Abstract
Objective
To determine the prevalence of illicit drug use and the impact on HIV treatment.
Design
Multivariable regression of cross-sectional data from 1163 HIV-infected and 294 controls from the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM).
Methods
An analysis of (1) prevalence of specific illicit drug use (ever, current), (2) being on HAART among those with an indication and (3) current HIV RNA and CD4 cell count among HAART users.
Results
Median age was 42 years, approximately 50% were non-Caucasian and 33% were women. Eighty-six percent of HIV-infected and 67% of controls reported ever using illicit drugs (P <0.0001); 28% of HIV-infected and 16% of controls reported current use (P = 0.0001). In adjusted models, current cocaine use and past heroin use were associated with not currently being on HAART. Among HAART users, those reporting past heroin use were as likely to have an undetectable HIV viral load as those who had never used heroin. Current and past cocaine use and current heroin use was associated with lower odds of undetectable HIV RNA. Past amphetamine use was associated with having an undetectable HIV. Similar results were seen for CD4 lymphocyte counts.
Conclusion
Illicit drug use in the US is common, although far fewer report current use than past use. Among HIV-infected patients, understanding of the type of illicit drugs used and whether drug use was in the past or ongoing is important, because of their differential effects on HIV treatment outcomes.
Keywords: amphetamines, cocaine, heroin, HIV, street drugs, viral load
Introduction
HIV infection is epidemic in the US and illicit drug use, a major risk factor for HIV acquisition, is highly prevalent [1]. Once infected, the continued use of illicit drugs contributes to the ongoing transmission of HIV infection [2]. Some data in HIV-infected patients suggest that current use of illicit drugs is predictive of a poorer HIV outcome. A longitudinal study of HIV-infected participants in Baltimore, Maryland found that the risk of developing new opportunistic infections was two-fold higher for both intermittent drug users during periods of active use and for persistent drug users. During periods of illicit drug abstinence, however, the risk of opportunistic infection was similar to that for those who never used drugs [3].
Furthermore, not all illicit drugs appear to carry the same risk of nonadherence to HAART. Tucker and colleagues, using data from the HIV Cost and Services Utilization Study, found that use of cocaine, marijuana, amphetamines, or sedatives in the previous month was associated with approximately two-fold odds of nonadherence [4]. In another small study of 85 HIV-infected current and former drug users, the strongest predictor of nonadherence to HAART was active cocaine use. In that study active cocaine users were less likely to maintain viral suppression than nonusers during the six-month study period [5].
Using self-reported, anonymous data from a large, nationally representative multicenter cohort of HIV-infected individuals and controls, we report the prevalence of specific illicit drug use, including ever having used, current use, and the amount of illicit drugs participants reported using over their lifetime (lifetime use). In addition, we report on the association between use of specific illicit drugs and being on HAART, and among those on HAART, the association between specific illicit drug use and both HIV viral load and CD4 lymphocyte count.
Methods
Outcome measurements
The study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) enrolled 1183 HIV-infected persons and 297 controls from June 2000 to September 2002. HIV-infected participants were recruited from sixteen participating HIV, infectious disease clinics, or cohorts. Participating HIV sites were selected based upon geographic diversity and the ability to perform the protocol. The 16 HIV clinical sites included in FRAM were affiliated to academic medical centers, and most were highly experienced in conducting HIV clinical trials. The FRAM population is representative of patients with HIV infection in the US [6]. Control participants were recruited from two centers (Birmingham, Alabama and Oakland, California) of the Coronary Artery Risk Development in Young Adults (CARDIA) study that followed participants longitudinally enrolled in the Visceral Fat and Metabolic Rate in Young Adults (VIM) ancillary study of CARDIA. CARDIA participants were originally recruited as a population-based sample of healthy 18 to 33-year-old Caucasian and African American men and women from four cities in 1986 for a longitudinal study of cardiovascular risk factors. The VIM ancillary study recruited participants from two of the four CARDIA Centers in 1995–96. VIM enrolled approximately 100 CARDIA participants from each of the race-gender groups with BMI distributed similarly above and below race–gender specific medians of the population-based CARDIA study. The CARDIA methodology has also been previously reported [7,8]. Eighty-three percent of CARDIA participants approached agreed to participate in FRAM at the year 15 CARDIA study visit. The FRAM protocol was approved by institutional review boards at all sites.
