Abstract
Contemporary laboratory animal guidance suggests that tail biopsy of laboratory mice can be performed before 21 d of age without anesthesia, whereas older mice must receive anesthesia before biopsy. Our objective was to determine whether administration of isoflurane anesthesia before tail biopsy produced a measurable effect on the behavior of mice (n = 196). We evaluated C57BL/6 and BALB/c mice at 21 to 24 (weaning), 28 to 31 (delayed weaning), and 42 to 45 (adult) d of age. Mice were observed at the time of biopsy and then twice within the first hour after a sham or tail biopsy. Anxiety-like responses were assessed by using an elevated plus-maze. Activity was evaluated remotely for 120 min. Isoflurane did not diminish acute responses to tail biopsy in mice 31 d or younger compared with sham-biopsied animals but had a significant effect in C57BL/6 biopsied adult mice. In addition, mice of all ages and strains that received anesthesia, regardless of biopsy, spent more time in the enclosed maze arms and had decreased activity up to 5 h after isoflurane exposure. Although tail biopsy should be performed in young mice to avoid transection of distal mature vertebrae, our experimental paradigm indicates that isoflurane anesthesia does not appreciably enhance wellbeing over that of mice biopsied without anesthesia at weaning ages. The influence of inhaled isoflurane was demonstrable and indicated that acute and prolonged alterations in anxiety and activity must be considered when interpreting the impact of anesthesia on tail biopsy across various ages and strains of laboratory mice.
Abbreviation: B6, C57BL/6; BALB, BALB/c; EPM, elevated plus-maze
Contemporary guidance for laboratory animal practice suggests that tail biopsy of mice for tissue genotyping can be performed before 21 d of age without anesthesia, whereas older mice must receive anesthesia prior to biopsy. Refinement of tissue sampling, genotyping methodology, and the effect of tail biopsy on animals has been a focused area of investigation in recent years.1,8,14,16,22,25,30,35 Prior studies have considered the most appropriate age of animals at the time of tissue sampling, the type of tissue to harvest, the quantity of tissue to collect, and whether anesthesia or topical analgesia ameliorate discomfort associated with the biopsy procedure.
Although multiple tissues, including hair, blood, mucosa, ear, digits, and tail can be sampled successfully,3,8,14 biopsy of the distal end of the tail remains one of the most commonly used procedures. Caudal vertebral development progresses at different rates, depending on mouse strain genetics.16 Regardless of genetic background, developing tail tissue in the mouse contains mature (ossified) vertebrae within the distal 5 mm before typical weaning age (approximately 21 d old). A limited number of systematic studies have been published that directly assess the effects of anesthesia on mice for tail biopsy,1,22 despite the prevalent guidance to administer anesthesia to mice older than weaning age.
In this study, we exposed 2 strains of mice to inhalant anesthesia at the time of tail biopsy. The main objective was to determine whether inhaled anesthesia had any measurable beneficial effects on responses to the biopsy procedure in mice. Specifically, we examined acute responses, anxiety-like behavior, and locomotor activity when comparing biopsied with sham-biopsied mice. We chose C57BL/6 (B6) and BALB/c (BALB) mice of standard weaning age and older because of their frequent use in scientific research and their documented differences in tail development and responses to tail biopsy.16 In brief, B6 mice undergo more rapid vertebral development of the tail than do BALB mice, leading to increased and prolonged behavioral responses to tail biopsy collection with increased age. We selected isoflurane as the anesthetic of choice for the tail biopsy procedure because of its high safety profile compared with nonvolatile anesthetics and ubiquitous application in contemporary clinical and laboratory animal practice.
Materials and Methods
Animals and testing paradigm.
All animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals,17 and experiments were IACUC-approved. Facilities housing the animals were AAALAC-accredited. Mature male and female mice (C57BL/6NCrl and BALB/cAnNCrl) were procured from an approved vendor (Charles River Laboratories, Wilmington, MA) at approximately 6 wk of age. Mice were housed in trios (2 female, 1 male) to facilitate breeding. Resulting litters were sexed and grouped into 3 age-specific cohorts for experimental purposes. From these trios, offspring (n = 196; 95 female, 101 male) were tested over a 6-mo period at predetermined age-specific time points (Table 1). The 4 experimental treatments tested at each age included: biopsy, sham biopsy, biopsy with anesthesia, and sham with anesthesia.
