Abstract
Introduction
Venous thrombus resolution may be regulated by an angiogenic process that involves the surrounding vein wall. The aims of this study were to determine whether: (i) thrombosis stimulates activation of the angiogenic transcription factor, hypoxia-inducible factor (HIF) 1α, and downstream expression of growth factors in vein wall; and (ii) upregulation of HIF1α in vein wall leads to increased growth factor expression and enhanced thrombus resolution.
Materials and methods
HIF1α, vascular endothelial growth factor (VEGF), and placental growth factor (PLGF) were quantified in mouse inferior vena cava (IVC) at days 1, 3, 7, and 14 after thrombus formation (n = 10-13 per group). An additional group of thrombosed mice were treated with the prolyl-hydroxylase domain (PHD) inhibitor, L-mimosine (L-mim) or vehicle control. HIF1α, VEGF, and PLGF in IVC were measured at days 1 and 7; and vein recanalisation and thrombus resolution were measured at days 7 and 10 (n = 6-7 per group).
Results
HIF1α was expressed in thrombosed IVC and its levels remained relatively constant throughout natural resolution. The levels of VEGF in thrombosed IVC were elevated at days 1 (P < 0.0001) and 3 (P < 0.05); and PLGF at days 1 (P < 0.0001), 3 (P < 0.0001), and 7 (P < 0.0001). Treatment with L-mim led to: increased HIF1α (P < 0.05), VEGF (P < 0.005), and PLGF (P < 0.001) levels in the IVC; decreased thrombus size (P < 0.01); and increased vein recanalisation (P < 0.001).
Conclusions
HIF1α levels in vein wall are not affected by thrombosis and it appears that the angiogenic drive in the vein surrounding resolving thrombus is regulated independently of HIF1α. Stimulating HIF1α levels in the vein wall leads to an increased angiogenic drive and promotes vein recanalisation and thrombus resolution.
Abbreviations: HIF1α, hypoxia-inducible factor 1α; VEGF, vascular endothelial growth factor; PLGF, placental growth factor; IVC, inferior vena cava; L-mim, L-mimosine
Keywords: Hypoxia, Hypoxia-inducible factor, Angiogenesis, Thrombus resolution
Deep vein thrombosis (DVT) has an incidence of 1 in 500 per year [1] and approximately 1 in 3 DVT patients develop post-thrombotic syndrome (PTS) within 5 years of thrombosis [2]. PTS is expensive to treat and symptoms include leg pain, swelling, and chronic ulceration [3,4].
Venous thrombi resolve naturally by a slow process of organisation [5]. Veins that recanalise more rapidly following DVT have reduced incidence of outflow obstruction and valvular reflux, which is associated with improved clinical outcome [6–8]. Anticoagulation does not accelerate resolution or reduce the incidence of PTS, and carries a risk of pathological bleeding. Alternative treatments that accelerate thrombus resolution without haemorrhagic side-effects may arise from a better understanding of the mechanisms that control natural resolution.
The formation of neovascular channels both within and around the thrombus, together with thrombus contraction, leads to vein recanalisation [9–11]. This neovascularisation may be driven by the expression of potent angiogenic factors within the thrombus [12–14], and increasing their levels in the thrombus enhances recanalisation [14–17].
We have recently shown that newly-formed and resolving thrombus is hypoxic compared with venous blood [18]. The remodeling response that normally follows hypoxia is controlled primarily by hypoxia-inducible factor 1 (HIF1), which is a heterodimeric nuclear transcription factor composed of α and β subunits [19]. HIF1β is constitutively expressed but HIF1α is regulated in an oxygen-dependent manner [20]. Prolyl hydroxylase domain (PHD) enzymes hydroxylate HIF1α under normoxia, which targets this subunit for rapid degradation via the ubiquitin-proteosome pathway. Under hypoxic conditions, however, PHD activity is compromised and HIF1α translocates to the nucleus, where it forms the active HIF1 complex with HIF1β [21]. HIF1 binds to the hypoxia-responsive element (HRE), causing transcriptional upregulation of its target genes, such as vascular endothelial growth factor (VEGF) [22].