Measurements
Illicit drug use was assessed by a standardized self-administered questionnaire developed for the CARDIA study that included questions regarding use of crack, cocaine, heroin, amphetamines, and marijuana [8,9]. The questionnaire was covered by an NIH Certificate of Confidentiality. For prevalence of illicit drug use, use was categorized as current (within the last 30 days), ever or never. Adherence was based on the AIDS Clinical Trials Group measure of 3 days prior to the study visit. For analysis of illicit drug and the association with receipt of HAART, HIV RNA levels and CD4 lymphocyte count, drug use was categorized as current, past but not current, or never. Based on the CARDIA questionnaire, amount of current use was further divided into <10 days per month versus ≤10 days per month. Lifetime drug use was divided into ≥10 times versus greater than 10 times; lifetime marijuana was divided into ≤100 times versus greater than 100 times. Age, gender, ethnicity, medical history, and risk factors for HIV were determined by self-report. Income was stratified into tertiles: <US$12 000, US$12 000–35 000, and >US$35 000 per annum.
In HIV-infected participants, CD4 lymphocyte count and HIV RNA quantification were measured centrally, and research associates performed a detailed chart abstraction of all antiretroviral medications as well as medical history. Participants were considered to have AIDS if they had a CD4 lymphocyte count less than 200 cells/μl or a history of an AIDS-defining illness.
Data from the FRAM sites were categorized geographically into four regions: (1) south-east: Atlanta VA Center, Atlanta, Georgia; University of Alabama, Birmingham, Alabama; Johns Hopkins University School of Medicine, Baltimore, Maryland; University of North Carolina, Chapel Hill, North Carolina and the Washington DC VA Center, Washington, DC; (2) north-east: New York Harbor VA Center, New York, New York; Tufts University, Boston, Massachusetts and St. Lukes-Roosevelt Hospital Center, New York, New York; (3) central: Indiana University, Indianapolis, Indiana; University Hospitals of Cleveland, Cleveland, Ohio; University of Colorado HSC, Denver Colorado and Washington University, St Louis, Missouri; and (4) west coast: Stanford University: Palo Alto, California; University of California, San Diego Owen Clinic, San Diego, California; University of California, San Francisco, California and the University of California, Los Angeles, California.
Statistical analysis
Analyses were restricted to 1163 HIV-infected and 294 control men and women, after exclusion of those with missing illicit drug use questionnaires (n = 12 HIV-infected and n = 3 controls). Analyses that compared HIV-infected persons with controls were restricted to those between the ages of 33 and 45 (n = 620 HIV-infected), since the control population did not include participants outside this age range. Characteristics of the HIV-infected participants and controls were compared and tested for statistical significance using the Mann–Whitney U test for continuous variables, and Fisher’s exact test for categorical variables.
Among HIV-infected patients in the full cohort (n = 1163), multivariable logistic and linear regression models were used to examine the association of illicit drug use with being on HAART, HIV viral suppression (defined as HIV RNA <400 copies/ml), and current CD4 lymphocyte count. The analysis of the association between illicit drug use and current HAART was restricted to patients with AIDS by CD4 cell count or prior AIDS-defining illness, because at the time of our study, national guidelines recommended the initiation of HAART when the CD4 cell count either fell below 200 cells/μl or there was a history of AIDS. The associations of illicit drug use with HIV viral load and CD4 cell count were analyzed in current HAART users.
These regression models were built using stepwise regression with P = 0.05 for entry and retention. Gender, age, and ethnicity were included in every model. Models were built in a sequence of steps, where each step built on the prior ones, controlling for (1) demographic factors only; (2) illicit drugs and socio-economic factors; (3) hepatitis C virus (HCV) status (by HCV RNA level); and (4) antiretroviral adherence (for HIV viral load and CD4 outcomes). At each stage, a factor was dropped from the model if it did not reach statistical significance or if it worsened model fit as assessed by standard statistical measures [e.g., adjusted R2, Akaike information criteria (AIC), area under the receiver operating characteristic curve (AROC), etc.]. We separately analyzed the use of speed, heroin and cocaine/crack, as there was incomplete overlap among the use of these categories of drugs; however, we pooled crack and cocaine because they are chemically similar and had overlap of use. We tested for a dose–response relationship of illicit drugs used, both for current amount of drug used and, separately, for the amount of lifetime use.
Interactions between gender, ethnicity, and other factors in the model were assessed; no statistically significant interactions were identified. The linearity assumption for continuous predictors was also tested. Candidate variables considered for inclusion in the multivariable models included illicit drug use (marijuana, amphetamines, heroin, crack, cocaine, and the combination of crack or cocaine use), socio-economic factors (income, education, homeless status, and adequate food intake), HCV status, HAART use, and antiretroviral adherence (for the 3 days prior to the study visit). In model step (2), illicit drug use was categorized as current, past, or never.