Table 1.
Categorization of 196 experimental mice by strain, age, and sex
| B6 |
BALB |
|||
| Cohort no. and age | Female | Male | Female | Male |
| 1: 21–24 d | 19 | 20 | 22 | 10 |
| 2: 28–31 d | 14 | 17 | 13 | 19 |
| 3: 42–45 d | 13 | 17 | 14 | 18 |
| Total: | 46 | 54 | 49 | 47 |
The testing paradigm was modified from similar classical behavioral studies.21,24 Each mouse underwent all of the following tests in consecutive order: experimental treatment, 2 acute observations spaced 1 h apart, placement on the elevated plus-maze (EPM) for 5 min, and 120 min (light, 30 min; dark, 90 min) of locomotor activity recording; the length of time for completion of the entire paradigm was approximately 5 h per mouse. Further randomization was incorporated into the study design by the ability to enroll only 8 mice simultaneously in the testing paradigm, due to locomotor box availability. Therefore, for each tested cohort of animals, the strain, age, and treatments of mice varied. Of the 24 experimental groups, a limited number (n = 4) had 7 mice counted in the final analysis due to malfunctions in specific locomotor recordings that were detected at the time of data review. The majority of groups (n = 18) had 8 mice per group, and 2 groups contained 11 or 12 animals per group. Animals were added to replace those mice that unexpectedly jumped from the EPM platform prior to the completion of trial recording, based on published recommendations that incomplete trials should be excluded from analyses.34
Caging and husbandry.
Animals were housed on an 12:12-h light:dark cycle in individual static microisolation cages (Max 75, Alt Design, Siloam Springs, AR) that were autoclaved with bedding (diameter, 0.12 in.; Bed-O-Cob, Animal Specialties and Provisions, Quakertown, PA) prior to use. Mice received water by autoclaved water bottles and were provided ad libitum access to autoclaved chow (LabDiet 5010, Animal Specialties and Provisions). Cotton nesting pads (Nestlets, Ancare, Bellmore, NY) and shelters (Shepherd Shacks, Shepherd Specialty Papers, distributed by Animal Specialties and Provisions) were provided to all breeding cages and weanling cages.
Sentinel mice at our institution were tested inhouse for 3 quarters per year and were found to be free from fur mites and pinworms (Syphacia spp. and Aspiculuris spp. by cecal exam). Sentinel mice also tested negative for antibodies to pathogens including mouse hepatitis virus, mouse parvoviruses, rotavirus, ectromelia virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, and Sendai virus. One quarter per year, sentinels from the housing facility were tested by an outside contract laboratory and were found to be free from all pathogens contained on a comprehensive assessment panel (HM Assessment Plus panel, Charles River Laboratories).
Experimental design.
Tail biopsy.
All mice were selected randomly from age-appropriate litters and uniquely identified by permanent ink markings at the tail base. Individual mice were exposed to isoflurane delivered by vaporizer (SurgiVet, Smith Medical, Dublin, OH) into an induction chamber (Braintree Scientific, Braintree, MA) at a concentration of 5% with 0.75 L/min O2. Mice underwent a 5-mm tail biopsy or sham biopsy (brief pressure placed at 5 mm proximal to the tail tip). Experimental mice receiving anesthesia were placed in the induction box and exposed to isoflurane for less than 1 min to render them unconscious, as determined by a loss of righting reflex and nonresponsiveness to toe pinch. All mice were manually restrained by using a one-handed grip before placement onto a plastic block with permanent grooves denoting 5-mm increments to provide consistency in sampling lengths. The tail was held in position next to the measured grooves and wiped briefly with alcohol. A single-use scalpel blade was held perpendicular to the tail to make a transverse biopsy cut. To promote hemostasis after biopsy, manual pressure was applied by using a disposable gauze sponge. Mice were returned to clean standard shoebox cages for acute behavioral assessments and regained consciousness within 45 s after removal from the induction chamber.
Acute observations.
