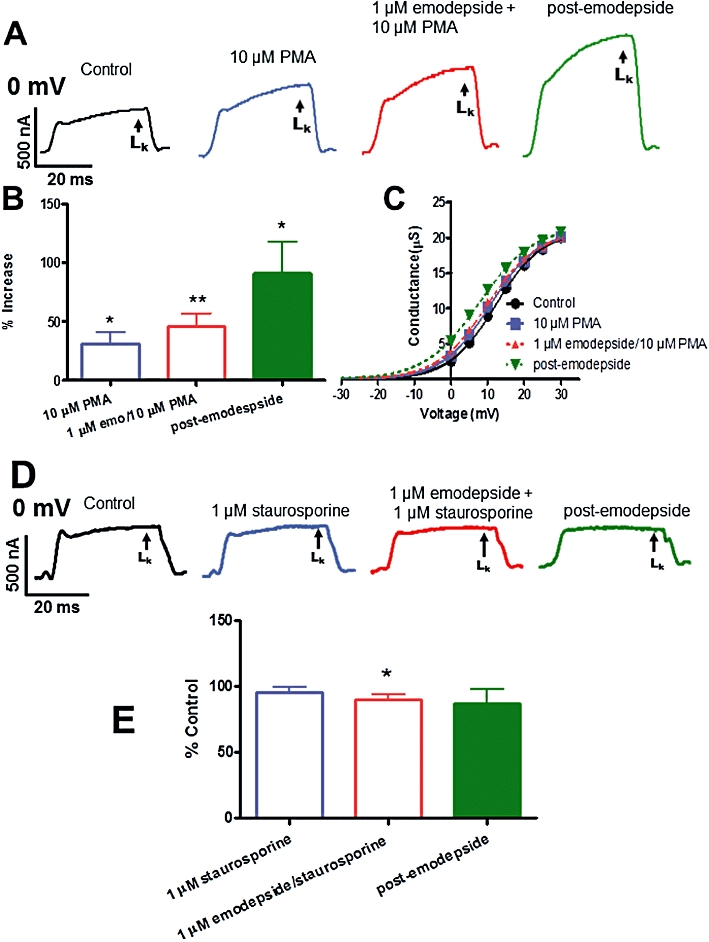
Effects of emodepside (1 µM) plus PKC modulators on the voltage-activated LK currents. (A) Representative traces of control LK current, the effects of PMA (10 µM) alone, emodepside + PMA and the post-emodepside period on the LK current. (B) Bar chart (mean ± SEM) of the % increases in LK currents caused by PMA (P < 0.05, n = 5, paired t-test), emodepside + PMA (P < 0.01, n = 5, paired t-test) and post-emodepside (P < 0.05, n = 3, paired t-test). Comparison was made with the % control LK current. (C) Conductance-voltage plots were fitted to the Boltzmann equation. In the activation curves shown, PMA and emodepside + PMA shifted the V50 in the hyperpolarization direction. Essentially no change in Gmax was observed. (D) Representative traces showing the effects of emodepside (1 µM) + staurosporine (1 µM) on the LK current. (E) Bar chart (mean ± SEM) of % change in currents caused by emodepside + staurosporine. Comparison was made with the % control LK current. In the presence of staurosporine, emodepside decreased the LK (P < 0.05, n = 4, paired t-test) and the decrease was sustained during the post-emodepside period. (C) In the presence of staurosporine, emodepside had no significant effects on the V50 but significantly reduced the Gmax (P < 0.05, n = 4, paired t-test).
The PKC inhibitor staurosporine (1 µM) by itself had no significant effect on the K currents (at 30 min, mean decrease 4.4 ± 4.0%, P > 0.05, n = 3). However, staurosporine inhibited the action of emodepside (1 µM). Figure 8 shows effects on representative K currents and Figure 8E shows summary bar-charts. Staurosporine, at 10 min, had no effect on the LK current. In the presence of staurosporine, emodepside (1 µM) was no longer activating, but was inhibitory, with a mean decrease of 10% (P < 0.05, n = 4, Figure 8E). The effects of PMA mimicking emodepside, and the loss of emodepside activation when pretreated with staurosporine, together indicated a role for PKC during the action of emodepside. The inhibitory action of emodepside following staurosporine suggests additional actions of emodepside but these were not explored further.