We have shown that hypoxia and HIF1α stabilization is associated with increased levels of angiogenic HIF1 targets in the naturally resolving thrombus and preventing HIF1α degradation in the thrombus enhances vein recanalisation, thrombus neovascularisation, and thrombus resolution [18]. Thrombus resolution is also regulated by interaction with the vein wall to which it adheres, and an angiogenic response within the wall may be a source of many of the channels that appear within the thrombus [10]. The contribution of the vein wall to the impetus for neovascularisation, in the context of HIF1 expression and angiogenic target gene induction is, however, unknown. We hypothesised that formation of occlusive thrombus causes a relative hypoxia in the surrounding vein wall, leading to increased levels of HIF1α, which upregulates the expression of angiogenic factors that promote vein recanalisation and thrombus resolution. The aims of this study were to determine whether: (i) HIF1α is induced in the vein wall following thrombus induction; (ii) this is associated with an increased angiogenic drive; and (iii) increased HIF1α levels in the vein wall enhances vein recanalisation and thrombus resolution.
Materials and methods
Mouse model of venous thrombosis
All experiments were performed under the Animals (Scientific Procedures) Act, 1986, and approved by the local ethics committee. Experimental venous thrombi were induced in the inferior vena cava (IVC) of 8 week old male BALB/C mice using a combination of blood flow restriction and endothelial disturbance as previously described [9,23,24].
Localisation of HIF1α in IVC during natural thrombus resolution
Transverse paraffin-sections (5 μm) of 1 and 7 day old thrombosed IVC (n = 3) were immunostained for HIF1α (anti-HIF1α, Stratech, UK) following antigen retrieval by pressure cooking sections in citrate buffer (0.3% sodium citrate, pH 6). Primary antibody binding was detected using biotinylated rabbit anti-rat (Dako, UK) and extravidin horseradish peroxidase complex (HRP, Sigma, UK). Isotype-matched IgG was used as a negative control. Peroxidase activity was visualised using an HRP substrate (SG, Vector, UK), which showed positive staining as dark blue/black, and sections were counter-stained using nuclear fast red.
Measurement of HIF1α, VEGF, and PLGF expression in IVC during natural thrombus resolution
Thrombosed mice were anaesthetised 1 day (n = 13), 3 days (n = 10), 7 days (n = 13), and 14 days (n = 10) after thrombus induction and a laparotomy was performed. IVC was immediately separated from the thrombus, excised, snap frozen in liquid nitrogen, and stored at − 80 °C. The IVC from 13 sham operated mice was harvested 24-hours post-operation to determine whether the presence of thrombus affects HIF1α, VEGF, and PLGF expression in the IVC. The infrarenal aorta was also harvested from these mice and HIF1α measured.
As HIF1β is constitutively expressed, the rate-limiting factor for HIF1 activation is accumulation and translocation of HIF1α to the nucleus. Both the nuclear and cytoplasmic fractions of IVC samples were therefore extracted using an extraction kit according to manufacturer's instructions (NE-PER Extraction Kit, Pierce, UK) [25–27]. Soluble protein concentrations in each fraction were measured using the Coomassie Plus modified Bradford assay (Pierce, UK).
The levels of HIF1α in nuclear fractions (active HIF1α) were measured using a human/mouse HIF1α ELISA (R&D Systems, UK). The levels of VEGF and PLGF (potent angiogenic factors that have been shown to be upregulated in hypoxic thrombus [18]) were measured in cytoplasmic fractions using mouse VEGF and PLGF ELISAs (R&D Systems, UK).
Effect of L-mimosine on HIF1α, VEGF, and PLGF expression in IVC
L-mimosine (L-mim) is a PHD inhibitor that has been used to increase HIF1α levels in vitro and in vivo [28,29]. Thrombi were formed in 34 mice and L-mim or vehicle control was administered as previously described (n = 17 per group) [18]. IVC was excised and fractionated 1 day (n = 10 per group) or 7 days (n = 7 per group) after thrombus induction. HIF1α expression was measured in nuclear fractions; while VEGF and PLGF expression were measured in cytoplasmic fractions. The concentrations of all factors were normalized against the soluble protein concentration and expressed in pg/mg.
Effect of L-mimosine on thrombus resolution
Thrombi were formed in 26 mice and L-mim or vehicle control was administered as previously described (n = 13 per group) [18]. The IVC containing thrombus was harvested at day 7 (n = 6 per group) or 10 (n = 7 per group) after thrombus formation and fixed in 10% formalin. Transverse paraffin sections (5 μm) were taken at 300 μm intervals along the entire length of the thrombus and stained with haematoxylin and eosin. Images of whole tissue sections were obtained in a blinded fashion using Image Pro Plus (Media Cybernetics, USA). Estimates of thrombus size and IVC recanalisation (mm2) were obtained as previously described [13,30].