Due to its skewed distribution, current CD4 lymphocyte count was log-transformed in all linear regression analyses; the results were back-transformed to produce estimated percentage effects. Confidence intervals in linear regression analyses were determined using the bias-corrected accelerated bootstrap method [10], with P-values defined as one minus the highest confidence level that still excluded zero; this was necessary because the error residuals appeared to be non-Gaussian. Logistic regression models were assessed at each stage using the Hosmer–Lemeshow goodness of fit test [11]. All analyses were conducted using the SAS system, version 9.1 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
Participant demographics
TABLE 1 displays the demographic information for the patients included in this analysis. The characteristics of the twelve HIV-infected patients excluded because they did not fill out the drug use questionnaires were similar to those included, other than excluded patients having a higher rate of AIDS-defining illness in comparison with those included (17 versus 2%, P = 0.036); however, the 17% represents only two out of the 12 patients. Nearly half of the FRAM participants’ risk for HIV infection was men who have sex with men (MSM); CARDIA participants were not queried about HIV-related risk factors.
TABLE 1.
Demographic and clinical characteristics of HIV-infected and control individuals.
| HIV+(ARa) | Control | P-value | HIV+(not ARa) | |
|---|---|---|---|---|
| n | 620 | 294 | 1163 | |
| Age (years) | ||||
| Median | 40.0 | 41.0 | 0.014 | 42.0 |
| IQR | 36.5–43.0 | 37.0–43.0 | 37.0–48.0 | |
| Gender | ||||
| Female | 30% | 48% | <0.0001 | 29% |
| Male | 70% | 52% | 71% | |
| Ethnicity | ||||
| Caucasian | 49% | 51% | <0.0001 | 48% |
| African American | 38% | 49% | 39% | |
| Hispanic | 11% | 0 | 10% | |
| Other | 1% | 0 | 3% | |
| Ever homeless | 9% | NA | 8% | |
| Income | ||||
| <US$12 000 | 38% | 7% | <0.0001 | 39% |
| US$12 000–35 000 | 35% | 25% | 34% | |
| >US$35 000 | 27% | 69% | 27% | |
| Education | ||||
| Elementary | 3% | 0 | <0.0001 | 3% |
| High School | 42% | 20% | 40% | |
| College | 48% | 63% | 46% | |
| Graduate school | 8% | 17% | 10% | |
| Current smoker | 46% | 17% | <0.0001 | 42% |
| HCV RNA+ | 19% | 2% | <0.0001 | 22% |
| Current methadone | 3% | 0 | 0.0005 | 4% |
| Current CD4 cell count (cells/μl) | ||||
| Median | 364 | NA | 350 | |
| IQR | 214–531 | 214–539 | ||
| CD4 cell count <200 cells/μl | 24% | NA | 23% | |
| HIV RNA (1000 copies/ml) | ||||
| Median | 0.4 | NA | 0.4 | |
| IQR | 0.4–15.2 | 0.4–12.5 | ||
| Detectable HIV RNA | 51% | NA | 49% | |
| HAART status | ||||
| Current | 78.5% | NA | 76.4% | |
| Past | 11.5% | 12.7% | ||
| Never | 10.0% | 11.0% | ||
| Antiretroviral adherenceb | 71.8% | NA | 75.7% | |
| Duration HIV (years) | ||||
| Median | 8.2 | NA | 8 | |
| IQR | 5.4–11.8 | 5.2–11.7 | ||
| HIV risk factors | ||||
| MSM | 48% | NA | 46% | |
| IDU | 19% | 21% | ||
| Other | 8% | 9% | ||
| Heterosexual | 25% | 24% | ||
| Recent OI | 3% | NA | 2% | |
| AIDS by CD4 cell count <200 cells/μl or OI | 74% | NA | 73% | |
HCV, hepatitis C virus; IDU, injection drug user; IQR, interquartile range; MSM, men who have sex with men; NA, not applicable; OI, opportunistic infection.
AR, age restricted;
Antiretroviral adherence was based on the 3 days prior to the study visit.
