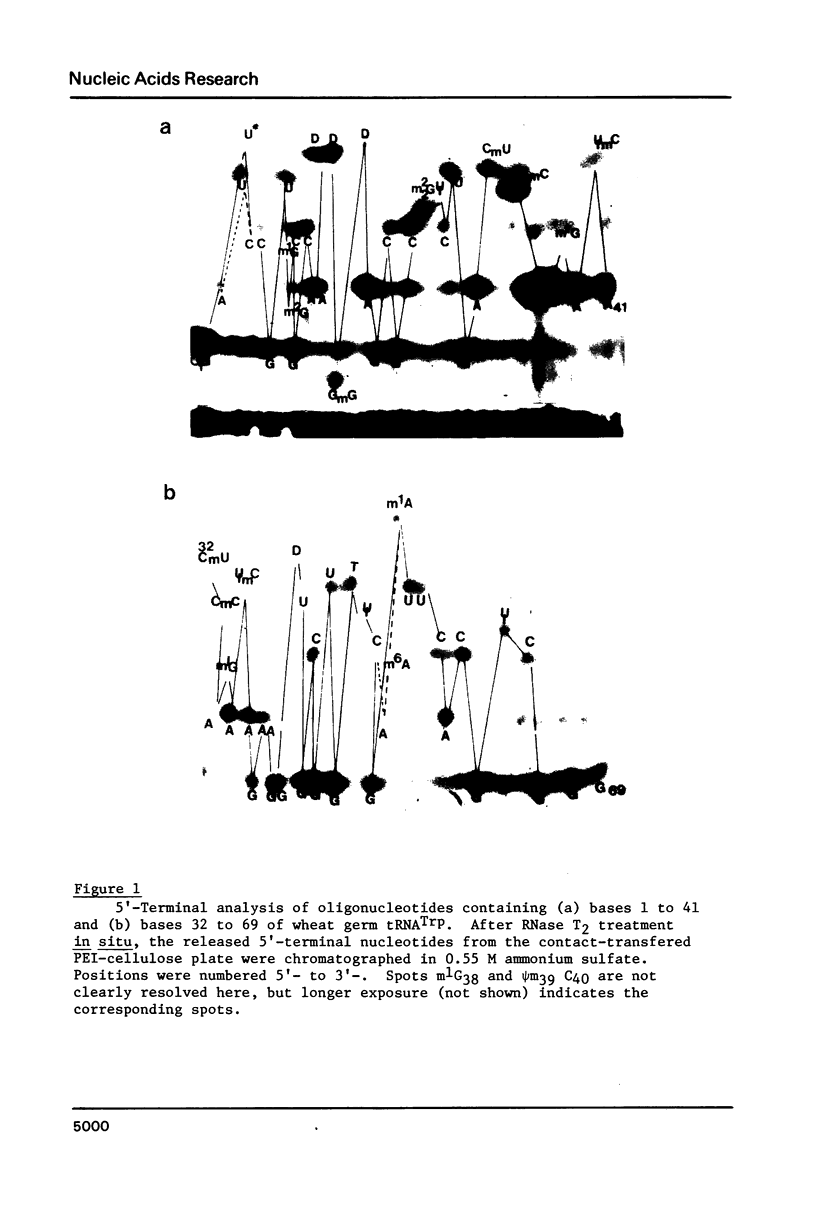
Abstract
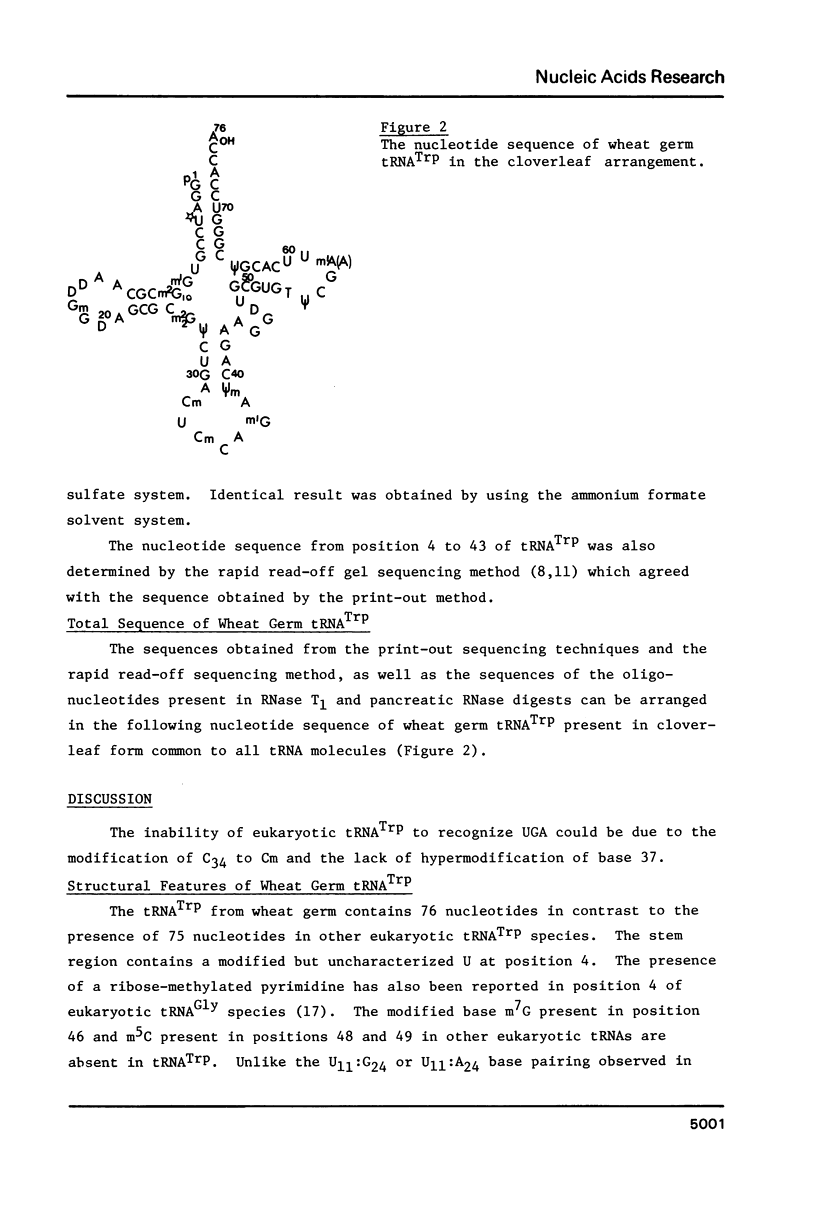
The coding properties of tRNATrp from yeast and wheat germ were studied. Unlike E. coli tRNATrp or mitochondrial tRNATrp, eukaryotic tRNATrp did not recognize the UGA codon in vitro. The sequence of wheat germ tRNATrp as determined by [32P] post-labelling techniques is: [sequence in text] The interesting features are: (i) Presence of a C11:G24 base pair in contrast to the U11:G24 in E. coli Su- tRNATrp. (ii) The anticodon sequence is -CmCA- compared to -CCA- in E. coli tRNATrp. (iii) Lack of a hypermodified base i6A adjacent to the 3'-end of the anticodon. (iv) Presence of -T psi CG- sequence instead of -psi psi CG- sequence present in mammalian tRNATrp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Chirikjian J. G. Structural requirements for binding of bovine tRNATrp with avian myeloblastosis virus DNA polymerase. Nucleic Acids Res. 1980 Jan 11;8(1):57–66. doi: 10.1093/nar/8.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet A. L., Caskey C. T. Mammalian peptide chain termination. II. Codon specificity and GTPase activity of release factor. Proc Natl Acad Sci U S A. 1971 Mar;68(3):619–624. doi: 10.1073/pnas.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Swanstrom R., Goodman H. M., Bishop J. M. tRNATrp as primer for RNA-directed DNA polymerase: structural determinants of function. J Biol Chem. 1979 Mar 25;254(6):1866–1874. [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P. Role of modified nucleosides in transfer ribonucleic acid. Effect of removal of the modified base