Abstract
β-cell replacement by allogeneic islet transplantation is a promising approach for patients with type 1 diabetes, but the shortage of organ donors requires new sources of β cells. Islet regeneration in vivo and generation of β-cells ex vivo followed by transplantation represent attractive therapeutic alternatives to restore the β-cell mass. In this paper, we discuss different postnatal cell types that have been envisaged as potential sources for future β-cell replacement therapy. The ultimate goal being translation to the clinic, a particular attention is given to the discrepancies between findings from studies performed in rodents (both ex vivo on primary cells and in vivo on animal models), when compared with clinical data and studies performed on human cells.
1. Introduction
Type 1 diabetes results from the specific destruction of β-cells by the immune system. The insulin-producing β-cells are the most abundant cell type residing in the islets of Langerhans, which are microorgans that are scattered throughout the exocrine tissue and represent only 1 to 2% of the total organ mass. β-cell replacement is considered the best therapeutic option, given the capacity of this particular cell type to accurately respond to highly variable changes in blood glucose level and as such to maintain glucose homeostasis. Islet transplantation via the portal vein of the liver is a promising approach and considerably less invasive than total organ transplantation. However, among other difficulties, the scarcity of donor material will always remain a major hurdle [1, 2].
Adult β-cell mass is known to be dynamic and to be able to respond to physiological changes in insulin demand such as obesity, pregnancy, and starvation. In theory, β-cell mass adaptation can occur via modification of cell size (hypertrophy versus atrophy) or modification of cell number (proliferation of existing mature β-cells or formation of new β-cells from progenitor cells versus apoptosis).
The origin of newly formed β-cells has long been debated [3–9], part of the observations reported remaining controversial and partial. In addition, many statements rely on studies performed in animal models or animal cell(s) (lines). As the ultimate goal is translation to the clinic, the aim of this paper will be to compare rodent and human data regarding postnatal cell types envisaged as potential sources to generate β-cells during the past decade.
We will focus on the replication capacity of the β-cell itself then address the unexpected recent plasticity of differentiated cell types developmentally close to β-cells. For many years the dogma was upheld that terminally differentiated cells were committed to a specific function and could no longer change their identity. In contrast, progenitor/stem cells are expected to retain some multipotency capacities and therefore be more suitable for tissue replacement strategies. Nowadays more and more examples of efficient “transdifferentiation” have been reported, without an apparent need for a progenitor cell intermediate stage. Finally, given their various capacities, adult mesenchymal stem cells (MSC) are increasingly considered for clinical use in many different applications. We will review the recent reports about both pancreatic and extrapancreatic sources of MSC and the possible application of these cells for type 1 diabetes treatment.
Several groups reported on a successful derivation of both murine and human embryonic stem cells (ESs) to β-cell-like cells able to revert hyperglycemia in diabetic mouse models (for detailed review see [10]). Mimicking normal pancreatogenesis appears to be the best strategy to differentiate ES cells toward the endocrine lineage, although large differences in differentiation capacity exist between different ES cell lines using similar protocols [11]. Importantly, the risk of teratomas formation in vivo, inherent to ES cells when they remain undifferentiated, as well as ethical principles about any therapeutical use of human ES cells currently hold back extensive clinical applications of these cells. Recent developments on technology to generate induced pluripotent stem cells (iPS) hold great promise [12–16]. However, safety issues due to genetic and epigenetic abnormalities during reprogramming or in subsequent cell culture are a major hurdle for clinical transplantation. A more immediate application of ES and iPS cells is more likely to be their use in modeling human disease [17]. Therefore, in this paper, we chose to limit ourselves to alternative postnatal cell sources The data of this part are summarized in Table 1.
Table 1.
Potential sources of de novo β-cells among differentiated adult cell types.
| Rodent | Human | |||
|---|---|---|---|---|
| in vitro | in vivo | in vitro | in vivo | |
| Endocrine | ||||
| β -cells | +++ | +++ | − | −/+ |
| α-cells | ? | ++ | ? | ? |
|
| ||||
| Exocrine | ||||
| Ductal cells | ++ | + | + | ++ |
| Acinar cells | + | −/+ | ? | ? |
|
| ||||
| Extra-pancreatic | ||||
| Liver cells | ? | ++ | + | ? |
2. Generation of β-Cells from Mature Differentiated Cells?
2.1. β-cells
During adult life, β-cell replication appears to be a predominant mechanism of β-cell mass expansion in healthy mice (Figure 1). Dor et al. demonstrated that preexisting β-cells, rather than multipotent stem cells, are the major source of new β-cells during adult life and after partial pancreatectomy [18]. The study relies on a transgenic mouse model carrying a rat insulin gene promoter-controlled expression cassette encoding a tamoxifen-dependent CRE recombinase. A pulse treatment of tamoxifen irreversibly induced the expression of a reporter gene (human placental alkaline phosphatase (HPAP)) through CRE-mediated excision of a STOP sequence specifically in β-cells. Intriguingly, the percentage of HPAP-positive β-cells immediately after tamoxifen treatment and 4, 6, 9, and 12 months later remained very similar, indicating that during normal turnover in murine β-cells originate exclusively from preexisting β-cells. In islet injury models, either by pancreatectomy or by β-cell ablation using β-cell specific expression of Diphtheria toxin A resulting in 70% and 80% reduction in β-cell mass, respectively [18, 19], new β-cells appeared to be formed largely from genetically labeled preexisting β-cells and not from neogenesis or from expansion of non-β-cell precursors. Nevertheless, murine β-cell replication capacity appears to decline with age [20–22]. Furthermore, the question was addressed whether all β-cells contribute equally to growth and maintenance of β-cell mass or if distinct subpopulations exist. Label-retaining techniques were applied. Brennand et al. performed an in vivo pulse-chase labeling experiment using Histone 2B-GFP as reporter gene [23]. A uniform label across the entire β-cell population was observed. Next, a clonal analysis of dividing β-cells revealed that all clones were of similar sizes. Altogether this suggests that, in mice, the pool of β-cells is homogenous and all cells are able to replicate at the same rate. Therefore all β-cells can be candidate for in vitro expansion. Similar conclusions were obtained from a parallel study, in which a DNA analog-based lineage tracing method was developed to detect sequential cell division in vivo [24]. Recently Dor and colleagues further investigated the replication dynamics of adult murine β-cells and showed that replicated β-cells are able to reenter the cell division cycle shortly after mitosis. This short quiescence period of several days was found to be lengthened with advanced age [25] and shortened during injury-driven β-cell regeneration and following treatment with a pharmacological activator of glucokinase [25, 26]. The data of this part are summarized in Table 1.
Figure 1.
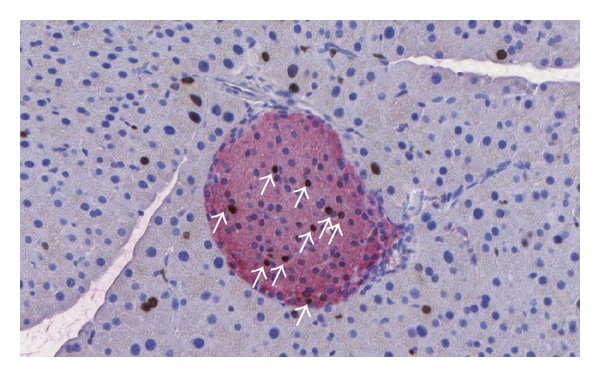
Section of murine pancreas stained with anti-insulin (red) and anti-BrdU (brown) antibodies. C57BL/6 mice were on a high-fat diet for 6 weeks. BrdU was injected for one week by s.c. injections, thereby labeling all proliferating cells. A number of replicating β-cells (arrows) and acinar cells are detected.
In humans, Meier et al. investigated the β-cell mass from infancy to adulthood by performing immunohistochemistry and morphometric analyses on pancreas obtained at autopsy from 46 donors (from 2 weeks to 21 years of age) and determined pancreas volume by CT scan in 135 individuals of similar age [27]. The authors concluded that the predominant expansion of β-cell mass in humans occurs in early childhood (2.6% of β-cells positive for Ki67), without secondary growth phase of β-cell mass during adolescence, in contrast to rodents. Interestingly, the islets appear to increase in size rather than in number. Moreover, although the authors identified some β-cells (both individual cells and in small clusters) near the ducts, the number of these insulin-positive cells increased to a similar extent as the overall expansion of β-cell numbers in the islets, consistent with the hypothesis of β-cell replication. It is important to note that an efficient β-cell mass regeneration approach in a therapeutic perspective would require a similar rate of β-cell replication. Other studies confirmed these results. Kassem et al. reported that β-cell replication decreased progressively from 3.2% at 17–32 weeks of gestation to 1.1% perinatally [28]. After birth, levels of β-cell replication would drop further to reach less than 0.1% in young adults [29]. These data also correlate with the findings that β-cell mass is established by the first two or three decades of human life as determined by measuring accumulation of lipofuscin bodies as a marker to estimate β-cell longevity [30] or by in vivo thymidine analog incorporation and radiocarbon dating [31]. Remarkably, the adaptive increase of β-cell mass in adult humans appears to be modest in response to obesity (2- versus 10-fold) [32], as well as during pregnancy (1.4 fold versus 2 to 5-fold) compared to rodents [33, 34]. In contrast to rodents again, in both obese subjects and pregnant women, the adaptive increase in β-cell number was accompanied by an increased number of small new islets, indicative of neogenesis (cf. paragraph 2.3), rather than an increase in islet size or number of β-cells per islet, characteristic for β-cell replication. Finally a study performed on human pancreatic tissue collected from 13 patients who underwent a partial pancreatectomy showed that, unlike in rodents, a 50% pancreatectomy does not trigger any β-cell regeneration in adult humans. This corroborates with the high incidence of diabetes after partial pancreatectomy [35]. In summary, in vivoβ-cell replication capacity in humans appears to be mostly limited to the very early postnatal period, and the triggers of such a process are still unknown.