The 4-aminopyridine-sensitive K current includes Ia as well as the emodepside-activated current
In the presence of 4-aminopyridine (5 mM), emodepside (1 µM) had no detectable effect on K currents (P > 0.05, n = 8). When we applied 4-aminopyridine after emodepside, and measured the emodepside-activated K current as a proportion of the total 4-aminopyridine-sensitive K current, it was only 50 ± 16% (n = 6). Martin et al. (1992) have shown that 5 mM 4-aminopyridine selectively inhibits Ia–like K currents but not IK–like K currents in A. suum under Ca free conditions. The sensitivity of the emodepside K currents to 4-aminopyridine showed that emodepside did not activate IK currents. The presence of Ca here, allows K currents to be activated by emodepside. The total 4-aminopyridine-sensitive current was bigger than the emodepside-activated current, indicating that 4-aminopyridine was not selective and inhibits the emodepside K current as well as the Ia–like K current.
Effects of iberiotoxin
We examined the effects of iberiotoxin; a selective inhibitor of high conductance Ca-activated K (SLO-1) channels with IC50 values often in the sub-nanomolar ranges (Galvez et al., 1990; Candia et al., 1992; Gruhn et al., 2002). Iberiotoxin (10 nM) was used to minimize effects on other channels (Sones et al., 2009) and this inhibitor produced a significant decrease (−23.7%, P < 0.05, n = 4) in the control LK currents (Figure 9), without a detectable change in membrane potential, indicating the presence of iberiotoxin-sensitive SLO-1-like K channels in A. suum. The application of emodepside (1 µM) was able to counter some of the effects of iberiotoxin, producing hyperpolarization (−4.1 ± 0.2 mV, P < 0.05, n = 4) and a return of the LK currents towards control levels, Figure 9, but emodepside no longer increased the K current above the control (P > 0.05). Thus iberiotoxin reduced (but did not entirely block) effects of emodepside indicating that iberiotoxin-sensitive SLO-1-like channels are involved.
Figure 9.
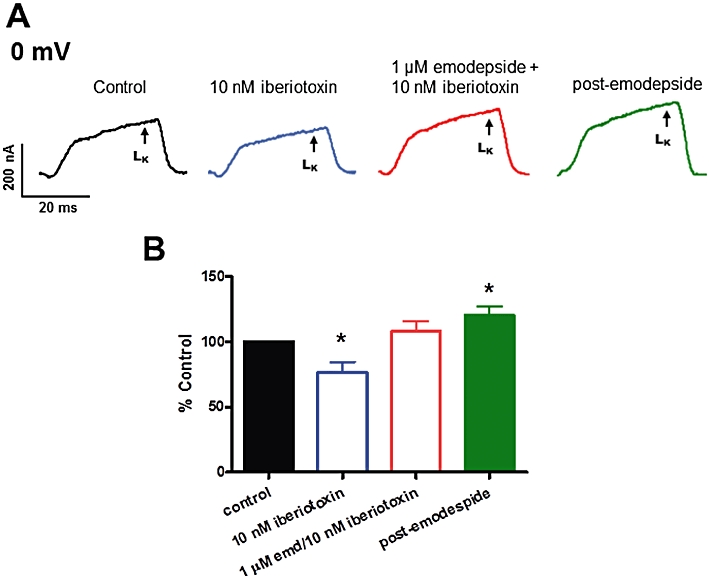
Effects of emodepside (1 µM) and iberiotoxin (10 nM) on the voltage-activated LK currents. (A) Representative traces of control LK current, 10 nM iberiotoxin effect on the LK currents, emodepside + iberiotoxin effect on the LK currents and the post-emodepside LK currents. (B) Current, expressed as percent control, at 0 mV showing the inhibitory effect of iberiotoxin and emodepside on the LK currents.
Emodepside effects on voltage-activated Ca currents
We investigated the effects of emodepside (1 µM) on voltage-activated Ca currents. The inhibitory neuropeptide PF1, which emodepside may mimic, has been shown to significantly inhibit the voltage-activated Ca currents in A. suum (Verma et al., 2009). In all nine recordings, we observed no significant effect of emodepside on the Ca currents (Figure 10).
Figure 10.