Statistical analysis
The Kolmogorov-Smirnov test was used to confirm that all data were normally distributed. Unpaired t-tests were used to test differences in HIF1α, VEGF, or PLGF between non-thrombosed and thrombosed IVC at days 1, 3, 7, and 14 after thrombus formation. One-way analysis of variance (ANOVA) was used to test whether there was a relationship between time after thrombus induction and HIF1α, VEGF, or PLGF in thrombosed IVC. If a relationship was present, Bonferroni's post-hoc was used to test differences between groups. Unpaired t-tests were used to compare differences between L-mim-treated mice versus controls. The relationships between HIF1α and VEGF or PLGF in the thrombosed IVC of L-mim-treated mice were tested using Pearson's correlation. P values of less than 0.05 were considered significant. Data are expressed as means ± standard error (SE).
Results
Localisation and measurement of HIF1α, VEGF, and PLGF in IVC during natural thrombus resolution
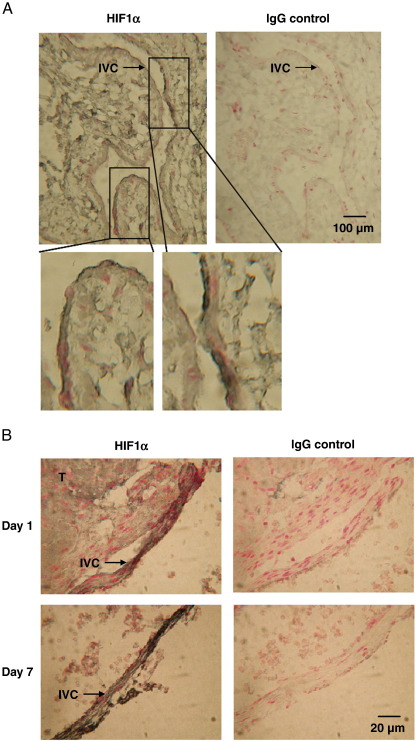
Non-thrombosed IVC stained positively for HIF1α (Fig. 1A). Thrombosed vein also stained positively for HIF1α at day 1 and 7 after thrombus induction (Fig. 1B).
Fig. 1.
HIF1α staining of thrombosed IVC. Nucleated cells within the (A) non-thrombosed and (B) thrombosed (T) IVC stained positively for HIF1α (black).
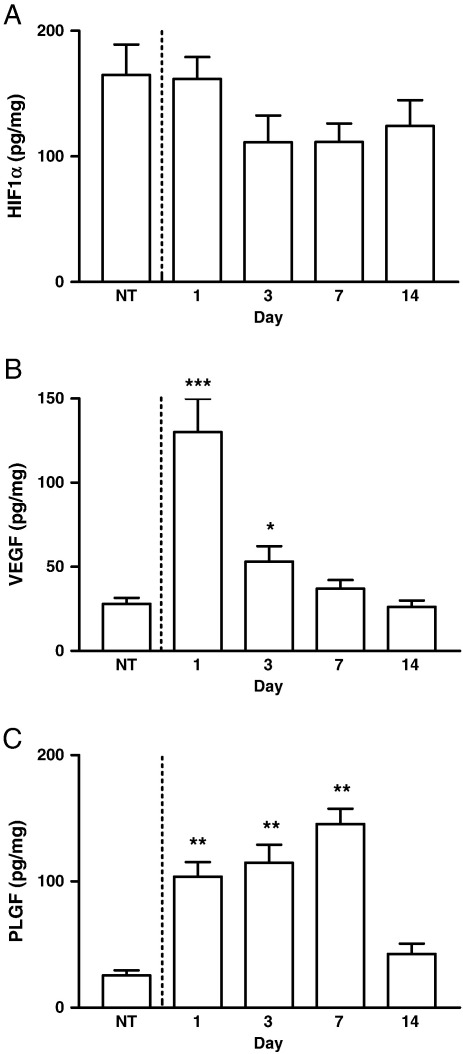
The level of HIF1α in the non-thrombosed IVC wall (165 ± 28 pg/mg) was almost 2-fold greater than that found in the infrarenal aorta (88 ± 15 pg/mg, P < 0.05). There was however, no difference in HIF1α expression between the non-thrombosed IVC (165 ± 28 pg/mg) and the thrombosed IVC at days 1 (162 ± 17 pg/mg), 3 (111 ± 21 pg/mg), 7 (111 ± 15 pg/mg), or 14 (124 ± 21 pg/mg) after thrombus formation and there was no significant temporal trend in HIF1α expression within the wall (Fig. 2A).