Prevalence of self-reported drug use
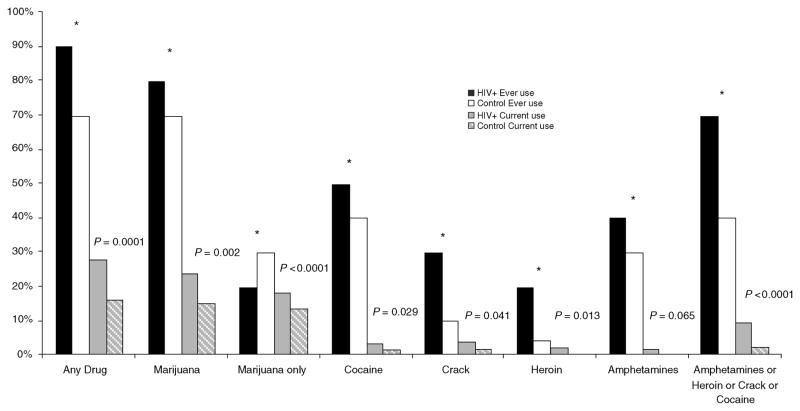
Figure 1 displays self-reported use of illicit drugs of study participants. The majority reported a history of illicit drug use. More HIV-infected patients reported ever using any illicit drug than controls (86 versus 67%, P <0.0001), but the majority was marijuana. Although report of marijuana use was more common in HIV-infected patients in comparison with controls (83 versus 66%, P <0.0001), fewer HIV-infected patients reported using nothing other than marijuana (19 versus 26%, P <0.0001). Among those who reported ever use of marijuana, 22% of HIV-infected patients and 1.6% of controls reported they used marijuana for medical reasons. History of use of other illicit drugs (amphetamines, heroin, crack or cocaine) was more common in HIV-infected patients in comparison with controls (67 versus 41%, P <0.0001).
Fig. 1. Prevalence of self-reported illicit drugs (age restricted).
*P <0.001 for all comparisons of ever use in HIV versus Control. P-values provided for comparison of current use in HIV-infected patients versus control individuals.
The trends for current drug use were similar to those for ever having used drugs, although current drug use was much less prevalent than ever drug use. More HIV-infected patients reported current use of illicit drugs (28 versus 16%, P = 0.0001) and current use of illicit drugs other than marijuana (10 versus 2%, P <0.0001).
For those who report only marijuana use, current marijuana only use was 39% among HIV-infected patients and 20% among control individuals. Among those who reported any current use of marijuana, 29% of HIV-infected and 4% of controls reported using marijuana for medical reasons.
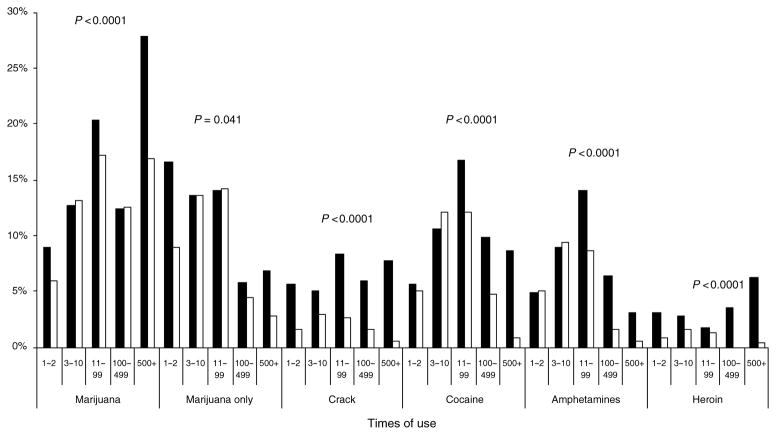
Figure 2 displays the self-reported use of illicit drugs, categorized by the number of times used over the lifetime. Categories were based on the previously validated CARDIA instrument. Again, reported lifetime use of each drug was greater in those with HIV infection.
Fig. 2.
Self-reported lifetime use of illicit drugs (age restricted).
Gender and ethnic differences
Prevalence of illicit drug use was also examined by gender and race. Among the HIV-infected patients, women and African Americans reported more lifetime crack and heroin use, whereas men and Caucasians reported more lifetime marijuana and amphetamine use. Among controls, it was found that men reported more lifetime use of cocaine, crack, and amphetamines. African Americans reported more crack use but less use of other drugs in comparison with Caucasians. Patterns of ever and current use showed similar results. When separated by gender and race, use of most drugs was higher in HIV-infected patients than in the respective controls.
Illicit drug use and HAART
Association of drug use with receipt of HAART in those with AIDS
We then evaluated whether illicit drug use was associated with currently being on HAART in those with an AIDS diagnosis, for whom treatment is clearly warranted. Table 2 displays the factors associated with current use of HAART in a multivariable logistic regression analysis. Women and current users of crack or cocaine were less likely to be on HAART, as were past users of heroin. Current heroin users were also less likely to be on HAART, but prevalence of current heroin use was low and the association did not reach statistical significance.
Table 2.