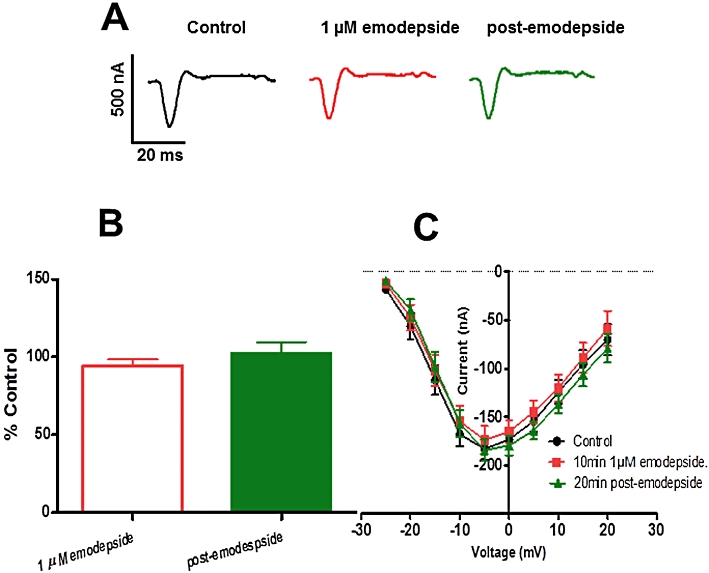
Effects of emodepside (1 µM) on the voltage-activated Ca currents. (A) Representative inward Ca current traces of control, 1 µM emodepside and the post-emodepside currents. (B) Bar chart (mean ± SEM) of the effects of emodepside on the inward Ca currents (green: 20 min post emodepside). Emodepside had essentially no effect on the voltage-activated inward Ca currents (P > 0.05, n = 9, paired t-test). (C) Current-voltage plot showing no effect of emodepside on the inward Ca2+ currents at the different voltage steps.
Discussion
Different mode of action
Emodepside is a cyclooctadepsipeptide anthelmintic that selectively inhibits muscle contraction of nematodes (Willson et al., 2003). It is effective against nematode isolates that have developed resistance to drugs from the other major classes of anthelmintic (Samson-Himmelstjerna von et al., 2005): ivermectin (an allosteric modulator of glutamate-activated Cl channels; Pemberton et al., 2001), levamisole (a nematode selective nAChR agonist; Qian et al., 2006; 2008;) and febantel (a selective ligand for nematode β-tubulin; Miro et al., 2006). Because emodepside retains its efficacy against these resistant isolates, it implies that emodepside has a different mode of action. In this paper we have examined effects of emodepside on the electrophysiology of A. suum muscle using two micropipette current- and voltage-clamp techniques with a view to determining its mechanism of action.
Emodepside is not a GABA receptor agonist
The earliest studies on the mechanisms of action of the cyclooctadepsipeptides used PF1022A. PF1022A seemed to exert its anthelmintic action on the nerves or muscle rather than affecting energy metabolism because low concentrations of PF1022A (<1 µM) depressed the motility of the nematode parasite, Angiostrongylus cantonensis (Terada, 1992). Chen et al. (1996) reported that PF1022A bound to GABA receptors of Ascaris suum muscle suggesting a direct effect on nematode GABA receptors. However, direct electrophysiological recording from Ascaris suum muscle found that PF1022A did not act like GABA, nor did it act as a cholinergic antagonist (Martin et al., 1996). Emodepside does not produce an increase in the muscle membrane conductance like GABA or piperazine (Martin, 1982) again showing that these compounds do not act as GABA agonists.
GeBner et al. (1996) reported that the depsipeptides PF1022A, PF1022-001 (antipode of PF1022A), valinomycin, enniatin A1 and beauvericin have ionophore activities, increasing the bilayer conductivity to monovalent ions like Na+, Li+, K+ and Cs+. However, they reported that only PF1022A exerts high paralytic effect on A. suum, suggesting that the anthelmintic effects of the cyclooctadepsipeptides are not attributable to ion-carrier activity.