Fig. 2.
HIF1α, VEGF, and PLGF expression in thrombosed IVC. (A) HIF1α expression did not change throughout thrombus resolution or when compared with the non-thrombosed (NT) IVC. (B) VEGF was elevated at days 1 and 3 but not days 7 and 14, compared with the non-thrombosed (NT) IVC and was greater at day 1 compared with days 3, 7, and 14. (C) PLGF was higher at days 1, 3, and 7 after thrombus induction compared with day 14 and the non-thrombosed (NT) IVC. *P < 0.01 vs. NT, day 7, and day 14. **P < 0.0001 vs. NT and P < 0.01 vs. day 14. ***P < 0.0001 vs. NT and P < 0.001 vs. days 3, 7, and 14.
There was a temporal pattern in both VEGF and PLGF expression in the IVC following thrombus induction (P < 0.0001). VEGF expression in thrombosed IVC was elevated at days 1 (130 ± 20 pg/mg, P < 0.0001) and 3 (53 ± 9 pg/mg, P = 0.01), but not 7 and 14 after thrombus induction compared with the non-thrombosed IVC (28 ± 4 pg/mg, Fig. 2B). VEGF in the thrombosed IVC was greater at day 1 compared with days 3, 7, and 14 (P < 0.001 for all comparisons). PLGF in the thrombosed IVC was raised at days 1 (104 ± 12 pg/mg, P < 0.0001), 3 (115 ± 14 pg/mg, P < 0.0001), and 7 (145 ± 12 pg/mg, P < 0.0001), but not at day 14 after thrombus induction compared with the non-thrombosed IVC (26 ± 4 pg/mg, Fig. 2C). PLGF in the thrombosed IVC was increased at days 1 (P < 0.01), 3 (P < 0.01), and 7 (P < 0.001) compared with day 14. There was no correlation between HIF1α and VEGF or PLGF in the non-thrombosed IVC or in the thrombosed IVC at day 1, 3, 7, or 14 after thrombus formation.
The effect of L-mim on HIF1α, VEGF, and PLGF in IVC
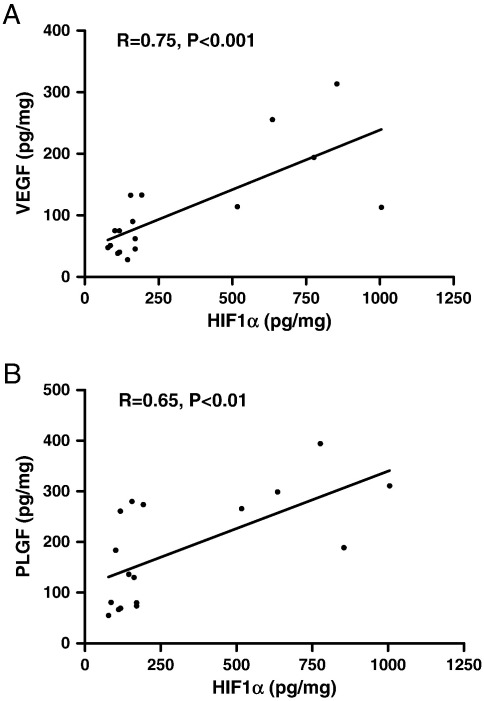
HIF1α expression was 2-fold greater in both the day 1 and day 7 thrombosed IVC of mice treated with L-mim compared with controls (P < 0.05, Table 1). HIF1α levels correlated positively with both VEGF (n = 17, R = 0.75, P < 0.0001, Fig. 3A) and PLGF (n = 17, R = 0.65, P < 0.01, Fig. 3B) in the IVC wall of L-mim-treated mice, whereas there were no significant correlations between HIF1α and either VEGF or PLGF in the IVC of control mice. VEGF expression was greater at day 1 (P < 0.05) and more than 2-fold greater at day 7 (P < 0.005, Table 1) in the thrombosed IVC of mice treated with L-mim compared with vehicle. PLGF expression was increased by over 3-fold at day 1 (P > 0.0001, Table 1) in the thrombosed IVC of L-mim-treated mice compared with controls.