Multivariable logistic regression analysis of demographic and illicit drug factors associated with being on HAART in HIV-infected patients with AIDS (not age restricted).
| Current HAART
|
|||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Demographics | |||
| Female versus male | 0.57 | (0.38, 0.85) | 0.006 |
| African American versus Caucasian | 0.85 | (0.56, 1.29) | 0.44 |
| Hispanic versus Caucasian | 0.95 | (0.49, 1.84) | 0.88 |
| Other versus Caucasian | 0.40 | (0.15, 1.08) | 0.071 |
| Age (per decade) | 1.24 | (0.98, 1.58) | 0.077 |
| Significant candidates | |||
| Heroin: current versus never | 0.42 | (0.14, 1.24) | 0.12 |
| Heroin: past versus never | 0.43 | (0.27, 0.69) | 0.001 |
| Crack/cocaine: current versus never | 0.40 | (0.19, 0.85) | 0.017 |
| Crack/cocaine: past versus never | 0.84 | (0.54, 1.30) | 0.43 |
CI, confidence interval; OR, odds ratio; Number of HIV-infected patients with AIDS: 834, with 82% receiving HAART.
Use of HAART in those with AIDS appeared similar by age and race. Associations between amount of each drug used in the past 30 days, or in a lifetime and being on HAART did not reach statistical significance in any models; there appeared to be no dose–response relationship.
Association of drug use with viral suppression and CD4 lymphocyte count among those on HAART
The factors associated with achievement of viral suppression (undetectable HIV RNA quantification) for patients currently on HAART were assessed (Table 3). Seventy-six percent of the HIV-infected FRAM participants were on HAART. Multivariable analysis demonstrated that African Americans were less likely than Caucasians to have an undetectable HIV RNA, as were current users of heroin and crack or cocaine in comparison with those who had never used each drug. Past crack/cocaine use was associated with lower odds of being suppressed, but not past heroin use.
Table 3.
Multivariable logistic regression analysis of demographic and illicit drug factors associated with HIV RNA suppression in current HAART recipients (not age restricted).
| Undetectable HIV RNA
|
|||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Demographics | |||
| Female versus male | 1.22 | (0.85, 1.74) | 0.27 |
| African American versus Caucasian | 0.60 | (0.43, 0.85) | 0.004 |
| Hispanic versus Caucasian | 0.87 | (0.52, 1.47) | 0.61 |
| Other versus Caucasian | 0.73 | (0.29, 1.87) | 0.51 |
| Age (per decade) | 1.09 | (0.92, 1.30) | 0.31 |
| Significant candidates | |||
| Adherent versus nonadherent | 1.70 | (1.19, 2.42) | 0.003 |
| Heroin: current versus never | 0.19 | (0.05, 0.74) | 0.017 |
| Heroin: past versus never | 1.12 | (0.71, 1.78) | 0.63 |
| Crack/cocaine: current versus never | 0.43 | (0.20, 0.91) | 0.027 |
| Crack/cocaine: past versus ever | 0.65 | (0.45, 0.94) | 0.021 |
| Amphetamines: current versus ever | 1.23 | (0.32, 4.78) | 0.77 |
| Amphetamines: past versus ever | 1.86 | (1.28, 2.69) | 0.001 |
CI, confidence interval; OR, odds ratio; Number of current HAART recipients: 885: 60% with undetectable HIV RNA.
Age and gender showed little or no association with viral suppression. In contrast, the data demonstrated that past use of amphetamines was associated with higher odds of having an undetectable HIV RNA. Not surprisingly, adherence was also associated with having an undetectable HIV RNA. Marijuana use did not appear to be associated with HIV treatment outcomes in any of the models (data not shown).
We also evaluated the factors associated with current CD4 lymphocyte counts (Table 4). African Americans had 24% lower CD4 lymphocyte counts than Caucasians, similar to the lack of suppression of viral replication. Women had 21% higher CD4 lymphocyte counts, similar to the slight but not statistically significant trend toward higher viral suppression in women. Lower income was associated with lower CD4 cell count; level of education did not appear to have an independent association with CD4 cell count. Current heroin use was associated with 53% lower CD4 lymphocyte counts. Similar to the analysis of viral suppression, past heroin use did not appear to be associated with a substantially lower CD4 lymphocyte count; nor did current or past cocaine use. In contrast, both current amphetamine use and past amphetamine use were associated with higher CD4 lymphocyte counts in comparison with those who had never used amphetamines. Adherence was associated with 15% higher CD4 lymphocyte counts, but did not reach statistical significance (P = 0.075). After multivariable adjustment, geographic region showed essentially no association with receipt of HAART, viral suppression, or CD4 lymphocyte counts.
Table 4.