adjacent to 3' end of the anticodon in codon-anticodon interaction. J Biol Chem. 1972 Jun 10;247(11):3369–3375. [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P., Simsek M., Raj Bhandary U. L. Initiator methionine transfer ribonucleic acid from wheat embryo. Purification, properties, and partial nucleotide sequences. J Biol Chem. 1974 Aug 10;249(15):4720–4729. [PubMed] [Google Scholar]
- Gupta N. K. Studies on polynucleotides. LXXXIX. A study of amino acid incorporation in a reticulocyte cell-free protein-synthesizing system with polyribonucleotides with repeating nucleotide sequences used as messengers. J Biol Chem. 1968 Oct 10;243(19):4959–4965. [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Roe B. A., Randerath K. The nucleotide sequence of human tRNAGly (anticodon GCC). Nucleic Acids Res. 1979 Oct 25;7(4):959–970. doi: 10.1093/nar/7.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Kato M., Sugisaki H., Nishimura S. Nucleotide sequence of starfish initiator tRNA. Nucleic Acids Res. 1979 Aug 10;6(11):3459–3469. doi: 10.1093/nar/6.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Ghosh H. P. A fast and sensitive method for the analysis of modified nucleosides in tRNA. Anal Biochem. 1974 Apr;58(2):578–591. doi: 10.1016/0003-2697(74)90227-9. [DOI] [PubMed] [Google Scholar]
- Stewart J. W., Sherman F., Jackson M., Thomas F. L., Shipman N. Demonstration of the UAA ochre codon in bakers yeast by amino-acid replacements in iso-1-cytochrome c. J Mol Biol. 1972 Jul 14;68(1):83–96. doi: 10.1016/0022-2836(72)90264-1. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]