Table 1.
The effect of L-mim on HIF1α and HIF1-mediated angiogenic factor expression in thrombosed IVC.
Values are pg/mg.
| Vehicle control | L-mimosine | Significance | ||
|---|---|---|---|---|
| Day 1 | HIF1α | 199 ± 6 | 452 ± 64 | P < 0.05 |
| VEGF | 81 ± 12 | 145 ± 29 | P < 0.05 | |
| PLGF | 76 ± 8 | 260 ± 15 | P < 0.0001 | |
| Day 7 | HIF1α | 80 ± 8 | 126 ± 14 | P < 0.05 |
| VEGF | 24 ± 4 | 51 ± 8 | P < 0.005 | |
| PLGF | 80 ± 9 | 87 ± 10 | P > 0.5 |
Fig. 3.
Associations between HIF1α and VEGF or PLGF expression in the thrombosed IVC of mice treated with L-mim. Positive correlations between HIF1α and (A) VEGF (n = 17, R = 0.75, P < 0.001) or (B) PLGF (n = 17, R = 0.65, P < 0.01).
The effect of L-mim treatment on thrombus resolution
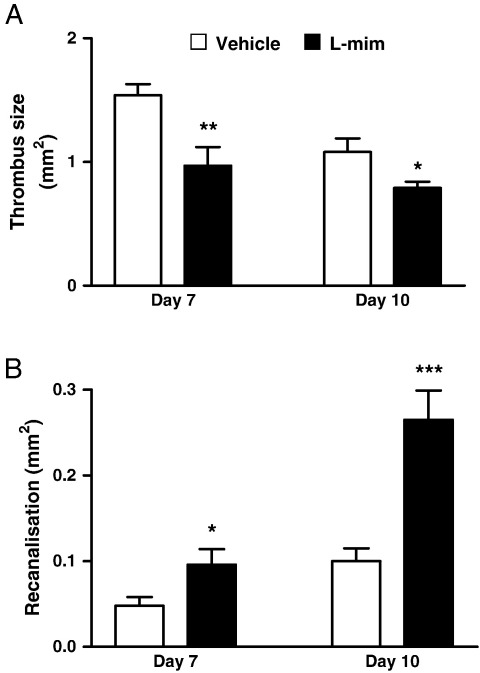
Thrombus size was decreased in L-mim-treated mice compared with controls at day 7 (1.0 ± 0.2 versus 1.5 ± 0.1 mm2, P < 0.01) and day 10 (0.8 ± 0.1 versus 1.1 ± 0.1 mm2, P < 0.05, Fig. 4A). Vein recanalisation was increased by 2-fold at day 7 (0.1 ± 0.02 versus 0.05 ± 0.01 mm2, P < 0.05) and by almost 3-fold day 10 (0.27 ± 0.03 versus 0.1 ± 0.02 mm2, P < 0.001, Fig. 4B) in L-mim-treated mice compared with controls.
Fig. 4.
Effect of L-mim treatment on thrombus resolution. Thrombus size (A) was decreased in L-mim-treated mice compared with controls at day 7 and day 10, while vein recanalisation (B) was increased at day 7 and day 10. *P < 0.05 vs. control. **P < 0.01 vs. control. ***P < 0.001 vs. control.
Discussion
Organisation and resolution of venous thrombus involves an angiogenic response from the vein wall that surrounds it [10]. We have previously shown that a number of angiogenic factors are temporally expressed within the resolving thrombus, including HIF1α, PLGF [18], monocyte chemotactic protein (MCP) 1, fibroblast growth factor (FGF), and VEGF [12–14]. In this study we examined whether: (i) presence of thrombus affects levels of HIF1α in vein wall; (ii) this correlates with angiogenic drive; and (iii) increasing these levels is associated with enhanced thrombus resolution and vein recanalisation.