Data available on β-cell proliferation in vitro are very limited when restricted to primary β-cells. An early report claimed that human β-cells were able to replicate efficiently (69% of BrdU/insulin double-positive cells) when exposed to a specific matrix (matrix produced by the rat bladder carcinoma cell line 804G) in the presence of hepatocyte growth factor/scatter factor (HGF/SF) [36]. However HGF induced a rapid decrease in insulin content [37]. This work was rapidly challenged by another report showing that the defined culture conditions were favorable for replication of ductal cells and not for β-cells [38]. In a comparative study between in vitro proliferation of purified human and rat β-cells, Parnaud et al. also failed to detect any β-cells that were positive for Ki67 or had incorporated BrdU even after 10 days of exposure [39]. However a clear proliferation of purified rat β-cells was observed and could be further enhanced by defined coatings or growth factors. Proliferation of human β-cells in (intact) isolated human islets was also assessed in the same study and remained undetectable. Therefore it appears that culture conditions for efficient human β-cell replication ex vivo have not been clearly identified.
2.2. α Cells
Differentiation of endocrine non-β-cells to β-cells is an interesting alternative mechanism for increasing the β-cell mass. A limited number of studies supported this new concept. Collombat et al. showed in various transgenic mouse models that forced expression of Pax4 at different stages (in pancreatic progenitor cells, endocrine precursor cells, or in mature α cells) resulted in a shift of all endocrine lineages toward a β-cell fate [40]. A bicistronic vector (Pax4-IRES-β-galactosidase) was used in order to follow cells of interest by staining for β-galactosidase activity. Importantly, the authors observed an age-dependent increase in islet size and in number of insulin/β-galactosidase double-positive cells and a concomitant decrease in α-cell content. These observations indicate that ectopic Pax4 expression can also force conversion of adult glucagon-expressing cells into β-cells. Remarkably, the subsequent decrease in glucagon was found to activate the differentiation of ducts-associated progenitor cells α cells (via an intermediate stage of Ngn3 positive cells). However the newly formed α cells failed to correct the hypoglucagonemia since they were shown to be rapidly converted into β-cells upon Pax4 ectopic expression. Notably, the expression of Pax4 in glucagon-positive cells has been reported to be sufficient to restore a functional β-cell mass in diabetic mice, although only in the young animals. Thorel et al. developed a transgenic mouse model of near total β-cell ablation that relies on the specific expression of Diphtheria Toxin Receptor (DTR) in pancreatic β-cells (expression driven by the rat insulin promoter) [41]. After administration of Diphtheria Toxin, a rapid and extreme (>99%) β-cell destruction by apoptosis was observed resulting in a characteristic diabetes within a couple of weeks. If given insulin, the mice survived and showed a slow β-cell mass regeneration. Only after 5 months the regenerated β-cell mass was able to maintain glucose homeostasis without exogenous insulin administration. Using a doxycycline inducible α-cell lineage tracing system, the authors established that one month after β-cell ablation, a large but variable (32 to 81%) fraction of newly formed β-cells resulted from the transdifferentiation of about 5 to 10% of α cells. Importantly, almost all (~90%) were bihormonal (still expressing glucagon even as far as 10 months after ablation). Of note, no β-cells were found in extrainsular locations. Therefore, in contrast to previous studies [19], the β-cell mass regeneration was attributed to neogenesis through α-cell transdifferentiation, rather than to the slow self-replication mechanism of preexisting mature β-cells. The authors suggested that the amount of β-cell loss and the type of injury would determine the mechanism of regeneration. This theory of unexpected pancreatic cell plasticity triggered by extreme β-cell loss was supported by a parallel study done by Chung and colleagues [42]. The model used was a combination of pancreatic duct ligation (PDL) with specific elimination of preexisting β-cells by alloxan [42]. No lineage tracing method was used in this work, and results are based on immunohistochemistry stainings performed at 7 and 14 days. The authors reported a rapid regeneration of β-cell mass within weeks and suggested that the newly formed β-cells resulted mainly from conversion of adult α cells. Interestingly the authors also observed that under injury conditions, α cells were able to replicate. However no α-cell division was required for conversion into β-cells. Importantly the regenerated β-cell mass was not sufficient to revert hyperglycemia, possibly due to the persistent inflammation caused by the PDL. In summary, three independent studies performed in transgenic mouse models showed that α- to β-cell conversion can occur. One could wonder how this relates with the developmental process. In rodents, the glucagon gene is expressed in the earliest endocrine cells that can be detected [43]. However, Herrera et al. demonstrated that, during murine embryogenesis, mature glucagon- and insulin-producing cells share a common precursor but belong to separate developmental lineages [44].
In human cells, the embryologic situation appears to be different, since insulin-positive cells are the first to be detected during development [45]. Therefore it is not known whether this extraordinary islet cell plasticity exists in adult human α cells in vitro or in vivo. If so, α cells, especially if their capacity to replicate under injury condition is confirmed, could be an ideal intraislet source for in vivo regeneration of β-cells.
2.3. Ductal Cells
Pancreatic ductal cells have long been thought to be the main source for progenitor cells in the pancreas (cf. reviews [3, 8]).
Several rodent studies based on immunohistochemical observations in animal models, suggested a possible mechanism of islet neogenesis via recapitulation of the embryological development after activation and differentiation of ductal progenitors [46–50]. Recently a number of lineage tracing studies in transgenic mouse models have been performed in order to identify putative endocrine progenitors in adults, but provided contradictory conclusions. Bonner-Weir and collaborators made use of the human carbonic anhydrase II (CAII) promoter, specific for differentiated ductal cells, combined to a tamoxifen inducible CRE-ER/Lox recombination system [51]. CAII-expressing cells within the pancreas acting as precursor cells gave rise to both pancreatic endocrine and exocrine tissues after birth and after injury. After PDL 25% of β-cells were labeled. The authors proposed that ductal cells represent a pool of homogenous cells from which a fraction can dedifferentiate into progenitor cells that are able to regenerate both endocrine and exocrine tissues. In a parallel study, Heimberg and colleagues suggested a slightly different model: a rare subpopulation of endocrine progenitor cells, located in the ductal lining, could be activated upon PDL in adult mouse pancreas and subsequently started to express an embryonic key endocrine developmental factor, Ngn3 [52]. Differentiation of an Ngn3-positive subpopulation gave rise to all islet cell types, including glucose responsive β-cells both in situ and when cultured in embryonic pancreatic explants. However, in a subsequent study a lineage tracing of HNF1β-positive ductal cells was performed. 65% of pancreatic ductal cells were labeled. The authors show that the ductal epithelium does not make a significant contribution to acinar or endocrine cells during neonatal growth (6-month observation period) or upon regeneration condition (PDL or Alloxan followed by EGF/gastrin treatment) [53]. Of interest, earlier lineage studies by Melton's group pointed out on the heterogeneity in developmental potential among “duct-like structures” in early embryo [54]. Lineage tracing experiments suggested that Ngn3-positive cells are indeed islet progenitors but distinct from duct progenitors. On the other hand, the authors could not rule out the possibility that a minor population of mature ductal cells is able to transiently activate Ngn3 gene expression and subsequently contributes to islets neogenesis. Lineage tracing of Muc1 ductal/acinar cells confirmed that Muc1-positive cells give rise to endocrine cells in utero, in line with embryological development [55]. However, after birth, Muc1 lineage-labeled cells were found to be restricted to the exocrine compartment, with no detectable contribution to islet cells. No injury or regeneration models have been tested in that particular model. Along the same line, another independent lineage labeling using the Sox9 promoter (active in early pancreatic progenitors) and resulting in 70% of labeled pancreatic ductal cells established that very few nonendocrine cells continue to arise from Sox9-positive precursors in early postnatal life, but no endocrine or acinar cell neogenesis from Sox9-positive cells occurs during adulthood [56]. Intriguingly, in contrast with an earlier study [52], the authors found that after PDL, Sox9-positive cells give rise to Ngn3-positive cells but these cells do not contribute to islets. Thus, although the function of ductal cells as endocrine progenitors in embryonic development is commonly accepted, more insight is needed into the potential of cells from the ductal compartment as an alternative source for the formation of new β-cells in adult rodents.