K-dependent hyperpolarization by releasing inhibitory neuropeptides (PF1/PF2)
Willson et al. (2003) tested the effects of emodepside on Ascaris suum muscle contraction and electrophysiology. They observed that 10 µM emodepside had a much slower inhibitory action on muscle contraction than GABA and produced a slow hyperpolarization with no detectable change in input conductance. The inhibitory neuropeptide, PF2, was also reported to cause slow inhibition of contraction of A. suum muscle, similar to emodepside (Fellowes et al., 2000; Willson et al., 2003). In this study we have observed similar, but concentration-dependent, effects with emodepside. Willson et al. (2003) showed that the K channel blocker 4-aminopyridine inhibited the effect of emodepside on membrane potential and suggested that emodepside may stimulate the release of inhibitory neuropeptides, such as PF1 or PF2, to produce its effects. PF1 causes slow, non-reversible, concentration-dependent membrane hyperpolarization that is significantly blocked by 4-aminopyridine (Franks et al., 1994; Verma et al., 2009). Here, we have observed similar effects of emodepside on the membrane potential. Despite the similarity in the effects of PF1 and emodepside on the membrane potential, we have demonstrated clear differences in their mode of action. Unlike PF1, which inhibits voltage-activated Ca currents (Verma et al., 2009), emodepside had no effects on voltage-activated Ca currents. We also observed that emodepside inhibited the ryanodine-induced spiking more slowly than PF1, probably because of the lack of emodepside effect on the voltage-activated Ca currents. Though there are similarities in the effects of emodepside and the inhibitory neuropeptides PF1 and PF2, our body of evidence would not support the proposal that emodepside stimulated the release of a PF1-like neuropeptide.
Latrophilin receptors
In 2004, Willson et al. described observations on the C. elegans pharynx with emodepside (100 nM) acting as an agonist to stimulate exocytosis and elicit pharyngeal paralysis. The paralysis of the pharynx produced by emodepside depended on the presence of LAT-1 with emodepside resistance appearing in lat-1 null mutants. We observed evidence of expression of Asu-lat-1 in adult muscle flaps and PKC and NO signalling pathways suggesting activation of G-protein receptor(s) by emodepside. Muhlfeld et al. (2009) used surface plasmon resonance to show that the neuropeptides AF1, AF10 and PF2 bind, albeit with low affinities, to HC110-R, implying that these neuropeptides may be putative natural ligands of the latrophilin-like receptor. They observed no reasonable binding characteristics with PF1 and other neuropeptides. Our observations, however, do not rule out a possible direct effect of emodepside on K channels.
SLO-1 as a target for emodepside in C. elegans
Guest et al. (2007) described use of a mutagenesis screen to generate alleles of slo-1 that encodes for a Ca-activated K channel in C. elegans. They observed that slo-1, but not slo-2, null mutants were more resistant to the inhibitory effects of emodepside than lat-1 and lat-2 (latrophilin receptor) double mutants. Guest et al. (2007) proposed that emodepside either directly or indirectly activates SLO-1 that is present in body wall muscle and motor neurons to produce its inhibitory effects in nematodes. Interestingly when slo-1 was selectively expressed in the pharyngeal muscle of slo-1 null mutants, there was no effect of emodepside on the frequency of pharyngeal pumping contrary to what might be expected if emodepside exerted a direct effect on SLO-1. However, slo-1 may regulate other features of the pharyngeal activity such as pump duration and pump interval and the effect of emodepside on these parameters remains to be investigated.
SLO-1 as a target for emodepside in A. suum
We have observed expression of Asu-slo-1, an evolutionarily conserved homologue of the slo-1 gene in adult A. suum body muscle flaps; and have also observed a high-conductance 250 pS K channel present in the A. suum bag membrane (unpublished single-channel observations). There is a Ca-dependent and voltage-activated K channel present in A. suum muscle which is affected by the inhibitory neuropeptide PF1 (Verma et al., 2009). Our studies here indicate that the target of emodepside includes SLO-1-like K channels. Evidence supporting this possibility includes the following findings: a Ca-dependent and voltage-activated K current activated by emodepside; the inhibitory effects of 4-aminopyridine (5 mM) on this current (which also blocks Ca-independent Ia- but not Ca-independent IK-like K currents in A. suum, Martin et al., 1992); inhibitory effects of 10 nM iberiotoxin (which has selective effects on SLO-1 K currents, Galvez et al., 1990; Candia et al., 1992; Gruhn et al., 2002). Not all of the emodepside K current was blocked by iberiotoxin which may be due to different types of β-subunits present with the SLO-1 α-subunits that mediate iberiotoxin sensitivity (Meera et al., 2000) or effects on K channels other than SLO-1, Ia or IK.