Multivariable logistic regression analysis of demographic and illicit drug factors associated with current CD4 lymphocyte count in HIV-infected patients on current HAART (not age restricted).
| Current CD4 lymphocyte count
|
|||||
|---|---|---|---|---|---|
| % Effect | 95% CI | P-value | Median CD4 lymphocyte count | ||
| Demographics | |||||
| Gender | Male | 338 | |||
| Female versus male | 20.9 | (2.3, 41.1) | 0.029 | Female | 376 |
| Ethnicity | |||||
| African American versus Caucasian | −23.6 | (−34.2, −10.9) | <0.0001 | African American | 291 |
| Hispanic versus Caucasian | −18.0 | (−36.2, 2.6) | 0.084 | Hispanic | 301 |
| Other versus Caucasian | 1.0 | (−30.8, 43.3) | 0.95 | Other ethnicity | 343 |
| Age (per decade) | 4.0 | (−3.4, 12.2) | 0.34 | Caucasian | 406 |
| Significant candidates | |||||
| Heroin | Heroin: never use | 359 | |||
| Current versus never | −53.0 | (−85.1, −18.7) | 0.003 | Heroin: current use | 261 |
| Past versus never | −6.6 | (−24.0, 13.5) | 0.51 | Heroin: past use | 335 |
| Amphetamines | Amphetamines: never use | 332 | |||
| Current versus never | 101.4 | (41.8, 198.4) | <0.0001 | Amphetamines: current use | 466 |
| Past versus never | 15.5 | (0.3, 32.0) | 0.044 | Amphetamines: past use | 376 |
| Income | Income: >US$35 000 | 424 | |||
| <US$12 000 versus >US$35 000 | −28.5 | (−38.3, −15.5) | <0.0001 | Income: <US$12 000 | 286 |
| US$12 000–35 000 versus >US$35 000 | −15.1 | (−27.7, −1.5) | 0.032 | Income: US$12 000–35 000 | 336 |
CI, confidence interval. Outcome is log-transformed CD4 lymphocyte count; results are back-transformed. Number of current HAART recipients: 885.
Discussion
In this large, nationally representative multicenter study of HIV-infected and control individuals, we found a high prevalence of past illicit drug use, but the majority was marijuana. Few other cohorts have been as large and systematically recruited as FRAM. Nearly all of the HIV-infected patients and two-thirds of control participants reported a history of illicit drugs, mainly marijuana, but only 28% of HIV-infected and 16% of control individuals reported current use of any illicit drug. History of crack, cocaine, and heroin use was more common in HIV-infected patients, consistent with data that suggests that use of these drugs is associated with HIV acquisition [12]. We also observed that specific illicit drugs had different associations with being on HAART, and among those on HAART, achieving viral suppression.
Even though significantly fewer FRAM patients report current use of illicit drugs in comparison with the number who reported ever using, the prevalence of current illicit drug use in both HIV-infected and control individuals in our study remained substantially higher than the approximate 8% prevalence reported in the 2005 National Survey on Drug Use and Health in patients of similar age [13]. The higher rates of illicit drug use in the FRAM study at our control sites may be explained in part by the fact that our study sites were mainly focused around US urban centers. Although there were small geographic regional differences in illicit drug use (data not shown), they were smaller than the differences between HIV patients and control individuals. The majority of HIV-infected men in our study were men who have sex with men. Another study limited to men who have sex with men (regardless of HIV status) using an Internet survey conducted over a 6-month period found a 30% prevalence of marijuana use and 7% prevalence of cocaine use in the past 6 months [14]. Finally, our finding of less reported current illicit drug use in comparison with past use only is consistent with national trends suggesting that people generally stop using illicit drugs over time or as they age [13,15]. The reason for the decreased use of current illicit drugs is not clear, however. Whereas one factor could be aging in both HIV-infected and control individuals, other factors such as concern about health and HIV treatment may play a role. These data suggest that future research into the reasons for stopping use should be considered.
As illicit drug use is a major risk factor for HIV acquisition, it is critical to understand the impact of use of specific drugs on HIV treatment outcomes. Several studies have found that active heroin use is associated with poorer HIV outcomes, whether due to poorer treatment adherence [16,17] and/or inadequacy of the social support network [18]. Cocaine use has also been associated with less adherence coupled with worse virologic and clinical outcomes [5,19,20]. Consistent with these studies, we report that current heroin and crack/cocaine users who were on HAART were less likely to achieve viral suppression. We have, however, observed that past heroin users who were on HAART were as likely as nonheroin users to achieve viral suppression. In FRAM, past heroin users were less likely to be on HAART, suggesting a possible bias against heroin users in receiving HAART. Some studies show that those in remission from substance abuse have higher rates of receipt of HAART [21]. Treatment of narcotic addiction with methadone maintenance therapy or buprenorphine/naloxon improves adherence and/or suppression of HIV viral load [22–24]. It will be important for future studies to evaluate the impact of drug treatment in HIV care centers.