Two observers were involved in the study and were assigned randomly to conduct experiments; therefore, mice were scored by direct observation by a single observer. Observers trained together to ensure consistency in grading by using a printed scoring sheet to avoid introduction of subjective bias. Blinding of observers to biopsy and strain (due to hair coat color differences) was not possible for the described testing paradigm; however, exposure to isoflurane and specific ages of mice were blinded by using coding of animals (as described earlier).
Observations of mouse behavior began at the actual moment of biopsy; a point was recorded if mice made an audible response or flicked the tail from the restrained position at the time of tissue transection. Mice then were observed immediately and continuously after biopsy for a 10-min period and then again at 60 min after biopsy (for a second 10-min period) to ascertain the ‘acute observation’ responses, as described previously.16 In summary, if mice were observed to be grooming, licking at the tail, or were subdued (not exploring a new cage environment), a point was recorded, and all points were tallied (at the end of a 10-min period) to generate a cumulative score, hereafter referred to as the acute observation score. After completion of the first 10-min observation time point, mice were relocated to the behavioral testing area and left undisturbed in cages until completion of the acute observation recordings.
EPM.
The maze apparatus, used for evaluating anxiety-like behaviors, consisted of 2 open and 2 enclosed arms (each 5 cm wide, 50 cm long; 15 cm high for enclosed arms) positioned at 90° angles and elevated 1 m above the floor. Individual mice were placed in the center of the 4-arm maze and were permitted to investigate the EPM for a period of 5 min. Animals were tested only once to remove bias of acclimation to the maze.32 The number of entries into each arm and amount of time spent in different parts of the maze were quantified by using a videotracking system (ViewPoint, Champagne au Mont d'or, France). Between recordings, the maze arms were wiped clean by using 95% ethanol to dilute and remove olfactory cues. Room lighting was diminished during testing. To better characterize complex mouse behaviors, conventional spatial parameters (time spent in open and enclosed arms) were augmented by assessments of ethologically relevant behaviors,2,4 based on animals’ perception of risk. These quantified parameters included head dips over open edges and stretch–attend postures into adjacent areas. Video clips, dated and coded by the computer system, were saved and later analyzed manually by a single observer blinded to conditions beyond overt tail biopsy.
We defined a stretch–attend posture as the firm plantation of the hindlimbs while the front half of the body moved forward in an exploratory manner, without the mouse fully leaving the arm in which it was currently. Head dips generally are viewed as an exploratory behavior, categorized as a motion in which the mouse tilts its head, from any of the arms or center area, over the side of an open arm.
Locomotor activity.
After EPM evaluations, mice were moved to an adjacent behavior testing room for locomotor testing, which provides a measure of physical health and coordination. Individual mouse assessments were made in clean autoclaved standard mouse cages, with a single layer of corncob bedding, inserted into an adjustable height frame (30 × 24 × 8 cm) to permit infrared motion detection by 2 levels of sensors. The sensors were arranged in an 8-beam array strip with approximately 1.5-cm spacing. This arrangement allowed detection of both horizontal and vertical movements within the cage. Mice were placed randomly into available boxes, locomotion assessments were conducted in a procedure room without reviewers present, and recordings were coded by the computer program. Mice were recorded over 120 min (light, 30 min; dark, 90 min), and beam break data were collected directly by using specialty software (MED Associates, St Albans, VT).
Statistical analysis.
Data were analyzed graphically for potential significant effects by using GraphPad Prism (GraphPad Software, La Jolla, CA), and subsequent statistical analyses were performed by using the MATLAB Statistics Toolbox software suite (Mathworks, Natick, MA). Given a behavioral measure of interest for an experimental group of fixed age or strain, the effects of treatment (biopsy or sham) and anesthesia were analyzed by using 2-way ANOVA with treatment, anesthesia, and treatment × anesthesia interaction as main effects. To compare behavioral measures between specific experimental groups, one-way ANOVA was used when comparing between age groups (21 to 24 d, 28 to 31 d, and 42 to 45 d), and a 2-sample Student t test was used for all other comparisons between 2 groups (for example, mice exposed to isoflurane anesthesia compared with control mice). Results were considered to be statistically significant at a P value less than 0.05.
Results
Acute observations.