We have shown that the untreated IVC contains more active HIF1α than both the thrombus that it surrounds (~ 12-fold increase) [18] and the infrarenal aorta (~ 2-fold increase). The presence of thrombus had no effect on HIF1α level in the IVC, although both VEGF and PLGF exhibited a temporal pattern of expression. Treatment with L-mim increased the levels of HIF1α in the IVC and these levels correlated with an increased angiogenic drive within the wall. The increased levels of HIF1α, VEGF, and PLGF were also associated with enhanced vein recanalisation and thrombus resolution. This led us to speculate that the effect of L-mim treatment on vein recanalisation and thrombus resolution [18] may, at least in part, be driven by HIF1-induced angiogenic gene expression (e.g. VEGF) in the IVC.
The similar levels of HIF1α in the IVC during natural thrombus resolution and in non-thrombosed IVC suggests that oxygen tension and HIF1α in the IVC is not affected by the presence of a thrombus or the extent to which the thrombus has resolved. The mouse IVC is a thin structure containing very few feeder microvessels (vena venora). Oxygenation of the IVC is therefore likely to occur through diffusion of oxygen from the blood in the vein lumen, which has a lower oxygen tension than arterial blood. The high levels of active HIF1α in the IVC may therefore be the result of chronic poor oxygenation.
The distinct temporal patterns of VEGF and PLGF and lack of correlation with HIF1α in the IVC during thrombus resolution suggests that VEGF and PLGF expression is HIF1-independent in the IVC. Possible explanations for this may be that chronic exposure of the IVC to a relatively hypoxic environment reduces the oxygen tension threshold at which HIF1 activation leads to transcriptional upregulation of its target genes in cells in this tissue. Expression of VEGF, for example, can also be regulated by other transcription factors including the transcriptional coactivator peroxisome-proliferator-activated receptor-gamma coactivator 1 (PGC1), which is an oxygen sensor that acts independently of HIF1 [20]. In the presence of thrombus there is also an increased inflammatory cell infiltrate [31], cells that could release a number of growth factors (including VEGF and PLGF) in an oxygen- and HIF1-independent manner. Treatment with L-mim (that acts by an oxygen-independent mechanism) resulted in increased HIF1α levels in the IVC and these levels were positively correlated with both VEGF and PLGF expression.
VEGF is a potent angiogenic growth factor produced by endothelial cells, macrophages, and neutrophils [32], which are present in the IVC and infiltrate the thrombus during its resolution [10]. VEGF expression has been localised to macrophages and endothelial cells at the periphery of the resolving thrombus and surrounding the newly-formed vascular channels within the thrombus [13]. Increasing VEGF levels in the thrombus increased vein recanalisation and thrombus resolution [16]. Enhancing the levels of angiogenic factors such as VEGF could promote thrombus resolution and vein recanalisation in a number of ways. These factors could increase: (i) local angiogenesis by promoting the outgrowth of new vessels into the thrombus from the vein wall; (ii) urokinase-type (uPA) and tissue-type plasminogen activator (tPA) activity in endothelial cells, which increases local fibrinolysis [33]; (iii) survival or mobilization of endothelial cells or their progenitors [34,35]; (iv) recruitment and activation of monocytes and neutrophils [36]; and (v) activation of monocytes, expressing proteolytic enzymes that remodel and organize the thrombus [37]. PLGF expression in the IVC may promote vein recanalisation and thrombus resolution by increasing; (i) growth, migration, and survival of endothelial cells directly via VEGFR1 [38]; (ii) monocyte recruitment; (iii) endothelial progenitor cell mobilisation [39]; and (iv) expression of synergistically-acting angiogenic factors including FGF, PDGF, and VEGF [40,41]. PLGF may also enhance VEGF signalling by displacing it from VEGFR1 and allowing it to bind with VEGFR2 [42].
This study shows that the angiogenic drive derived from the vein wall surrounding naturally-resolving thrombus is regulated independently of HIF1α and provides data to support the notion that increasing HIF1α levels in the environs of the thrombus promotes an angiogenic milieu that drives resolution.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
CE was funded by a PhD studentship (FS/06/079) from the British Heart Foundation.