There are somewhat limited and conflicting data on the impact of specific stimulant use on HIV treatment outcomes. In a longitudinal study of 150 HIV-infected individuals, of whom 102 tested positive for recent illegal drugs, those using stimulants, especially cocaine with methamphetamines, had a four-fold greater risk of nonadherence, particularly among those who had used within the past 3 days [25]. One study reported that methamphetamine use, in particular, was associated with increased risk-taking behavior, poorer adherence and worse clinical outcomes [26]. In contrast, another study did not find an association between methamphetamine use alone and adherence [27]. Using actual HIV RNA levels and CD4 cell count to assess treatment efficacy, past amphetamine users in FRAM were more likely to have an undetectable viral load and increased CD4 cell count, and current amphetamine users were more likely to have an increased CD4 cell count. The prevalence of current amphetamine use was low, with large confidence intervals. Given the limitations, we are hesitant to speculate on possible biological reasons. One small study did find that HIV-infected men who used methamphet-amines had a lower prevalence of HIV-related diarrhea [28]. We report our results and look forward to additional studies to either confirm or refute these findings. Men who have sex with men (MSM) were just as likely to ever use amphetamines as all other HIV-positive men (39% of all HIV-positive versus 37% of MSM), suggesting an independent amphetamine effect. Our study, however, did not distinguish methamphetamines from other types of amphetamines. Finally, marijuana use showed no association with any HIV treatment outcome. Among HIV-infected patients, nearly one-third reported that they used marijuana for medical reasons. Our data confirm the importance of clinicians determining a patient’s specific drug-use history and especially current use rather than assuming that all drug users are the same.
Our study has certain limitations. The data are self-reported and therefore subject to recall and reporting bias. As the control individuals were population-based, they differ in demographic factors from the HIV-infected patients in our study. Our adherence evaluation was based upon a single point in time, limiting our ability to fully explore this parameter. The strikingly lower current use of drugs in the last 30 days compared to ‘ever’ use is of importance in understanding the effects of past and present drug use. A caveat is, however, that we may not be able to adjust for all factors that might confound current drug use. Furthermore, we did not ask about amyl nitrate, recreational use of prescription drugs, or other drugs often considered to be ‘party drugs’. The contribution of illicit drug use to current CD4 cell count levels is difficult to define in our study as many variables impact current CD4 cell counts (including nadir CD4, time on antiretroviral therapy, and when HAART was initiated) that may not be fully accounted for in the multivariable model. Finally, it should also be noted that women had a lower odds of receiving HAART, although women who did receive HAART were slightly more likely than men to have a suppressed viral load. Future study into potential gender bias is needed.
Our study suggests that illicit drug use is common in the US, although far fewer people report current use compared to ever having used drugs. Among HIV-infected patients, an understanding of the type of illicit drugs used and whether drug use was in the past or is ongoing is important, because of their differential effects on HIV treatment outcomes. Past use of some illicit drugs, especially heroin and amphetamines, and current use of marijuana, may not be a barrier to achieving a therapeutic benefit from antiretroviral therapy. Our study contributes to the growing literature suggesting that HIV treatment programs need to incorporate treatment of current heroin and cocaine addiction.
Acknowledgments
This study was supported by NIH grants RO1-DK57508, HL74814, andHL53359, and NIH GCRC grants M01- RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, and RR00865. The funding agency had no role in the collection or analysis of the data.
Sites and investigators
University Hospitals of Cleveland (Barbara Gripshover, MD); Tufts University (Abby Shevitz, MD and Christine Wanke, MD); Stanford University (Andrew Zolopa, MD and Lisa Gooze, MD); University of Alabama at Birmingham (Michael Saag, MD and Barbara Smith, PhD); John Hopkins University (Joseph Cofrancesco and Adrian Dobs); University of Colorado Heath Sciences Center (Constance Benson, MD and Lisa Kosmiski, MD); University of North Carolina at Chapel Hill (Charles van der Horst, MD); University of California at San Diego (W. Christopher Mathews, MD and Daniel Lee, MD); Washington University (William Powderly, MD and Kevin Yarasheski, PhD); VA Medical Center, Atlanta (David Rimland, MD); University of California at Los Angeles (Judith Currier, MD and Matthew Leibowitz, MD); VA Medical Center, New York (Michael Simberkoff, MD and Juan Bandres, MD); VA Medical Center, Washington DC (Cynthia Gibert, MD and Fred Gordin, MD); St Luke’s-Roosevelt Hospital Center (Donald Kotler, MD and Ellen Engelson, PhD); University of California at San Francisco (Morris Schambelan, MD and Kathleen Mulligan, PhD); Indiana University (Michael Dube, MD); Kaiser Permanente, Oakland (Stephen Sidney, MD); University of Alabama at Birmingham (Cora E. Lewis, MD).