No mice exhibited a tail flick away from the platform at the time of tail transection. A small subset of animals (9 of 196, 4.59%) vocalized briefly at the time of biopsy or, in sham animals, with application of pressure; mice that vocalized at the time of restraint were not included in this subset. The 3 BALB mice that vocalized were from cohorts 1 (21 to 24 d) and 2 (28 to 31 d); 2 were biopsied (1 with anesthesia), and the remaining mouse underwent sham biopsy without anesthesia. The 6 B6 mice that vocalized were in cohorts 2 (28 to 31 d) and 3 (42 to 45 d), and all received biopsy without anesthesia (data not shown). Strain-related differences in vocalization were deemed to be negligible.
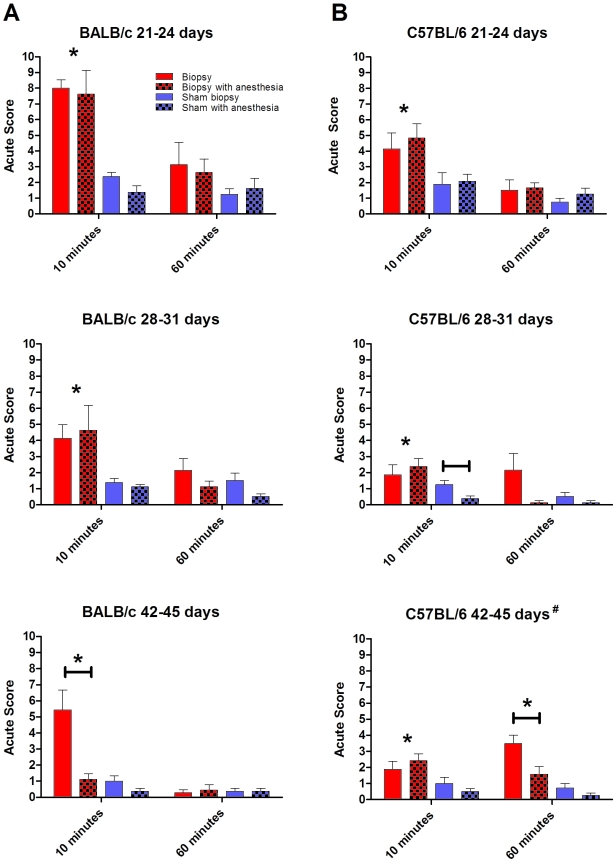
BALB.
In all cohorts at the 10-min point, the number of acute responses was significantly (P < 0.01) higher in biopsied BALB mice than in mice that had a sham-biopsy (Figure 1 A). In cohorts 1 and 2, the application of anesthesia in mice that received a biopsy had no effect on the acute observation score during the first 10 min after the procedure. In the oldest group (cohort 3) at 10 min, the application of anesthesia significantly (P < 0.05) reduced the acute score in biopsied animals. No significant differences were noted between the experimental groups at 60 min after biopsy/sham in any cohort.
Figure 1.
Strain-, age-, and anesthesia-associated changes in acute behavioral responses after tail biopsy of mice. Acute observation scores were recorded for the first 10 min after biopsy and again at 60 min after biopsy for all cohorts of mice. Isoflurane anesthesia did not diminish acute responses in younger mice (cohorts 1 and 2) for either (A) BALB or (B) B6 mice. Horizontal bars above the columns highlight significant (P < 0.05) differences between pairs within the same treatment group. Asterisks denote that all biopsied animals at the designated time point, regardless of anesthesia, had significantly (P < 0.05) higher acute scores than did sham-biopsied animals. The unique designator (#) for the oldest cohort of B6 mice indicates that no significant difference was found between acute scores of biopsied animals at 10 and 60 min. Red bar, biopsy; red checked bar, biopsy with anesthesia; blue bar, sham biopsy; and blue checked bar, sham with anesthesia.
B6.
In all cohorts at 10 min, B6 mice that received a biopsy had significantly (P < 0.01) higher acute scores than did sham-biopsied animals (Figure 1 B). When age was excluded as a variable, administration of anesthesia significantly (P < 0.05) reduced the acute observation score at 60 min in biopsied animals. Within cohort 3, the responses of biopsied mice with the administration of anesthesia were significantly (P < 0.01) diminished at 60 min, with response levels on par with those of the sham-biopsy animals at the same time point. Cohort 3 was the only group in which biopsied animals had a significantly (P < 0.05) increased acute observation score at 60 min compared with that of sham-biopsied animals. In addition, cohort 3 was the only group in which the 10- and 60-min acute observation scores of biopsied mice were not significantly different from each other.