Immunohistochemistry stainings performed on tissues obtained at autopsy or after pancreatectomy are obviously the only possible technique to evaluate the β-cell mass in humans. The occurrence of neogenesis is suggested by the presence of insulin-positive cells in the ductal area combined to the absence of replication of this cell population (ruling out the expansion of a preexisting insulin-positive ductal cell population) (Figure 2). Careful quantification and repeated observations in different contexts can result in strong indications. Meier et al. analyzed pancreatic tissues obtained at autopsy from 46 children aged from 2 weeks to 21 years and observed about 0.5% of insulin-positive ductal cells [27]. A similar percentage has been reported by Reers et al. that studied pancreatic tissues from 20 donors aged from 7 to 66 years [57]. The rate of islet neogenesis does not seem to be affected by aging. Importantly, Butler et al. observed that ductal cells positive for insulin were increased by 2- and 3-fold during obesity and pregnancy, respectively [32, 33]. Therefore it appears that in humans islet neogenesis from ductal cells is stimulated during metabolic situations that require an increase in insulin level. It should be noted that during both obesity and pregnancy the adaptive increase of β-cell mass is much more limited in humans compared to rodents. In pathophysiological condition such as chronic pancreatitis, Phillips et al. also observed a significant increase of insulin-positive ducts in adult human pancreas of 11 patients compared to control group [58]. In another study, the question was addressed whether β-cell neogenesis occurs in the transplanted pancreas of type 1 diabetic patients who had received a simultaneous pancreas-kidney transplant (SPK) [59]. Pancreatic tissues from 9 SPK patients and 16 nondiabetic organ donors were examined by immunohistochemistry. Remarkably, high numbers 33 to 90% of ductal cells were found to be insulin positive, and 17% to 95% of the ducts harboured insulin-positive cells in SPK patients with recurrent autoimmunity and diabetes. A high degree of neogenesis was observed in patients with the most severe β-cell loss, whereas a low degree of neogenesis was observed in normoglycaemic patients, suggesting that in the particular context pancreas transplantation, hyperglycemia and chronic inflammation may strongly stimulate β-cell neogenesis from ductal cells. Intriguingly, a study by Bouwens et al. analyzing pancreatic tissues from nine adult donors revealed that 15% of all β-cells were located in units with a diameter less than 20 mm and without associated glucagon-, somatostatin-, or pancreatic polypeptide cells [29]. These single β-cell units were situated in or along ductules, from which they appear to bud as previously noticed in fetal and neonatal pancreas. Furthermore, simultaneous presence of Ki67-positive ductal cells (0.05%) and absence of Ki67-immunoreactive budding β-cells suggested that β-cell neogenesis depends on ductal cell proliferation and differentiation in humans.
Figure 2.
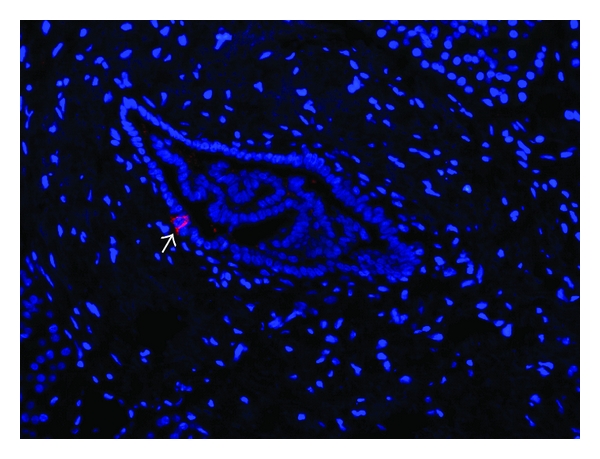
Section of human pancreatic exocrine tissue stained with an anti-insulin antibody (red). Nuclei are stained by DAPI (blue). An insulin-positive cell (arrow) is detected in the duct lining suggesting a process of β-cell neogenesis.
About ten years ago murine ductal cells cultured in vitro were reported to form 3D clusters that differentiate to functional islet cells, which are able to respond to a glucose challenge and to reverse diabetes in mice [60]. An interesting strategy for the prospective isolation of putative progenitors from an enriched ductal cell population is also being pursued by Taniguchi and colleagues [61, 62]. The approach combines immunohistochemical analysis of mouse pancreas to define new phenotypic markers and flow cytometry cell sorting to isolate clonal cell populations that are able to differentiate toward the endocrine lineage in vitro or in vivo. The data suggest that a population of progenitor cells was present among CD133-positive ductal cells.
Koblas et al. confirmed that a subpopulation of CD133-positive cells in human islet-depleted tissue was able to differentiate into functional insulin-producing cells in vitro and to secrete insulin in a glucose-dependent manner [63]. Furthermore, Bonner-Weir and collaborators showed that human primary ductal cells could be isolated from islet-depleted pancreatic tissue, expanded in culture, and triggered to differentiate towards glucose responsive islet-like clusters [64]. These results were confirmed by Gao et al. who further characterized the nature of these pancreatic progenitor cells [65]. During monolayer expansion, two subpopulations of proliferating cells were observed, CK19-positive ductal cells at an early time point (day 3) and nestin-positive cells at a later time point (day 7). Under serum-free conditions and Matrigel covering of the cells, the CK19-positive cells, but not the Nestin-positive cells, were able to form islet-like clusters that contain insulin- and glucagon-positive cells. When transplanted under the kidney capsule of nude mice, one out of five grafts demonstrated further growth with foci of both endocrine and exocrine cells. Next, Bonner-Weir and colleagues used magnetic cell sorting and antibodies raised against the ductal surface marker CA19-9 to isolate ductal cells from islet-depleted tissue [66]. Transplantation experiments of purified ductal cells versus unpurified preparations (56% CK19-positive cells only) into normoglycemic NOD/SCID mice revealed that differentiation of ductal cells to insulin-producing cells was dependent on the presence of nonductal cells, probably pancreatic stromal cells as suggested by the authors. Of interest, in vitro islet-to-duct plasticity has also been reported for human cells [67, 68].
Although some lineage tracing studies in rodents have provided contradictory results, most in vivo and in vitro data from both human studies indicate that cells from the ductal compartment are an attractive putative cell source for β-cell replacement strategies.
2.4. Acinar Cells
Besides the ductal origin hypothesis, a number of studies show that acinar cells might also display a certain degree of plasticity.
Melton and colleagues suggested that acinar cells and endocrine cells share a common progenitor after the ductal cell lineage has already separated [54]. On the other hand, Bouwens and colleagues proposed a variant of the ductal origin hypothesis, in which acinar cells would be able to transdifferentiate towards β-cells, through an intermediate dedifferentiated ductal-like stage [49, 69]. This model was supported by more reports. β-cell mass regeneration has been studied after streptozotocin treatment in a transgenic mouse model expressing interferon gamma under the control of the insulin promoter [70]. New β-cells appeared to result primarily from the formation of new islets from small pancreatic ducts. However, interestingly, some putative transitional cells were identified harboring both exocrine and endocrine granules, indicative of acinar cells as possible precursor cells. Similar observations were made in a parallel study after PDL [71]. Furthermore, in order to visualize better possible intermediate stages during acinar-to-ductal transdifferentiation in a PDL injury model, Lardon et al. took advantage of the fact that dexamethasone treatment inhibits the loss of amylase from acinar cells [72]. Putative transitional cells coexpressing acinus-specific (amylase) and duct-specific (CK20) markers were identified in vivo. Furthermore, acinus-to-islet conversion was confirmed in vitro after isolation of acini and identification of putative transitional cells coexpressing acinus-specific (amylase) and β-cell-specific (insulin) markers. Several groups recently performed some in vivo lineage tracing analyses using acinus-specific promoters (amylase and elastase). Replication of preexisting acinar cells is seen as the major mechanism for regeneration of the acinar tissue. Moreover, acinus-to-duct transdifferentiation has been reported to occur in vivo, although at a very low frequency, in mouse models for pancreatitis [73], and in a mouse model that develops insulin-positive cell-containing hyperplastic ducts in response to the growth factor TGFα [74]. However, the same authors also showed that the insulin positive cells adjacent to acinus-derived ductal cells arose from preexisting insulin-positive cells and not from acinar cells. Along the same line, Stoffers and collaborator failed to observe any acinus-to-β-cell transdifferentiation in adult mice under normal conditions and after 70% pancreatectomy, PDL, or caerulein-induced pancreatitis [75]. On the other hand, Melton et al. reported that adenovirus-mediated coexpression of Pdx1, Ngn3, and MafA in vivo in pancreas from adult mice was sufficient to induce the transdifferentiation of mature exocrine cells into β-cells that are indistinguishable from endogenous islet β-cells in size, shape, and ultrastructure [76]. However the question remains open whether acinar cells or other possible precursor cells were reprogrammed in these experiments.