We observed that the effect of emodepside was very slow in onset and increased over a period of more than 10 min. For example, in our ryanodine-induced spiking experiments, we observed the speed of onset of the inhibitory action of emodepside was slower than the onset of action of PF1. The slowly developing effect of emodepside may be due to the highly lipophilic nature of this compound and a membrane partitioning effect. Indeed, the potency of a similarly lipophilic anthelmintic, ivermectin also follows a slow time-course in electrophysiological experiments in A. suum (Kass et al., 1980). Alternatively the slow time-course and persistence may be because emodepside does not directly activate the K channels but acts though a signalling cascade. We found that the emodepside effect was potentiated by a NO donor and a PKC activator, while antagonism of iNOS and inhibition of PKC with staurosporine inhibited the effects of emodepside. Interestingly, these signalling molecules are known activators of SLO-1 in other cells (Bolotina et al., 1994; Mistry and Garland, 1998; Wang et al., 1999; Holden-Dye et al., 2007) and therefore emodepside may act through either or both of these signalling cascades and the signalling cascades may be in series or parallel. A number of studies on the mammalian orthologues of SLO-1 show that they are directly and alternately regulated by complex, multiple signalling cascades, involving NO and diacylglyerol or Ca-dependent PKC activation (Ghatta et al., 2006; Salkoff et al., 2006). Although emodepside is lipophilic, and this is consistent with the observed lack of a washout effect (Willson et al., 2003; Schurmann et al., 2007), we observed that the effect of emodepside on voltage-activated K currents continued to increase following washout. This is similar to PF1 (Verma et al., 2009) and, therefore, an additional or alternative explanation may be the involvement of a second messenger(s) signalling cascade.
To conclude, we found that Asu-slo-1 and Asu-lat-1 genes were expressed in body muscle flaps of the nematode parasite A. suum. Emodepside induced a 4-aminopyridine-sensitive hyperpolarization of the muscle membrane potential and potentiated voltage- and Ca-dependent, 4-aminopyridine- and iberiotoxin-sensitive, SLO-1-like currents that were affected by modulators of NO and PKC signalling pathways. Emodepside had no effect on voltage-activated Ca currents. The effects are consistent with a model in which NO, PKC and emodepside signalling pathways converge on the K channels, and/or a model in which emodepside activates the K channels through an NO or a PKC signalling cascade. Further experiments on heterologous expression systems are required to distinguish between these modes of action.
Acknowledgments
The research project culminating in this paper was funded by National Institute of Allergy and Infectious Diseases (NIH) grant R 01 A1 047194 to RJM, the Iowa Center for Advanced Neurotoxicity (ICAN) seed grant to APR and INRA (the French national institute for Agricultural Research) to CLC and CN. We are grateful to Professor Dr Achim Harder (Bayer HealthCare AG, Animal Health, R & D-Parasiticides, Building 6700, 40789 Monheim, Germany) for generously providing the emodepside. We are grateful to Professor Robert J Walker and Professor Lindy Holden-Dye, University of Southampton, UK for critical reading of the manuscript.
Glossary
Abbreviations
- NNLA
N-nitro L-arginine
- PKC
protein kinase C
- SNP
sodium nitroprusside
Note added in Proof
The following two manuscripts have appeared recently describing further effects of emodepside and the role of SLO-1.
Crisford, A. et al. (2011) Selective toxicity of the anthelmintic emodepside revealed by heterologous expression of human KCNMA1 in Caenorhabditis elegans. Molecular Pharmacology. 79, 1031–1043.
Welz, C., et al. (2011). SLO-1-channels of Parasitic Nematodes Reconstitute locomotor Behaviour and Emodepside Sensitivity in Caenorrhabditis elegans slo-1 Loss of Function Mutants. PloS Pathog. April; 7(4): e100133.
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Ethidium bromide stained agarose gel showing patterns revealed by RT-PCR for transcription of Asu-slo-1 and Asu-lat-1 from adult stage muscle flap. M: Molecular marker.