For B6 mice in cohort 3, unanesthetized biopsied animals had prolonged behavioral responses at 60 min that were significantly (P < 0.01) different from those in BALB cohort 3 mice, although BALB initially were more responsive (P < 0.05) at 10 min. In conclusion, regardless of strain, isoflurane anesthesia did not diminish biopsy-related behaviors in the youngest cohorts but was linked to diminished responses in cohort 3 BALB mice at 10 min and cohort 3 B6 mice at 60 min.
EPM.
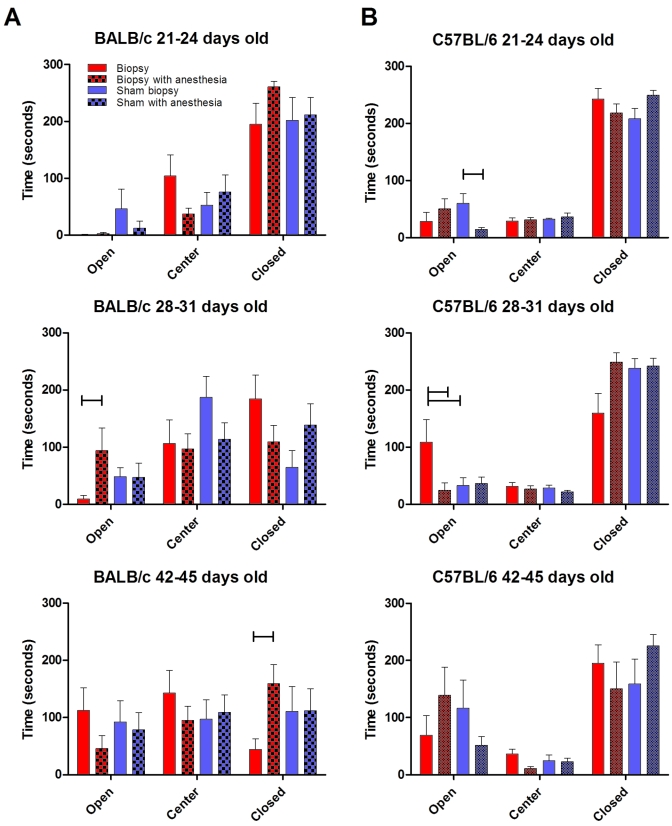
BALB.
No significant effect of tail biopsy was noted in BALB mice, regardless of age or anesthesia exposure, in their performance on the EPM (Figure 2 A). In cohort 1, the majority of time, regardless of treatment group, was spent in the enclosed arms. Mice in cohort 2 were more exploratory; mice that received a biopsy with anesthesia spent more time in the open arms than did mice that were biopsied without anesthesia (P < 0.05). In contrast to cohort 2, mice in the oldest group (cohort 3) that were biopsied under anesthesia spent significantly (P < 0.05) more time in the enclosed arms than did mice that were biopsied without anesthesia. On average, BALB mice exposed to anesthesia spent 29.7 s longer in the closed arm than did BALB mice that were not exposed to anesthesia. Administration of isoflurane anesthesia in all BALB cohorts significantly (P < 0.05) increased the number of stretch–attend postures (results not shown).
Figure 2.
Strain-, age-, and anesthesia-associated effects on time spent in the areas of the elevated plus-maze after tail biopsy. Individual mice of strain background (A) BALB or (B) B6 were recorded for a total of 5 min. Horizontal bars above the columns highlight significant (P < 0.05) differences between pairs within the same treatment group. For cohort 2 B6 mice, the longer bar indicates a significant (P < 0.05) difference between the conscious biopsied and sham-biopsied animals in the open arms. No significant effect of the biopsy itself was noted for BALB or B6 mice. Red bar, biopsy; red checked bar, biopsy with anesthesia; blue bar, sham biopsy; and blue checked bar, sham with anesthesia.
B6.