In vitro, rat exocrine cells treated with dexamethasone can convert to hepatocyte-like cells. In contrast, when cultured in low serum medium (1%) in the presence of EGF and LIF, rat exocrine cells can transdifferentiate to functional β-cells [77]. In that particular study, 10% of dedifferentiated acinar cells expressed insulin, with a total insulin content of 40 to 90% of primary β-cells, and transplantation of about 100,000 of these insulin-positive cells was sufficient to revert hyperglycemia in a diabetic nude mouse model. In an independent study, Minami et al. showed that suspension culture in presence of EGF and nicotinamide converted 5% of adult murine acinar cells to glucose responsive insulin-producing cells [78]. An adenovirus mediated in vitro lineage tracing study using the acinus-specific amylase or elastase promoter confirmed the identity of the starting population. In addition, acinus-to-duct transdifferentiation was shown to occur, in response to EGF-receptor signalling, through an intermediate nestin-positive stage in an in vitro culture of pancreatic explants [79].
Regarding human cells, there are no data available about a possible acinus-to-islet cell plasticity. One possible explanation is the selective death by apoptosis of human acinar cells when cultured in vitro. In contrast, human ductal cells can survive, adhere to plastic culture dish, and proliferate [80]. Nevertheless the possible acinus-to-duct transdifferentiation suggested by others [81] cannot be ruled out in this study.
2.5. Liver Cells
Liver and pancreas have a common embryological origin, both arising from the primitive gut. Therefore liver cells have been hypothesized to constitute a potential cell source for β-cell generation [82].
Ferber and collaborators demonstrated that an adenovirus-mediated expression of the key pancreatic transcription factor Pdx1 in mouse liver (intravenous infusion) resulted in transdifferentiation of hepatocytes to β-cell-like cells [83]. Pdx1 expression found in 60% of hepatocytes resulted in a 3-fold increase of plasma insulin levels, 59% as insulin and 41% as proinsulin. These data indicate that proinsulin was processed which was substantiated by expression of the prohormone convertase 1/3. The amount of insulin produced was sufficient to reduce hyperglycemia in a diabetic mouse model. A similar approach was successfully reported with adenovirus-mediated expression of Pdx1 or Ngn3 [84] and the coexpression of NeuroD1/Beta2 and betacellulin [85] in the liver. Of note, although the overexpression of Pdx1 was able to revert the elevated blood glucose of diabetic mice, the animals died from liver inflammation most likely due to the exocrine-differentiating activity of Pdx1. Interestingly Yechoor et al. showed that gene transfer of a pancreatic key transcription factor (Ngn3 in this study) in liver leads to long-term diabetes reversal in mice [86]. However the authors demonstrated that, although insulin expression was transiently induced in terminally differentiated hepatocytes, the long-term diabetes reversal obtained in these mice was resulting from the differentiation of hepatic progenitors able to generate islet-like clusters. The phenomenon was described as “transdetermination,” that is, lineage switching in lineage-determined, but not terminally differentiated, cells.
Human adult liver cells were shown to expand in vitro and to transdifferentiate towards an endocrine pancreatic lineage after Pdx1 overexpression [87]. Pdx1-expressing human liver cells were found to express insulin that is stored in secretory granules, which are released in a glucose-regulated manner. When transplanted under the kidney capsule of diabetic immunodeficient mice, these cells ameliorated hyperglycemia for prolonged periods of time. Similar studies using human fetal progenitor liver cells were reported [88, 89]. Since harvesting and propagating significant numbers of primary hepatocytes from patients with diabetes would be theoretically feasible, the liver can be considered as an interesting extrapancreatic source for β-cell replacement therapy.
3. Generation of β-Cells from Adult Mesenchymal Stem Cells?
Mesenchymal stem cells (MSC) were originally identified in bone marrow by Friedenstein in 1976 as a rare, heterogeneous, non hematopoietic, and multipotent stromal population able to differentiate to mesenchymal lineages including bone, fat, and cartilage. MSC are virtually present in all organs, including the pancreas and the islets of Langerhans. It is now generally accepted that the perivascular area harbors the MSC, explaining the ubiquitous distribution of these cells in the body [90]. Interestingly, MSC can be obtained from live donors (and potentially from the patient itself) and are easily expandable in vitro. Therefore, despite the fact that their identity and their exact role in vivo have not been clearly defined yet, mesenchymal stem cells have been envisaged for a broad range of therapeutic applications including type 1 diabetes [91, 92]. The data of this part are summarized in Table 2.
Table 2.
Potential sources of de novo β-cells among differentiated adult MSC: BM: bone marrow, AT: adipose tissue; UCB: umbilical cord blood.
| Rodent | Human | |||
|---|---|---|---|---|
| in vitro | in vivo | in vitro | in vivo | |
| Pancreatic | ||||
| Islet-(derived) MSC | ? | ? | + | ? |
| Exocrine-(derived) MSC | ? | ? | −/+ | ? |
|
| ||||
| Extra-pancreatic | ||||
| BM-MSC AT-MSC UCB-MSC |
+ | −/+ | −/+ | − |
3.1. Islet(-Derived) MSC
We and others reported on the expansion of MSC-like cell population from isolated human islets: human islet-derived precursor cells (IPC) [93–95], NIP/Nestin-positive Islet-derived Progenitors [96], PIDM/Pancreatic Islet-Derived Mesenchymal cells [97], PHID/Proliferating Human Islet-Derived cells [98], and more [99, 100]. These cells are able to proliferate ex vivo and can be passaged. Interestingly, most of the groups reported a common characteristic, which is aggregation into clusters ranging in sizes between 50 and 200 μm (similar range as primary islets) under serum starvation and a subsequent increase in expression of endocrine markers. However others failed to reproduce these data of partial differentiation [101, 102]. In any case, the endocrine markers remained at very low level when compared to freshly isolated human islets. Davani et al. reported a further capacity of IPC to differentiate into functional β-cells that secrete human C-peptide in response to glucose after reimplantation of 4-day-old aggregates in mice [94].
The origin of these cells remains elusive and very controversial. Initially Gershengorn and colleagues proposed an Epithelial-to-Mesenchymal Transition (EMT) to occur from human β-cells [93]. However this idea was rapidly refuted by the same group and others after lineage tracing experiments performed in transgenic mouse models that failed to show any EMT of murine β-cells [103–106]. The overall conclusion of these four studies was that the proliferative cell population derived from cultured murine islets was not originating from β-cells, since no β-cell specific markers were identified in these cells. Efrat and collaborators recently developed a lineage tracing system similar to the techniques applied in transgenic mouse models, but now applied to human cells in vitro using lentiviral vectors [107]. The dual viral system relies on the β-cell specific expression of the CRE recombinase in one vector and a CMV-GFP reporter vector in another vector in which GFP expression is restricted by a “floxed” intermediate sequence. As lentiviral vectors integrate into the genome of the transduced cells, the reporter gene will remain expressed in all cells originating from the initial pool of labeled cells. In their follow-up study, Russ et al. slightly modified the system by using a tamoxifen-inducible CRE/ER recombinase, restricting the labeling period to the duration of a short (overnight) tamoxifen pulse [108]. Human β-cells were efficiently (50%) and specifically labeled by the dual lentiviral system. This powerful technique provided evidence that human β-cells can dedifferentiate to an MSC-like cell population and proliferate when cultured ex vivo, in contrast to mouse β-cells, as revealed earlier from the transgenic mice studies [103–106]. Intriguingly, 40% of cells exhibiting MSC markers in culture (likely to be the cell population previously named IPC, NIP, PIDM, or PHID by others) resulted from an EMT of β-cells. On the other hand, several groups suggested, but never unequivocally demonstrated, the presence of a mesenchymal stem cell population within human islets [94, 96, 97]. In a recent study, we investigated the presence of MSC(-like cells) in freshly isolated human islets, and we identified a double-positive CD90/CD105 population representing approximately 2% of the total islet cell population. The presence of these cells inside freshly isolated human islets was confirmed by confocal microscopy [95]. An independent study validated the presence of pancreatic MSC in the periacinar, perivascular, and periductal space of human pancreas [109]. The functional significance of the presence of these cells in the islets and the possible interplay between islet-MSC, endocrine cells, and the vascular system in human islets remain to be further clarified. Altogether these data suggest that the MSC-like cell population derived from human islets in culture results from subpopulations of at least two origins: proliferation of islet-MSC and proliferation of dedifferentiated β-cells. More studies, in particular detailed lineage-tracing experiments will be needed to confirm this model.
At the moment it remains unclear whether all mesenchymal stem(-like) cells are equal and whether MSC originating from islets would be more prone to differentiate toward the endocrine lineage. Two groups determined the gene expression profiles in human islet-derived MSC [110, 111]. Both studies confirmed the common mesenchymal character of the population with the archetypical bone marrow-MSC. Remarkably cultured islet-derived IPCs are different from bone marrow-MSC (BM-MSC) in that they express a set of islet-specific genes, although at low level. Upon differentiation, following the rather basic differentiation protocols developed so far for these cells, gene expression data showed that IPCs are able to go further along the endocrine pathways than BM-MSC.