Table S1 Primer sequences used for Asu-slo-1 and Asu-lat-1 RT-PCR experiments
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl. 1):S142. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bowman JW, Winterrowd CA, Friedman AR, Thompson DP, Klein RD, Davis JP, et al. Nitric oxide mediates the inhibitory effects of SDPNFLRFamide, a nematode FMRFamide-related neuropeptide, in Ascaris suum. J Neurophysiol. 1995;74:1880–1888. doi: 10.1152/jn.1995.74.5.1880. [DOI] [PubMed] [Google Scholar]
- Bowman JW, Friedman AR, Thompson DP, Maule AG, Alexander-Bowman SJ, Geary TG. Structure-activity relationships of an inhibitory nematode FMRFamide-related peptide, SDPNFLRFamide (PF1), on Ascaris suum muscle. Int J Parasitol. 2002;32:1765–1771. doi: 10.1016/s0020-7519(02)00213-8. [DOI] [PubMed] [Google Scholar]
- Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys J. 1992;63:583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Terada M, Cheng JT. Characterization of subtypes of gamma-aminobutyric acid receptors in an Ascaris muscle preparation by binding assay and binding of PF1022A, a new anthelmintic, on the receptors. Parasitol Res. 1996;82:97–101. doi: 10.1007/s004360050077. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellowes RA, Maule AG, Marks NJ, Geary TG, Thompson DP, Halton DW. Nematode neuropeptide modulation of the vagina vera of Ascaris suum: in vitro effects of PF1, PF2, PF4, AF3 and AF4. Parasitology. 2000;120:79–89. doi: 10.1017/s0031182099005260. [DOI] [PubMed] [Google Scholar]
- Franks C, Holden-Dye L, Williams R, Pang F, Walker R. A nematode FMRFamide-like peptide, SDPNFLRFamide (PF1), relaxes the dorsal muscle strip preparation of Ascaris suum. Parasitology. 1994;108:229–236. doi: 10.1017/s0031182000068335. [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- GeBner G, Meder S, Rink T, Boheim G, Harder A, Jeschke P, et al. Ionophore and anthelmintic activity of PF1022A, a Cyclooctadepsipeptide, are not related. Pest Sci. 1996;48:399–407. [Google Scholar]
- Ghatta S, Nimmagadda D, Xu X, O'Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharm Ther. 2006;110:103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Gruhn N, Boesgaard S, Eiberg J, Bang L, Thiis J, Schroeder TV, et al. Effects of large conductance Ca2+-activated K+ channels on nitroglyerin-mediated vasorelaxation in humans. Eur J Pharmacol. 2002;446:145–150. doi: 10.1016/s0014-2999(02)01826-5. [DOI] [PubMed] [Google Scholar]
- Guest M, Bull K, Walker RJ, Amliwala K, O'Connor V, Harder A, et al. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int J Parasitol. 2007;37:1577–1588. doi: 10.1016/j.ijpara.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Harder A, Holden-Dye L, Walker R, Wunderlich F. Mechanisms of action of emodepside. Parasitol Res. 2005;97:S1–S10. doi: 10.1007/s00436-005-1438-z. [DOI] [PubMed] [Google Scholar]
- Holden-Dye L, O'Connor V, Hopper NA, Walker RJ, Harder A, Bull K, et al. SLO, SLO, quick, quick, slow: calcium-activated potassium channels as regulators of Caenorhabditis elegans behaviour and targets for anthelmintics. Invert Neurosci. 2007;7:199–208. doi: 10.1007/s10158-007-0057-z. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CE, Hudson AL, Davey MW. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol. 2009;25:328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Kaplan RM. Anthelmintic resistance in nematodes of horses. Vet Res. 2002;33:491–507. doi: 10.1051/vetres:2002035. [DOI] [PubMed] [Google Scholar]
- Kass IS, Wang CC, Walrond JP, Stretton AOW. Avermectin B1a, a paralyzing anthelmintic that affects interneurons and inhibitory motorneurons in Ascaris. Proc Natl Acad Sci USA. 1980;77:6211–6215. doi: 10.1073/pnas.77.10.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Park BJ, Choi HS, Park CS, Eom SH, Ahnn J. Identification and characterization of a putative C. elegans potassium channel gene (Ce-slo-2) distantly related to Ca2+-activated K+ channels. Gene. 1999;240:35–43. doi: 10.1016/s0378-1119(99)00398-4. [DOI] [PubMed] [Google Scholar]
- Martin RJ. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982;77:255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. doi: 10.1017/S0031182007000029. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Thorn P, Gration KAF, Harrow ID. Voltage-activated currents in somatic muscle of the nematode parasite Ascaris suum. J Exp Biol. 1992;173:75–90. doi: 10.1242/jeb.173.1.75. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Harder A, Londershausen M, Jeschke P. Anthelmintic actions of the cyclic depsipeptide PF1022A and its electrophysiological effects on muscle cells of Ascaris suum. Pest Sci. 1996;48:343–349. [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- & Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro G, Mateo M, Montoya A, Vela E, Calonge R. Survey of intestinal parasites in stay dogs in the Madrid area and comparison of the efficacy of three anthelmintics in naturally infected dogs. Parasitol Res. 2006;100:317–320. doi: 10.1007/s00436-006-0258-0. [DOI] [PubMed] [Google Scholar]