Data coordinating center
University of Alabama, Birmingham (O. Dale Williams, PhD Heather McCreath, PhD, Charles Katholi, PhD, George Howard, PhD, Tekeda Ferguson, and Anthony Goudie)
Image reading center
St Luke’s-Roosevelt Hospital Center: (Steven Heymsfield, MD, Jack Wang, MS and Mark Punyanitya).
Office of the principal investigator
University of California, San Francisco, Veterans Affairs Medical Center and the Northern California Institute for Research and Development: (Carl Grunfeld, MD, PhD, Phyllis Tien, MD, Peter Bacchetti, PhD, Dennis Osmond, PhD, Andrew Avins, MD, Michael Shlipak, MD, Rebecca Scherzer, PhD, Erin Madden, MPH, Mae Pang, RN, MSN, Heather Southwell, MS, RD, and Yong Kyoo Chang, MS).
References
- 1.Anon Epidemiology of HIV/AIDS–United States, 1981–2005. MMWR Morb Mortal Wkly Rep. 2006;55:589–592. [PubMed] [Google Scholar]
- 2.Vu MQ, Steketee RW, Valleroy L, Weinstock H, Karon J, Janssen R. HIV incidence in the United States, 1978–1999. J Acquir Immune Defic Syndr. 2002;31:188–201. doi: 10.1097/00126334-200210010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 4.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 8.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoegerman GS, Lewis CE, Flack J, Raczynski JM, Caveny J, Gardin JM. Lack of association of recreational cocaine and alcohol use with left ventricular mass in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 1995;25:895–900. doi: 10.1016/0735-1097(94)00469-7. [DOI] [PubMed] [Google Scholar]
- 10.Efron B, Tibshirani R. An Introduction to the Bootstrap. London: Chapman and Hall; 1993. [Google Scholar]
- 11.Hosmer DJ, Lemeshow S. Applied logistic regression. 2. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 12.Cohen E, Navaline H, Metzger D. High-risk behaviors for HIV: a comparison between crack-abusing and opioid-abusing African-American women. J Psychoactive Drugs. 1994;26:233–241. doi: 10.1080/02791072.1994.10472436. [DOI] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies, National Clearinghouse for Alcohol and Drug Information (NCADI); 2006. [Google Scholar]
- 14.Hirshfield S, Remien RH, Humberstone M, Walavalkar I, Chiasson MA. Substance use and high-risk sex among men who have sex with men: a national online study in the USA. AIDS Care. 2004;16:1036–1047. doi: 10.1080/09540120412331292525. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon K, Kerr T, Lai C, Ishida T, Wood E, Montaner JS, et al. Nonadherence to antiretroviral therapy among a community with endemic rates of injection drug use. J Int Assoc Physicians AIDS Care (Chic Ill) 2005;4:66–72. doi: 10.1177/1545109705284353. [DOI] [PubMed] [Google Scholar]
- 17.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. Cmaj. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- 18.Knowlton A, Arnsten J, Eldred L, Wilkinson J, Gourevitch M, Shade S, et al. Individual, interpersonal, and structural correlates of effective HAART use among urban active injection drug users. J Acquir Immune Defic Syndr. 2006;41:486–492. doi: 10.1097/01.qai.0000186392.26334.e3. [DOI] [PubMed] [Google Scholar]
- 19.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004;29:117–127. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- 21.Wood E, Hogg RS, Kerr T, Palepu A, Zhang R, Montaner JS. Impact of accessing methadone on the time to initiating HIV treatment among antiretroviral-naive HIV-infected injection drug users. Aids. 2005;19:837–839. doi: 10.1097/01.aids.0000168982.20456.eb. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan LE, Barry D, Moore BA, Chawarski MC, Tetrault JM, Pantalon MV, et al. A trial of integrated buprenorphine/naloxone and HIV clinical care. Clin Infect Dis. 2006;43 (Suppl 4):S184–S190. doi: 10.1086/508182. [DOI] [PubMed] [Google Scholar]
- 23.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–270. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84:188–194. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug Use and Medication Adherence among HIV-1 Infected Individuals. AIDS Behav. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2:194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- 27.Mathews WC, Mar-Tang M, Ballard C, Colwell B, Abulhosn K, Noonan C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care STDS. 2002;16:157–172. doi: 10.1089/10872910252930867. [DOI] [PubMed] [Google Scholar]
- 28.Robinson L, Rempel H. Methamphetamine use and HIV symptom self-management. J Assoc Nurses AIDS Care. 2006;17:7–14. doi: 10.1016/j.jana.2006.07.003. [DOI] [PubMed] [Google Scholar]