In summary, islet-(derived) MSC could be of a valuable therapeutic significance since they appear to retain some genetic characteristic making them closer to endocrine cells than other sources of MSC such as bone-marrow-derived MSC. Further studies will be needed to verify this hypothesis. Furthermore, the observation that human β-cells can dedifferentiate ex vivo into a mesenchymal phenotype in contrast to murine β-cells illustrates again the discrepancy in plasticity of these cells between rodents and humans.
3.2. Exocrine/Islet Depleted Tissue(-Derived) MSC
Similarly to human islet cells cultured ex vivo, two independent studies reported that a population of MSC(-like) cells could be derived from human pancreatic exocrine tissue. Under defined conditions, these cells could show some sign of differentiation toward the endocrine lineage [112, 113].
The exact origin of these cells remains also unclear. On the one hand, epithelial-to-mesenchymal transition from exocrine cells has been proposed by Seeberger et al. [114]. Along the same line, Fanjul et al. described coexpression of ductal markers and mesenchymal markers both in cultured human ductal-like cells in vitro and in ductal cells in one pancreas from a nondiabetic subject and three pancreata from patients with type 2 diabetes [115]. Shin et al. demonstrated that the transdifferentiation capacity of human ductal cells was reduced after EMT [116]. On the other hand, Sordi et al. suggested that the mesenchymal stem cell population growing out of cultured pancreatic tissue (both endocrine and exocrine fractions were tested) would be mostly originating from proliferating “pancreatic MSC” that are partly derived from the bone marrow [109]. Total bone marrow transplantations from donor GFP transgenic mice in lethally irradiated recipient mice were performed. Surprisingly, after 12 weeks, GFP-labeled bone-marrow-derived cells were found to be localized preferentially in two organs, pancreas (4.82 ± 4% of total) and lung (4.43 ± 2.3% of total). 18.5 ± 4% of MSC derived in culture from these pancreata were found to be GFP positive.
However once again, in absence of data on pancreatic tissues after bone marrow transplantation in humans, we can wonder how these results would be translatable to the human situation. In a study of a pancreas allograft removed 8 months after transplantation, it was found that part of pancreatic MSC expressed recipient HLA, suggesting an extrapancreatic origin of these cells [109].
Besides their putative differentiation capacity, MSC display very interesting additional characteristics. Sordi et al. reported that pancreatic MSC extracted from both endocrine and exocrine tissue and cotransplanted in mice with a minimal pancreatic islet mass facilitated the restoration of normoglycemia and neovascularization of the islet graft [109].
In conclusion, MSC can be derived from human exocrine tissue but show limited capacity of differentiation towards the endocrine pathway. Similarly to islet(-derived) MSC, the origin and role of these cells are unclear.
3.3. Extrapancreatic Sources of MSC
Bone marrow, adipose tissue, and umbilical cord blood are the main sources of BM-MSC reported so far. Hess et al. reported that transplantation of murine bone marrow cells in streptozotocin-treated mice was able to reduce hyperglycemia by initiating endogenous pancreatic regeneration. A majority of bone-marrow-derived cells were found to be localized near ductal and islet structures. Quantitative analysis of the pancreas revealed a very low frequency of donor insulin-positive cells. However the presence of donor cells was accompanied by a rapid proliferation of recipient pancreatic β-cells and by neogenesis of insulin-positive cells of recipient origin within a week after transplantation. The mechanism behind this regeneration process remains to be clarified. The authors suggested that bone-marrow-derived endothelial cells could be involved by secreting factors that enhance tissue repair [117]. This model was confirmed by others [118]. Along the same line, Lechner et al. observed no evidence for significant transdifferentiation of labeled (GFP transgenic mice) murine bone marrow into pancreatic β-cells in vivo [119]. By using a mouse model for impaired bone-marrow-derived cell mobilization (Nos3 −/− mice), Hasegawa et al. demonstrated that homing of donor bone-marrow-derived cells in recipient bone marrow and subsequent mobilization into the injured periphery were required for β-cell regeneration. Interestingly, simple bone marrow cell infusion without preirradiation had no effects, suggesting that injury signals are involved in triggering this process [120]. Finally, in similar transplantation experiments, Urban et al. revealed that murine-bone-marrow derived mesenchymal stem cells cooperate with bone marrow cells since neither bone marrow cells nor MSC transplantation was effective alone [121]. In contrast to all these studies and using a lineage tracing system (Ins/CRE-LoxP/EGFP), Ianus et al. claimed that transplanted murine bone marrow cells were able to differentiate themselves into functional β-cells [122]. Four to six weeks after transplantation from male mice into lethally irradiated recipient female mice, recipient mice contained Y chromosome and EGFP double-positive cells in their pancreatic islets. Of note, β-cell specificity was verified since neither bone marrow cells nor circulating peripheral blood nucleated cells of donor or recipient mice had any detectable EGFP. Cell fusion was also ruled out in this experiment.
Regarding human cells, Prockop and colleagues evaluated the competence of bone marrow MSC in a similar type of experiment. Human bone marrow MSC were delivered via intracardiac infusions in diabetic NOD/SCID mice. Although rare β-cells were found to be of human origin (i.e., BM-MSC derived), blood glucose levels were found to be decreased after some weeks. The authors suggested that human BM-MSC were able to home to and promote repair of pancreatic islets and renal glomeruli in a diabetic mouse model [123]. In a parallel study, Sordi et al. showed that human BM-MSC express a restricted set of functionally active chemokine receptors (CXCR4, CX3CR1, CXCR6, CCR1, CCR7) capable of promoting migration to pancreatic islets [124]. Butler and collaborators studied 31 human pancreases obtained at autopsy from patients who had received a bone marrow-derived graft (26 cases), a peripheral blood-derived graft (4 cases), or a combination of both peripheral blood- and bone marrow-derived stem cells (1 case) [125]. More than 4000 islets were examined in this relatively large cohort, and no pancreatic β-cells were found to be derived from donor cells (including two cases of patients with type 2 diabetes). Therefore, it appears that in humans the bone marrow compartment does not contribute to pancreatic β-cell mass maintenance in healthy individuals. Nevertheless promising clinical results on glucose metabolism were recently reported. A clinical trial in 11 patients with type 1 diabetes was designed to test the safety and efficacy of intraportal coinfusion of insulin-producing adipose tissue-derived MSC and bone marrow cells. Differentiation of adipose tissue-derived MSC was initiated in vitro by a 3-day culture period in a defined medium described earlier [126]. A mean follow-up of about two years showed significant improvements of all clinical parameters related to diabetes (a decrease in insulin requirements, an increase in C-peptide levels, and absence of diabetic ketoacidosis) [127]. In a clinical trial involving 25 patients with type 2 diabetes, Ricordi and collaborators observed reduced insulin requirements and significant improvements of all metabolic variables (12-month follow-up) after an intrapancreatic infusion of autologous bone marrow cells [128]. However no data are available about the possible differentiation of MSC after implantation, and it is more likely that improved glucose metabolism is related to paracrine effects of MSC in this last study.
In vitro, several groups reported the successful derivation of functional β-cells from rodent bone marrow cells in the presence or absence of serum [129] and with addition of growth factors like nicotinamide and β-mercaptoethanol [130] and conophylline and betacellulin-delta4 [131]. After transplantation these cells can (partly) revert hyperglycemia in diabetic mice. Also murine adipose tissue-derived MSC could be efficiently converted reaching up to 48% of cells that expressed c-peptide, following a 3-stage and 10-day differentiation protocol involving activin A, sodium butyrate, β-mercaptoethanol, taurine, GLP-1, nicotinamide, and nonessential amino acids [132].
The approach is slightly different in human cells, involving the combination of defined medium and virus-mediated ectopic expression of proendocrine transcription factors. Karnieli et al. reported that overexpression of Pdx1 in BM-MSC from 9 of 14 donors can trigger their differentiation to a β-cell-like phenotype displaying about 1% of the regular insulin content and able to control the insulin release in a glucose-dependent manner in vitro [133]. The cells lacked expression of Beta2/NeuroD1. However transplantation into streptozotocin-treated mice resulted in further differentiation, including induction of Beta2/NeuroD1 and reduction of hyperglycemia. Similar results were obtained in a parallel study [134]. Human MSC from other sources than bone marrow were also evaluated. MSC isolated from the Wharton's jelly of the umbilical cord were differentiated to islet-like cell clusters through stepwise culturing in neuron-conditioned medium [135]. The clusters were found to express islet-specific genes and to be glucose responsive in vitro. Functionality was further verified after transplantation into the liver of streptozotocin-induced diabetic rats via laparotomy. The presence of characteristic secretory granules was observed by electron microscopy 12 weeks after transplantation.
In summary, the capacity of extrapancreatic mesenchymal stem cells to differentiate to β-cells in vivo appears to be very limited. Nevertheless, several groups reported a successful differentiation to functional β-cell-like cells in vitro especially from human MSC. MSC appear to display unique migratory and secretory properties (growth factors, cytokines) that make them attractive as “helper” cells for tissue repair (improve engraftment, viability, function) (for review see [92, 136]).