In all age groups, regardless of biopsy or anesthetic exposure, B6 mice spent the majority of their time in the enclosed arms of the EPM (Figure 2 B). Posthoc analysis identified significant differences for particular testing cohorts. In cohort 1, administration of isoflurane anesthesia decreased (P < 0.01) the amount of time spent in open arms by sham-biopsied mice only. Mice in cohort 2 that received a biopsy without anesthesia spent significantly (P < 0.05) less time in the closed arms than did conscious sham-biopsied mice and biopsied mice with anesthesia. On average, B6 mice receiving anesthesia spent 19.3 s longer in the closed arm than did B6 mice that were not exposed to anesthesia.
In summary, mice that received isoflurane anesthesia, regardless of strain, spent significantly (P < 0.05) more time (average, 26.3 s) in the enclosed arms of the maze, indicative of increased anxiety-like behavioral patterns. Regarding strain effects, BALB mice spent less time in enclosed arms than did B6 (P < 0.01), indicating that BALB were less anxious in our maze testing paradigm than were the B6 mice. In addition, BALB mice displayed more stretch–attend postures and head dips than B6 mice (P < 0.01; results not shown). Increases in both stretch–attend postures (P < 0.01) and head dips (P < 0.05) occurred in both strains with increased age, most likely related to increased activity with age, as later observed during time spent in locomotor activity boxes.
Locomotor activity.
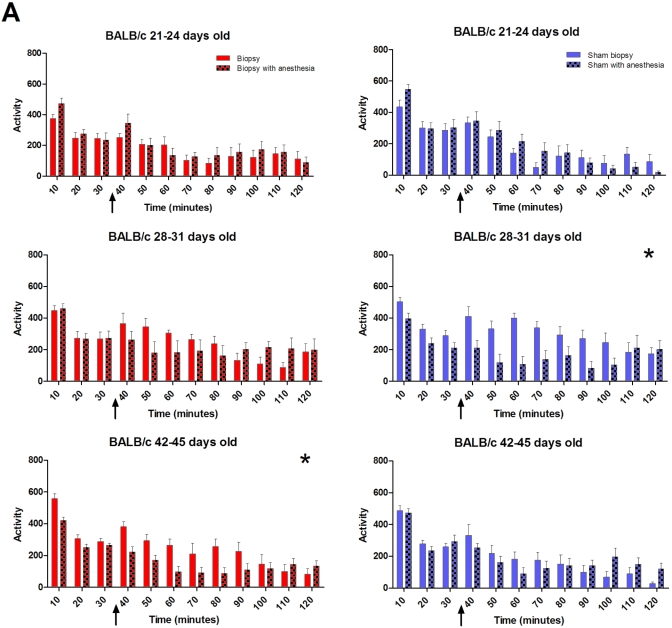
BALB.
Anesthesia led to overall decreases in activity in BALB mice in cohort 2 that had a sham-biopsy (P < 0.01) and cohort 3 biopsied animals (P < 0.05) compared with mice that did not receive anesthesia (Figure 3 A).
Figure 3.
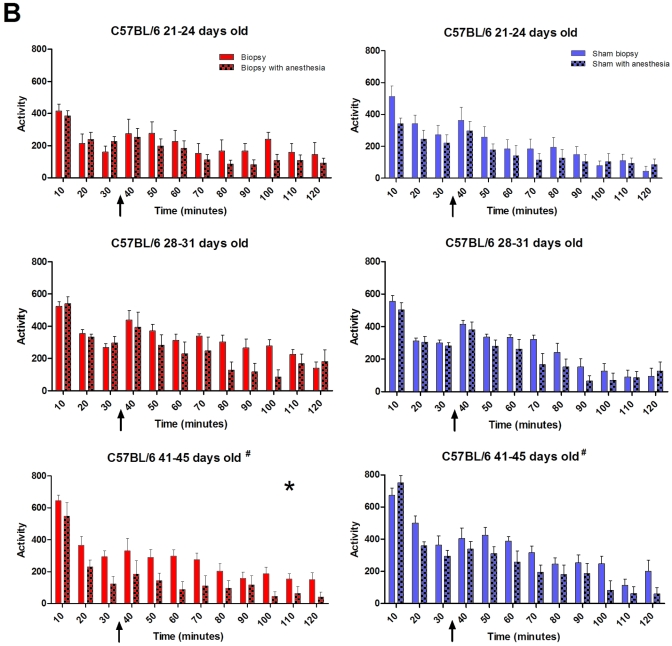
Strain-, age-, and anesthesia-associated effects on locomotor activity recorded for 120 min beginning at approximately 3 h after tail biopsy. Locomotion assessments provided a measure of physical health and coordination of the mice. Mice showed a transient increase in activity at 30 min (arrow) when overhead lights were extinguished. Both (A) BALB and (B) B6 had significant (P < 0.01) decreases in activity in those mice that received anesthesia across ages, regardless of biopsy. Asterisks indicate that for the identified cohort, mice that received anesthesia had significantly (P < 0.01) decreased locomotion averaged over the recording period. For B6 mice, the unique designator (#) by the cohort 3 graphs indicates that biopsied mice displayed a significant (P < 0.001) decrease in activity compared with sham animals, regardless of anesthesia. Red bar, biopsy; red checked bar, biopsy with anesthesia; blue bar, sham biopsy; and blue checked bar, sham with anesthesia. Figure continued on the next page.