- Mistry DK, Garland CJ. Nitric Oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlfeld S, Schmitt-Wrede H-P, Harder A, Wunderlich F. FMRFamide-like neuropeptides as putative ligands of the latrophilin-like HC110-R from Haemonchus contortus. Mol Biochem Parasitol. 2009;164:162–164. doi: 10.1016/j.molbiopara.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Pemberton D, Franks C, Walker R, Holden-Dye L. Characterization of glutamate-gated chloride channels in the pharynx of wild-type and mutant caenorhabditis elegans delineates the role of the subunit GluCl-alpha2 in the function of the native receptor. Mol Pharmacol. 2001;59:1037–1043. doi: 10.1124/mol.59.5.1037. [DOI] [PubMed] [Google Scholar]
- Prichard RK. Anthelmintic resistance in nematodes: extent, recent understanding and future directions for control and research. Int J Parasitol. 1990;20:515–523. doi: 10.1016/0020-7519(90)90199-w. [DOI] [PubMed] [Google Scholar]
- Prichard RK. Anthelmintic resistance. Vet Parasitol. 1994;54:259–268. doi: 10.1016/0304-4017(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Puttachary S, Robertson AP, Clark CL, Martin RJ. Levamisole and ryanodine receptors (II): an electrophysiological study in Ascaris suum. Mol Biochem Parasitol. 2010;171:8–16. doi: 10.1016/j.molbiopara.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L- and B-subtypes of nematode nAChR resolved at the single-channel in Ascaris suum. FASEB J. 2006;20:E2108–E2116. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- Qian H, Robertson AP, Powell-Coffman JA, Martin RJ. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J. 2008;22:3247–3254. doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeger B, Schmitt-Wrede H-P, Dehnhardt M, Benten WPM, Krucken J, Harder A, et al. Latrophilin-like receptor from the parasitic nematode Haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J. 2001;15:1332–1334. doi: 10.1096/fj.00-0664fje. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;5:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Samson-Himmelstjerna von G, Harder A, Sangster NC, Coles GC. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology. 2005;130:343–347. doi: 10.1017/s0031182004006523. [DOI] [PubMed] [Google Scholar]
- Sangster NC. Managing parasiticide resistance. Vet Parasitol. 2001;98:89–109. doi: 10.1016/s0304-4017(01)00425-3. [DOI] [PubMed] [Google Scholar]
- Sangster NC, Gill J. Pharmacology of anthelmintic resistance. Parasitol Today. 1999;15:141–146. doi: 10.1016/s0169-4758(99)01413-1. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takagi M, Yaguchi T, Miyadoh S, Okada T, Koyama M. A new anthelmintic cyclodepsipeptide, PF1022A. J Antibiot. 1992;45:692–697. doi: 10.7164/antibiotics.45.692. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann S, Harder A, Schnieder T, Samson-Himmelstjerna von G. Effects of emodepside on egg hatching, larval development and larval motility in parasitic nematodes. Parasitol Res. 2007;101:S45–S56. [Google Scholar]
- Sones WR, Leblanc N, Greenwood IA. Inhibition of vascular calcium-gated chloride currents by blockers of Kca1.1, but not modulators of Kca2.1 or Kca2.3 channels. Br J Pharmacol. 2009;158:521–531. doi: 10.1111/j.1476-5381.2009.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M. Neuropharmacological mechanism of action of PF1022A, an antinematode anthelmintic with a new structure of cyclic depsipeptide, on Angiostrongylus cantonensis and isolated frog rectus. Jpn J Parasitol. 1992;41:108–107. [Google Scholar]
- Verma S, Robertson AP, Martin RJ. Effects of SDPNFLRF-amide (PF1) on voltage-activated currents in Ascaris suum muscle. Int J Parasitol. 2009;39:315–326. doi: 10.1016/j.ijpara.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Determinant for β-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci USA. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou Y, Wen H, Levitan IB. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J Neurosci. 1999;19:1–7. doi: 10.1523/JNEUROSCI.19-10-j0005.1999. RC4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei A, Jegla T, Salkoff L. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 1996;35:805–829. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Willson J, Amliwala K, Harder A, Holden-Dye L, Walker RJ. The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 2003;126:79–86. doi: 10.1017/s0031182002002639. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