4. Mechanisms for Maintenance of the Cellular Identity
A crucial aspect common to all putative cell sources will be to uncover the mechanisms that preserve and control cell identity in order to enable successful manipulation of adult cell plasticity in clinical settings. Up to now, most efforts to influence the cell identity were focused on direct gene expression (overexpression/downregulation of key transcription factors) and growth-factor-mediated activation of specific signaling pathways. A new era has started aiming at better understanding the processes that regulate gene expression. Chromatin accessibility is a determining factor blocking or facilitating expression of specific genes. Cell identity is regulated by epigenetic factors that tightly regulate the activation or repression of genes including genomic DNA methylation, histone modifications, and noncoding RNA regulation (for review please see [137, 138]). Dhawan et al. recently demonstrated that pancreatic β-cell identity is maintained by DNA methylation-mediated repression of Arx [139]. The question whether all MSC(-like cells) are equal was recently addressed. Mutskov et al. investigated the patterns of histone modifications over the insulin gene in human islets and IPC (the MSC-like population derived ex vivo from human islets) compared to HeLa and BM-MSC [140]. Although neither IPC nor HeLa nor BM-MSC express insulin, IPC showed significant levels of active chromatin modifications, similarly to human islets although at a more moderate level. The probable multiorigin of the IPC might obscure the interpretation of these results. However these epigenetic marks absent in the unrelated cell types (HeLa and BM-MSC) might be part of a general mechanism whereby tissue-derived precursor/stem cells are committed to a distinct specification. Non-coding RNA (such as siRNA (short interference RNA), miRNA (microRNA), and lncRNA (long non-coding RNA)) are emerging as key players in regulation of development [141]. Joglekar et al. demonstrated that the miR-30 family of miRNAs contribute to the regulation of the dedifferentiation of human fetal β-cells through epithelial-to-mesenchymal transition by negatively regulating the translation of mesenchymal genes [142].
Epigenetic reprogramming of cell types with shared developmental history could be an effective strategy for pancreatic β-cell replacement therapies. The cells may display some intrinsic commitment to become islets even during adulthood and might thus require fewer triggers to differentiate/transdifferentiate towards a β-cell lineage. Along this line, in the field of reprogramming to iPS, the notion of epigenetic “memory” inherited from the parental cell is coming forward. Bar-Nur et al. observed a preferential lineage-specific differentiation in iPS derived from human β-cells [143]. These new insights in gene regulation should help to exploit the full potential of adult cell plasticity in the perspective of cell replacement therapies to treat diseases such as type 1 diabetes.
5. Conclusions and Discussion
In conclusion, it emerges that extreme caution should be taken when translating the findings obtained from rodent studies to the human situation. Regarding in vivo regeneration investigated under physiological conditions or under injury, it appears that β-cell replication is the predominant mechanism occurring in mice. In humans, this process seems to be restricted to the very early postnatal life, whereas during adulthood, neogenesis from (a subpopulation of) ductal and/or acinar cells seems to be responsible for the increase of β-cell mass required in physiological situations of higher insulin demands like obesity or pregnancy. Nevertheless, the unanswered question remains which cell type could be involved: progenitor cells present in the ductal area, or fully differentiated adult cells able to transdifferentiate, or a distinct subpopulation that is able to dedifferentiate to a progenitor-like intermediate stage followed by redifferentiation to an endocrine lineage. Remarkably, both the animal model and the degree of destruction appear to be key points, as the regeneration processes can be different: β-cell mass regeneration from α cells has been observed in models of near total ablation only. Regarding in vitro replicative capacity of β-cells, human β-cells cannot efficiently replicate, in contrast to rodent cells. However, contrary to murine β-cells again, human β-cells can dedifferentiate to a mesenchymal-like phenotype and proliferate. These discrepancies between findings from human versus rodent studies are not as unexpected as they seem, given the major differences observed in islet cell biology field such as timing of pancreatic developmental stages [45], islet architecture and composition [144, 145], and islet innervation [146]. In the stem cell field, murine and human stem cells are notoriously dissimilar. Finally, cultured human primary cells are more prone to replicative aging than murine cells as telomere shortening limits cell replication and leads to senescence.
However, up to now, there is no way to accurately evaluate the β-cell mass in humans, neither by imaging techniques nor by physiological measurements. Studies are limited to histological analysis performed on organs at autopsy or after pancreatectomy generating static pictures that could be misinterpreted, even if careful quantification and repeated observations in different contexts can still result in strong indications. Therefore, animal models become essential offering access to a broad range of technologies obviously not applicable to humans. Among others, the recently developed genetic approaches (such as lineage tracing systems, comprising of a tissue-specific promoter and a (tamoxifen-)inducible recombination system and a reporter gene) are valuable tools to follow a dynamic process and reinforce (or sometimes challenge) earlier theories suggested by more descriptive immunohistochemistry data. Nevertheless several limitations have to be taken into consideration: a possible leakiness of the recombination system, the relative specificity of a given promoter that can display some (transient) activity in nonspecific cells, and a limited penetrance (usually less than half of the cells are actually labeled) [147, 148]. Altogether these elements might contribute to the discrepancies between results obtained by different labs about the origin of β-cell regeneration for instance, and hopefully validation of some of the models by separate labs in different experimental contexts will clarify the situation in a near future.
Since stimulation of human β-cells replication is still elusive, other cell sources have been envisaged. Studies from the last decade revealed an unexpected aspect of plasticity from mature differentiated cells by dedifferentiation or transdifferentiation. Of interest, it appears that regeneration does not always require recapitulation of the embryological development as needed for efficient differentiation of ES cells. For instance, the recent discovery of α-to-β-cell plasticity does not appear to correlate with any developmental process [7]. Murine and human ductal cells, rodent acinar cells (human ones cannot be maintained in culture), and human liver cells could be efficiently converted to functional β-cells able to revert hyperglycemia in a diabetic mouse model. Although the potential contribution of MSC to islet regeneration in a physiological situation remains unclear and their origin and function are still elusive, islet-derived mesenchymal stem cells seem to display specific genetic and epigenetic marks that could make them more prone to differentiation towards the endocrine compartment than extrapancreatic sources of MSC. This suggests that all mesenchymal stem cells (-like cells) are not equal. In addition MSC, in particular from humans, revealed additional properties (like homing to injury site and secretion of favorable growth factors) that may be of clinical use. Therefore, further investigations will be required to determine if islet-(derived-) MSC can be stimulated to contribute in any way to β-cell mass regeneration. Another approach successfully tested has been to force expression of proendocrine transcription factors in vivo in the liver and in the exocrine tissue. However no lineage tracing experiments have been performed in these studies, and the origin of the newly formed β-cells needs to be identified.
Finally, there is currently no unique optimal alternative cell source for β-cell (re)generation. Therefore a crucial aspect common to all putative cell sources will be to further uncover the mechanisms that preserve and control cell identity in order to enable successful manipulation of adult cell plasticity for clinical application.
Acknowledgment
This work has been supported by the Dutch Diabetes Fonds, the Stichting DON, The Bontius Foundation and the DCTI consortium.