B6.
Cohort 3 B6 mice that were biopsied displayed a significant (P < 0.001) decrease in activity compared with that of sham animals, regardless of anesthesia (Figure 3 B). The most active animals were the B6 sham mice in cohort 3 that received anesthesia; the least active animals were the cohort 3 B6 mice that were biopsied under anesthesia.
Both strains at all ages showed brief and transient increases in activity at 30 min when the overhead lights were extinguished. Both strains and all ages of mice that received anesthesia showed significant (P < 0.01) decreases in activity, regardless of biopsy.
Discussion
Investigations of research methodologies for mice have led to updated guidance and refinements in the practice of harvesting distal tail tissue prior to genotyping. Current recommendations are that mice should be of young ages, with minimal lengths of tissue (no more than 2 mm of distal tail) collected, in order to ensure that tissue is obtained before tail ossification and to maximize the quality and quantity of extracted DNA.14,16 Tail biopsy, when compared with collection of other murine tissues for DNA isolation, has no measurable effects that indicate distress or considerable pain; in fact, routine handling for restraint, compared with all tissue collection methods, results in similar physiologic changes in tested animals.8 Further, recent work assessing serum corticosterone in mouse pups indicates that handling, independent of tissue sampling, is a major stressor for mice.29 Decreases in cage-climbing behavior within 30 min of a 2.5-mm tail biopsy in weaning-age mice have been reported, but these changes were transient effects of the tail biopsy procedure.30
Investigations on the potential for painful responses to tail biopsy in mice have been published.1,8 To determine anesthetic benefits for biopsy, one laboratory assessed administration of either methoxyflurane or ether to telemeterized mice prior to tail biopsy. 1 In that study, older mice (12 to 32 wk old) did not have prolonged physiologic alterations after biopsy and ether (which induced a 7% mortality rate) and methoxyflurane were not found to improve wellbeing after biopsy.1 We sought to assess inhalant anesthesia effects of agents currently in use at our institution. The use of isoflurane as a general anesthesia intervention to diminish potential adverse effects experienced by animals at the time of tissue collection is common in contemporary laboratory animal practice but has not been previously validated scientifically for tail biopsy procedures. We expected that brief isoflurane exposure would ameliorate abnormal acute behavioral responses and diminish anxiety-like behaviors after biopsy, and that routine locomotor activities would not be altered due to adequate alleviation of any perceived discomfort in biopsied mice.
The popularity of isoflurane use in laboratory rodents includes its ease of administration and manipulation of the depth of anesthesia, limited hemodynamic18 and cardiodepressive7 effects, rapid achievement of satisfactory muscle relaxation for surgical manipulations, and minimal biotransformation, such that the anesthetic is primarily eliminated by exhaled air without hepatic metabolism. Side effects can include respiratory depression, with hypercapnia, acidosis, and a marked decrease in respiratory rate, as measured in aged mice (16 to 32 wk old) that were maintained under isoflurane anesthesia for 50 min.6 Effects of 1% inhaled isoflurane anesthesia on spatial learning in mice have been noted up to 28 h after inhalation;31 however, animals in those studies were under anesthesia for 1 h, whereas our anesthetic exposure was less than 1 min per mouse. From a personnel safety perspective for those working in the laboratory animal environment, despite its slight odor, isoflurane is regarded as a nonirritant, nonexplosive, and nonflammable agent that can readily be scavenged to avoid waste anesthetic exposure.