References
- 1.Fowkes F.J., Price J.F., Fowkes F.G. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg. January 2003;25(1):1–5. doi: 10.1053/ejvs.2002.1778. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P., Lensing A.W., Cogo A., Cuppini S., Villalta S., Carta M. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. July 1 1996;125(1):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Anderson F.A., Jr., Wheeler H.B., Goldberg R.J., Hosmer D.W., Patwardhan N.A., Jovanovic B. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. May 1991;151(5):933–938. [PubMed] [Google Scholar]
- 4.Heit J.A., Silverstein M.D., Mohr D.N., Petterson T.M., Lohse C.M., O'Fallon W.M. The epidemiology of venous thromboembolism in the community. Thromb Haemost. July 2001;86(1):452–463. [PubMed] [Google Scholar]
- 5.Holmstrom M., Lindmarker P., Granqvist S., Johnsson H., Lockner D. A 6-month venographic follow-up in 164 patients with acute deep vein thrombosis. Thromb Haemost. August 1997;78(2):803–807. [PubMed] [Google Scholar]
- 6.Meissner M.H., Manzo R.A., Bergelin R.O., Markel A., Strandness D.E., Jr. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. October 1993;18(4):596–605. [PubMed] [Google Scholar]
- 7.Saarinen J., Kallio T., Lehto M., Hiltunen S., Sisto T. The occurrence of the post-thrombotic changes after an acute deep venous thrombosis. A prospective two-year follow-up study. J Cardiovasc Surg (Torino) June 2000;41(3):441–446. [PubMed] [Google Scholar]
- 8.Meissner M.H., Caps M.T., Zierler B.K., Polissar N., Bergelin R.O., Manzo R.A. Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. November 1998;28(5):826–833. doi: 10.1016/s0741-5214(98)70057-6. [DOI] [PubMed] [Google Scholar]
- 9.Modarai B., Burnand K.G., Sawyer B., Smith A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation. May 24 2005;111(20):2645–2653. doi: 10.1161/CIRCULATIONAHA.104.492678. [DOI] [PubMed] [Google Scholar]
- 10.Modarai B., Burnand K.G., Humphries J., Waltham M., Smith A. The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost. May 2005;93(5):801–809. doi: 10.1160/TH04-09-0596. [DOI] [PubMed] [Google Scholar]
- 11.Moldovan N.I., Asahara T. Role of blood mononuclear cells in recanalization and vascularization of thrombi: past, present, and future. Trends Cardiovasc Med. October 2003;13(7):265–269. doi: 10.1016/s1050-1738(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 12.Humphries J., McGuinness C.L., Smith A., Waltham M., Poston R., Burnand K.G. Monocyte chemotactic protein-1 (MCP-1) accelerates the organization and resolution of venous thrombi. J Vasc Surg. November 1999;30(5):894–899. doi: 10.1016/s0741-5214(99)70014-5. [DOI] [PubMed] [Google Scholar]
- 13.Waltham M., Burnand K.G., Collins M., Smith A. Vascular endothelial growth factor and basic fibroblast growth factor are found in resolving venous thrombi. J Vasc Surg. November 2000;32(5):988–996. doi: 10.1067/mva.2000.110882. [DOI] [PubMed] [Google Scholar]
- 14.Wakefield T.W., Linn M.J., Henke P.K., Kadell A.M., Wilke C.A., Wrobleski S.K. Neovascularization during venous thrombosis organization: a preliminary study. J Vasc Surg. November 1999;30(5):885–892. doi: 10.1016/s0741-5214(99)70013-3. [DOI] [PubMed] [Google Scholar]
- 15.Ali T., Humphries J., Burnand K., Sawyer B., Bursill C., Channon K. Monocyte recruitment in venous thrombus resolution. J Vasc Surg. March 2006;43(3):601–608. doi: 10.1016/j.jvs.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 16.Waltham M., Burnand K.G., Collins M., McGuinness C.L., Singh I., Smith A. Vascular endothelial growth factor enhances venous thrombus recanalisation and organisation. Thromb Haemost. January 2003;89(1):169–176. [PubMed] [Google Scholar]
- 17.Henke P.K., Wakefield T.W., Kadell A.M., Linn M.J., Varma M.R., Sarkar M. Interleukin-8 administration enhances venous thrombosis resolution in a rat model. J Surg Res. July 2001;99(1):84–91. doi: 10.1006/jsre.2001.6122. [DOI] [PubMed] [Google Scholar]
- 18.Evans C.E., Humphries J., Mattock K., Waltham M., Wadoodi A., Saha P., Modarai B., Maxwell P.H., Smith A. Hypoxia and upregulation of hypoxia-inducible factor 1α stimulate venous thrombus recanalization. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2443–2451. doi: 10.1161/ATVBAHA.110.215038. [DOI] [PubMed] [Google Scholar]
- 19.Hanze J., Weissmann N., Grimminger F., Seeger W., Rose F. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb Haemost. May 2007;97(5):774–787. [PubMed] [Google Scholar]