References
- 1.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 2.de Kort H, de Koning EJ, Rabelink TJ, Bruijn JA, Bajema IM. Islet transplantation in type 1 diabetes. British Medical Journal. 2011;342:p. d217. doi: 10.1136/bmj.d217. [DOI] [PubMed] [Google Scholar]
- 3.Granger A, Kushner JA. Cellular origins of β-cell regeneration: a legacy view of historical controversies. Journal of Internal Medicine. 2009;266(4):325–338. doi: 10.1111/j.1365-2796.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nature Reviews Endocrinology. 2010;6(3):139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 5.Collombat P, Xu X, Heimberg H, Mansouri A. Pancreatic beta-cells: from generation to regeneration. Seminars in Cell and Developmental Biology. 2010;21(8):838–844. doi: 10.1016/j.semcdb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung CH, Levine F. Adult pancreatic alpha cells: a new source of cells for beta-cell regeneration. Review of Diabetic Studies. 2010;7(2):124–131. doi: 10.1900/RDS.2010.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desgraz R, Bonal C, Herrera PL. β-Cell regeneration: the pancreatic intrinsic faculty. Trends in Endocrinology and Metabolism. 2011;22(1):34–43. doi: 10.1016/j.tem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domínguez-Bendala J, Inverardi L, Ricordi C. Stem cell-derived islet cells for transplantation. Current Opinion in Organ Transplantation. 2010 doi: 10.1097/MOT.0b013e32834252b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Hoof D, D’Amour KA, German MS. Derivation of insulin-producing cells from human embryonic stem cells. Stem Cell Research. 2009;3(2-3):73–87. doi: 10.1016/j.scr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Nostro MC, Sarangi F, Ogawa S, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. Journal of Biological Chemistry. 2008;283(46):31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Jiang W, Liu M, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Research. 2009;19(4):429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 15.Alipio Z, Liao W, Roemer EJ, et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(30):13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maehr R, Chen S, Snitow M, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mummery C. Induced pluripotent stem cells—a cautionary note. New England Journal of Medicine. 2011;364(22):2160–2162. doi: 10.1056/NEJMcibr1103052. [DOI] [PubMed] [Google Scholar]
- 18.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 19.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by β cell regeneration. Journal of Clinical Investigation. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes. 2005;54(9):2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 21.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in β-cell proliferation restricts the capacity of β-cell regeneration in mice. Diabetes. 2009;58(6):1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443(7110):453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 23.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biology. 2007;5(7):p. e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult β cells does not involve specialized progenitors. Developmental Cell. 2007;12(5):817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development. 2010;137(19):3205–3213. doi: 10.1242/dev.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metabolism. 2011;13(4):440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Meier JJ, Butler AE, Saisho Y, et al. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57(6):1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. β-Cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49(8):1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- 29.Bouwens L, Pipeleers DG. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia. 1998;41(6):629–633. doi: 10.1007/s001250050960. [DOI] [PubMed] [Google Scholar]
- 30.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modeling of lipofuscin accumulation. Diabetologia. 2010;53(2):321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- 31.Perl SY, Kushner JA, Buchholz BA, et al. Significant human β-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. Journal of Clinical Endocrinology and Metabolism. 2010;95(10):E234–E239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 33.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieck S, Kaestner KH. Expansion of β-cell mass in response to pregnancy. Trends in Endocrinology and Metabolism. 2010;21(3):151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menge BA, Tannapfel A, Belyaev O, et al. Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes. 2008;57(1):142–149. doi: 10.2337/db07-1294. [DOI] [PubMed] [Google Scholar]
- 36.Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS. Growth factor/matrix-induced proliferation of human adult β- cells. Diabetes. 1995;44(12):1458–1460. doi: 10.2337/diab.44.12.1458. [DOI] [PubMed] [Google Scholar]
- 37.Beattie GM, Cirulli V, Lopez AD, Hayek A. Ex Vivo Expansion of Human Pancreatic Endocrine Cells. Journal of Clinical Endocrinology and Metabolism. 1997;82(6):1852–1856. doi: 10.1210/jcem.82.6.4009. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre VH, Otonkoski T, Ustinov J, Huotari MA, Pipeleers DG, Bouwens L. Culture of adult human islet preparations with hepatocyte growth factor and 804G matrix is mitogenic for duct cells but not for β-cells: effect of HGF on human 2-cells. Diabetes. 1998;47(1):134–137. doi: 10.2337/diab.47.1.134. [DOI] [PubMed] [Google Scholar]
- 39.Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51(1):91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 40.Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell. 2009;138(3):449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28(9):1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 43.Gromada J, Franklin I, Wollheim CB. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine Reviews. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 44.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 45.Piper K, Brickwood S, Turnpenny LW, et al. Beta cell differentiation during early human pancreas development. Journal of Endocrinology. 2004;181(1):11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg L, Duguid WP, Vinik AI. The effect of cellophane wrapping of the pancreas in the Syrian golden hamster: autoradiographic observations. Pancreas. 1989;4(1):31–37. doi: 10.1097/00006676-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 48.Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993;118(1):33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 49.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38(12):1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, Zangen DH, Reitz P, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48(3):507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- 51.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, D’Hoker J, Stangé G, et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Developmental Cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129(10):2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 55.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Developmental Biology. 2010;10 doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopp JL, Dubois CL, Schaffer AE, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reers C, Erbel S, Esposito I, et al. Impaired islet turnover in human donor pancreata with aging. European Journal of Endocrinology. 2009;160(2):185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 58.Phillips JM, O’Reilly L, Bland C, Foulis AK, Cooke A. Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes. 2007;56(3):634–640. doi: 10.2337/db06-0832. [DOI] [PubMed] [Google Scholar]
- 59.Martin-Pagola A, Sisino G, Allende G, et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia. 2008;51(10):1803–1813. doi: 10.1007/s00125-008-1105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nature Medicine. 2000;6(3):278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53(8):2143–2152. doi: 10.2337/diabetes.53.8.2143. [DOI] [PubMed] [Google Scholar]
- 62.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132(2):720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 63.Koblas T, Pektorova L, Zacharovova K, et al. Differentiation of CD133-positive pancreatic cells into insulin-producing islet-like cell clusters. Transplantation Proceedings. 2008;40(2):415–418. doi: 10.1016/j.transproceed.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 64.Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52(8):2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 66.Yatoh S, Dodge R, Akashi T, et al. Differentiation of affinity-purified human pancreatic duct cells to β-cells. Diabetes. 2007;56(7):1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 67.Kerr-Conte J, Pattou F, Lecomte-Houcke M, et al. Ductal cyst formation in collagen-embedded adult human islet preparations: a means to the reproduction of nesidioblastosis in vitro. Diabetes. 1996;45(8):1108–1114. doi: 10.2337/diab.45.8.1108. [DOI] [PubMed] [Google Scholar]
- 68.Jamal AM, Lipsett M, Sladek R, Laganière S, Hanley S, Rosenberg L. Morphogenetic plasticity of adult human pancreatic islets of Langerhans. Cell Death and Differentiation. 2005;12(7):702–712. doi: 10.1038/sj.cdd.4401617. [DOI] [PubMed] [Google Scholar]
- 69.Baeyens L, Bouwens L. Can β-cells be derived from exocrine pancreas? Diabetes, Obesity and Metabolism. 2008;10(supplement 4):170–178. doi: 10.1111/j.1463-1326.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 70.Gu D, Arnush M, Sarvetnick N. Endocrine/exocrine intermediate cells in streptozotocin-treated ins- IFN-γ transgenic mice. Pancreas. 1997;15(3):246–250. doi: 10.1097/00006676-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Bertelli E, Bendayan M. Intermediate endocrine-acinar pancreatic cells in duct ligation conditions. American Journal of Physiology. 1997;273(5):C1641–C1649. doi: 10.1152/ajpcell.1997.273.5.C1641. [DOI] [PubMed] [Google Scholar]
- 72.Lardon J, Huyens N, Rooman I, Bouwens L. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Archiv. 2004;444(1):61–65. doi: 10.1007/s00428-003-0930-z. [DOI] [PubMed] [Google Scholar]
- 73.Strobel O, Dor Y, Alsina J, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133(6):1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blaine SA, Ray KC, Anunobi R, Gannon MA, Washington MK, Means AL. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development. 2010;137(14):2289–2296. doi: 10.1242/dev.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet β cell, regeneration. Journal of Clinical Investigation. 2007;117(4):971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48(1):49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- 78.Minami K, Okuno M, Miyawaki K, et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(42):15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Means AL, Meszoely IM, Suzuki K, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132(16):3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 80.Street CN, Lakey JR, Rajotte RV, et al. Enriched human pancreatic ductal cultures obtained from selective death of acinar cells express pancreatic and duodenal homeobox gene-1 age-dependently. Review of Diabetic Studies. 2004;1(2):66–79. doi: 10.1900/RDS.2004.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. Journal of Pathology. 1992;166(2):97–103. doi: 10.1002/path.1711660203. [DOI] [PubMed] [Google Scholar]