- 20.Fong G.H. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11(2):121–140. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 21.Kallio P.J., Pongratz I., Gradin K., McGuire J., Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci U S A. May 27 1997;94(11):5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke Q., Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. November 2006;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 23.Singh I., Smith A., Vanzieleghem B., Collen D., Burnand K., Saint-Remy J.M. Antithrombotic effects of controlled inhibition of factor VIII with a partially inhibitory human monoclonal antibody in a murine vena cava thrombosis model. Blood. May 1 2002;99(9):3235–3240. doi: 10.1182/blood.v99.9.3235. [DOI] [PubMed] [Google Scholar]
- 24.Singh I., Burnand K.G., Collins M., Luttun A., Collen D., Boelhouwer B. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: rescue by normal bone marrow-derived cells. Circulation. February 18 2003;107(6):869–875. doi: 10.1161/01.cir.0000050149.22928.39. [DOI] [PubMed] [Google Scholar]
- 25.Trotter K.W., Archer T.K. Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol Cell Biol. April 2004;24(8):3347–3358. doi: 10.1128/MCB.24.8.3347-3358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H.B., Loo K.K., Palaszynski K., Ashouri J., Lubahn D.B., Voskuhl R.R. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J Immunol. December 15 2003;171(12):6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 27.Yamada N.A., Farber R.A. Induction of a low level of microsatellite instability by overexpression of DNA polymerase Beta. Cancer Res. November 1 2002;62(21):6061–6064. [PubMed] [Google Scholar]
- 28.Warnecke C., Griethe W., Weidemann A., Jurgensen J.S., Willam C., Bachmann S. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J. June 2003;17(9):1186–1188. doi: 10.1096/fj.02-1062fje. [DOI] [PubMed] [Google Scholar]
- 29.Li N., Yi F., Sundy C.M., Chen L., Hilliker M.L., Donley D.K. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. January 2007;292(1):F207–F216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 30.Modarai B., Humphries J., Gossage J.A., Waltham M., Burnand K.G., Kanaganayagam G.S. Adenovirus-mediated VEGF gene therapy enhances venous thrombus recanalization and resolution. Arterioscler Thromb Vasc Biol. October 2008;28(10):1753–1759. doi: 10.1161/ATVBAHA.108.170571. [DOI] [PubMed] [Google Scholar]
- 31.McGuinness C.L., Humphries J., Waltham M., Burnand K.G., Collins M., Smith A. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. June 2001;85(6):1018–1024. [PubMed] [Google Scholar]
- 32.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat Med. June 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 33.Pepper M.S., Ferrara N., Orci L., Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. December 16 1991;181(2):902–906. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 34.Gerber H.P., McMurtrey A., Kowalski J., Yan M., Keyt B.A., Dixit V. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. November 13 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 35.Hattori K., Dias S., Heissig B., Hackett N.R., Lyden D., Tateno M. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. May 7 2001;193(9):1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barleon B., Sozzani S., Zhou D., Weich H.A., Mantovani A., Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. April 15 1996;87(8):3336–3343. [PubMed] [Google Scholar]
- 37.Clauss M., Gerlach M., Gerlach H., Brett J., Wang F., Familletti P.C. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. December 1 1990;172(6):1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer C., Mazzone M., Jonckx B., Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. December 2008;8(12):942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 39.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis. 2008;11(3):215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 40.Marcellini M., De L.N., Riccioni T., Ciucci A., Orecchia A., Lacal P.M. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am J Pathol. August 2006;169(2):643–654. doi: 10.2353/ajpath.2006.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy H., Bhardwaj S., Babu M., Jauhiainen S., Herzig K.H., Bellu A.R. Adenovirus-mediated gene transfer of placental growth factor to perivascular tissue induces angiogenesis via upregulation of the expression of endogenous vascular endothelial growth factor-A. Hum Gene Ther. December 2005;16(12):1422–1428. doi: 10.1089/hum.2005.16.1422. [DOI] [PubMed] [Google Scholar]
- 42.Park J.E., Chen H.H., Winer J., Houck K.A., Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. October 14 1994;269(41):25646–25654. [PubMed] [Google Scholar]