- 82.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nature Medicine. 2000;6(5):568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 84.Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or neurogenin-3 in the liver. Molecular Therapy. 2007;15(2):255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- 85.Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nature Medicine. 2003;9(5):596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 86.Yechoor V, Liu V, Espiritu C, et al. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Developmental Cell. 2009;16(3):358–373. doi: 10.1016/j.devcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sapir T, Shternhall K, Meivar-Levy I, et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(22):7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zalzman M, Gupta S, Giri RK, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the β-cell phenotype. Diabetes. 2005;54(9):2568–2575. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 90.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 92.Chen CW, Montelatici E, Crisan M, et al. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine and Growth Factor Reviews. 2009;20(5-6):429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 93.Gershengorn MC, Hardikar AA, Wei C, Ceras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306(5705):2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 94.Davani B, Ikonomou L, Raaka BM, et al. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25(12):3215–3222. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- 95.Carlotti F, Zaldumbide A, Loomans CJ, et al. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets. 2010;2(3) doi: 10.4161/isl.2.3.11449. [DOI] [PubMed] [Google Scholar]
- 96.Zulewski H, Abraham EJ, Gerlach MJ, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50(3):521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 97.Gallo R, Gambelli F, Gava B, et al. Generation and expansion of multipotent mesenchymal progenitor cells from cultured human pancreatic islets. Cell Death and Differentiation. 2007;14(11):1860–1871. doi: 10.1038/sj.cdd.4402199. [DOI] [PubMed] [Google Scholar]
- 98.Ouziel-Yahalom L, Zalzman M, Anker-Kitai L, et al. Expansion and redifferentiation of adult human pancreatic islet cells. Biochemical and Biophysical Research Communications. 2006;341(2):291–298. doi: 10.1016/j.bbrc.2005.12.187. [DOI] [PubMed] [Google Scholar]
- 99.Lechner A, Nolan AL, Blacken RA, Habener JF. Redifferentiation of insulin-secreting cells after in vitro expansion of adult human pancreatic islet tissue. Biochemical and Biophysical Research Communications. 2005;327(2):581–588. doi: 10.1016/j.bbrc.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 100.Eberhardt M, Salmon P, von Mach M-A, et al. Multipotential nestin and Isl-1 positive mesenchymal stem cells isolated from human pancreatic islets. Biochemical and Biophysical Research Communications. 2006;345(3):1167–1176. doi: 10.1016/j.bbrc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Beattie GM, Itkin-Ansari P, Cirulli V, et al. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes. 1999;48(5):1013–1019. doi: 10.2337/diabetes.48.5.1013. [DOI] [PubMed] [Google Scholar]
- 102.Kayali AG, Flores LE, Lopez AD, et al. Limited capacity of human adult islets expanded in vitro to redifferentiate into insulin-producing β-cells. Diabetes. 2007;56(3):703–708. doi: 10.2337/db06-1545. [DOI] [PubMed] [Google Scholar]
- 103.Morton RA, Geras-Raaka E, Wilson LM, Raaka BM, Gershengorn MC. Endocrine precursor cells from mouse islets are not generated by epithelial-to-mesenchymal transition of mature beta cells. Molecular and Cellular Endocrinology. 2007;270(1-2):87–93. doi: 10.1016/j.mce.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chase LG, Ulloa-Montoya F, Kidder BL, Verfaillie CM. Islet-derived fibroblast-like cells are not derived via epithelial-mesenchymal transition from Pdx-1 or insulin-positive cells. Diabetes. 2007;56(1):3–7. doi: 10.2337/db06-1165. [DOI] [PubMed] [Google Scholar]
- 105.Atouf F, Cheol HP, Pechhold K, Ta M, Choi Y, Lumelsky NL. No evidence for mouse pancreatic β-cell epithelial-mesenchymal transition in vitro. Diabetes. 2007;56(3):699–702. doi: 10.2337/db06-1446. [DOI] [PubMed] [Google Scholar]
- 106.Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic β-cells. Diabetes. 2007;56(5):1299–1304. doi: 10.2337/db06-1654. [DOI] [PubMed] [Google Scholar]
- 107.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human β-cells revealed by cell-lineage tracing. Diabetes. 2008;57(6):1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 108.Russ HA, Ravassard P, Kerr-Conte J, Pattou F, Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006417. Article ID e6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sordi V, Melzi R, Mercalli A, et al. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells. 2010;28(1):140–151. doi: 10.1002/stem.259. [DOI] [PubMed] [Google Scholar]
- 110.Kutlu B, Kayali AG, Jung S, et al. Meta-analysis of gene expression in human pancreatic islets after in vitro expansion. Physiological Genomics. 2009;39(1):72–81. doi: 10.1152/physiolgenomics.00063.2009. [DOI] [PubMed] [Google Scholar]
- 111.Limbert C, Ebert R, Schilling T, et al. Functional signature of human islet-derived precursor cells compared to bone marrow-derived mesenchymal stem cells. Stem Cells and Development. 2010;19(5):679–691. doi: 10.1089/scd.2009.0241. [DOI] [PubMed] [Google Scholar]
- 112.Seeberger KL, Dufour JM, James Shapiro AM, Lakey JRT, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Laboratory Investigation. 2006;86(2):141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 113.Baertschiger RM, Bosco D, Morel P, et al. Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas. 2008;37(1):75–84. doi: 10.1097/MPA.0b013e31815fcb1e. [DOI] [PubMed] [Google Scholar]
- 114.Seeberger KL, Eshpeter A, Rajotte RV, Korbutt GS. Epithelial cells within the human pancreas do not coexpress mesenchymal antigens: epithelial-mesenchymal transition is an artifact of cell culture. Laboratory Investigation. 2009;89(2):110–121. doi: 10.1038/labinvest.2008.122. [DOI] [PubMed] [Google Scholar]
- 115.Fanjul M, Gmyr V, Sengenès C, et al. Evidence for epithelial-mesenchymal transition in adult human pancreatic exocrine cells. Journal of Histochemistry and Cytochemistry. 2010;58(9):807–823. doi: 10.1369/jhc.2010.955807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shin JA, Hong OK, Lee HJ, et al. Transforming growth factor-β induces epithelial to mesenchymal transition and suppresses the proliferation and transdifferentiation of cultured human pancreatic duct cells. Journal of Cellular Biochemistry. 2011;112(1):179–188. doi: 10.1002/jcb.22929. [DOI] [PubMed] [Google Scholar]
- 117.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nature Biotechnology. 2003;21(7):763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 118.Gao X, Song L, Shen K, Wang H, Niu W, Qin X. Transplantation of bone marrow derived cells promotes pancreatic islet repair in diabetic mice. Biochemical and Biophysical Research Communications. 2008;371(1):132–137. doi: 10.1016/j.bbrc.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 119.Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic β-Cells in vivo. Diabetes. 2004;53(3):616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 120.Hasegawa Y, Ogihara T, Yamada T, et al. Bone marrow (BM) transplantation promotes β-cell regeneration after acute injury through BM cell mobilization. Endocrinology. 2007;148(5):2006–2015. doi: 10.1210/en.2006-1351. [DOI] [PubMed] [Google Scholar]
- 121.Urbán VS, Kiss J, Kovács J, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26(1):244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 122.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. Journal of Clinical Investigation. 2003;111(6):843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 125.Butler AE, Huang A, Rao PN, et al. Hematopoietic stem cells derived from adult donors are not a source of pancreatic β-cells in adult nondiabetic humans. Diabetes. 2007;56(7):1810–1816. doi: 10.2337/db06-1385. [DOI] [PubMed] [Google Scholar]
- 126.Timper K, Seboek D, Eberhardt M, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochemical and Biophysical Research Communications. 2006;341(4):1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 127.Vanikar AV, Dave SD, Thakkar UG, Trivedi HL. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells International. 2010;2010:p. 5. doi: 10.4061/2010/582382. Article ID 582382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Estrada EJ, Valacchi F, Nicora E, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplantation. 2008;17(12):1295–1304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- 129.Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans differentiating into insulin-producing cells for the treatment of type I diabetes. Laboratory Investigation. 2004;84(5):607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 130.Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World Journal of Gastroenterology. 2004;10(20):3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hisanaga E, Park KY, Yamada S, et al. A simple method to induce differentiation of murine bone marrow mesenchymal cells to insulin-producing cells using conophylline and betacellulin-delta4. Endocrine Journal. 2008;55(3):535–543. doi: 10.1507/endocrj.k07e-173. [DOI] [PubMed] [Google Scholar]
- 132.Chandra V, G S, Phadnis S, Nair PD, Bhonde RR. Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells. 2009;27(8):1941–1953. doi: 10.1002/stem.117. [DOI] [PubMed] [Google Scholar]
- 133.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25(11):2837–2844. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 134.Li Y, Zhang R, Qiao H, et al. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. Journal of Cellular Physiology. 2007;211(1):36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- 135.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS ONE. 2008;3(1) doi: 10.1371/journal.pone.0001451. Article ID e1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sordi V, Piemonti L. Mesenchymal stem cells as feeder cells for pancreatic islet transplants. Review of Diabetic Studies. 2010;7(2):132–143. doi: 10.1900/RDS.2010.7.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barrero MJ, Boué S, Izpisúa Belmonte JC. Epigenetic Mechanisms that Regulate Cell Identity. Cell Stem Cell. 2010;7(5):565–570. doi: 10.1016/j.stem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 138.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Developmental Cell. 2010;19(5):698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 139.Dhawan S, Georgia S, Tschen S-I, Fan G, Bhushan A. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Developmental Cell. 2011;20(4):419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mutskov V, Raaka BM, Felsenfeld G, Gershengorn MC. The human insulin gene displays transcriptionally active epigenetic marks in islet-derived mesenchymal precursor cells in the absence of insulin expression. Stem Cells. 2007;25(12):3223–3233. doi: 10.1634/stemcells.2007-0325. [DOI] [PubMed] [Google Scholar]
- 141.van Leeuwen S, Mikkers H. Long non-coding RNAs: guardians of development. Differentiation. 2010;80(4-5):175–183. doi: 10.1016/j.diff.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 142.Joglekar MV, Patil D, Joglekar VM, et al. The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets. 2009;1(2):137–147. doi: 10.4161/isl.1.2.9578. [DOI] [PubMed] [Google Scholar]
- 143.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9(1):17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 144.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. Journal of Histochemistry and Cytochemistry. 2005;53(9):1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 145.Bosco D, Armanet M, Morel P, et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metabolism. 2011;14(1):45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metabolism. 2010;11(1):2–3. doi: 10.1016/j.cmet.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Murtaugh LC. Stem cells and β cells: the same, but different? Cell Stem Cell. 2011;8(3):244–245. doi: 10.1016/j.stem.2011.02.010. [DOI] [PubMed] [Google Scholar]