Isoflurane did not reduce observed behavioral responses within mice of weaning age (21 to 31 d) for either the BALB or B6 strain. In particular, this noneffect was evident at the time of tail transection, when approximately 90% of mice had no audible response to the tissue cut and no animals flicked their tails away from the platform, regardless of age, strain, or anesthesia exposure. Importantly, with exposure to brief isoflurane, diminished undesirable behavioral responses were noted primarily in biopsied mice older than 1 mo. Adult B6 mice exhibited more prolonged responses to biopsy without anesthesia than did BALB mice. Prolonged behavioral responses in this cohort most likely were related to transection of mature caudal vertebrae in the B6 tail.16
The mouse strains tested in our experiments were selected for their tail development characteristics; however, inbred strain variability to behavioral testing has been scientifically validated.12,28 Despite concerted attempts to standardize all handling, housing, and testing conditions, different outcomes in the conduct of similar experiments have routinely been documented among laboratories.4,9,10,13,15,19,20,23,33 Historically, assessments of the plus-maze paradigm and exposure to novel environments for BALB can be summarized by behavioral and physiologic responses indicative of high anxiety, with diminished locomotor activity, increased stretching and sniffing, and avoidance of open areas.4,13 Conversely, B6 mice use a different strategy to cope with anxiogenic conditions, including a high level of locomotion and exploratory activity in the plus-maze paradigm, indicative of diminished anxiety.4 The EPM is noted to measure the conflict between exploration of ‘novelty’ and the avoidance of lighted open areas as an anxiety indicator; the measurement is taken according to how subjects partition their locations in the maze spaces.5,23,26,34 The initial period within a novel environment is known to elicit the greatest anxiety-related response.13 Anxiety is defined as a mental and physical state induced by the anticipation of threatening stimuli, with the potential outcome linked to preparing the organism for danger.13 In traditional behavioral modeling, mice that overcome fear will move away from the relative safety of the maze walls,20 and anxiolytics will increase the number of entries into and amount of time spent in the open arms.11
We sought to determine whether isoflurane anesthesia would diminish anxiety-like behaviors in mice that received tail biopsy compared with sham-biopsy controls, and we expected that we also would capture elements of inherent strain differences. Our findings showed that anesthetized mice spent more time in the enclosed EPM arms, regardless of strain, age group, or biopsy. Interestingly, this finding indicated that isoflurane exposure, whether from the agent itself or in response to the experience of losing consciousness, may have contributed further to anxious behaviors. In addition, BALB mice demonstrated a higher level of activity, exploring the open and closed EPM arms to a greater extent, than did the B6 animals, particularly among older mice. Our finding that BALB mice were less anxious in the EPM is in general agreement with previous studies28 but in contradiction to historical strain anxiety behaviors;4 these differences may be attributed to the relatively young ages at which our mice were tested. In attempts to better characterize complex mouse behaviors, conventional spatial parameters often are augmented by assessments of ethologic parameters, based on the animals’ perception of risk36 and potential for increased anxiety. By extrapolation, very high levels of these postures may be linked to exploratory behavior and an overcoming of anxiety. Interpretations of overall behavior must be considered cautiously, without dogmatic adherence to preconceptions of individual testing variables; instead, these variables (stretch–attend postures and head dips) should be interpreted as part of the overall behavioral profile.2 Our study was focused primarily on the effect of isoflurane anesthesia on behavioral responses, and the ambiguities of background genetic effects on behavior were of lesser importance for overall recommendations on tail biopsy.
Locomotion can be used as an indicator of general health, exploratory drive, novelty, and anxiety.33 In our assessment of activity after biopsy with or without isoflurane, we hypothesized that the more active animals also would be those with less discomfort after the procedure. Indeed, diminished activity between adult (cohort 3) conscious biopsied B6 mice, compared with the conscious sham animals, is indicative of prolonged discomfort related to the biopsy. Regardless of age and strain, animals that received anesthesia had an overall decrease in activity that was not linked to any prolonged biopsy effect. The diminished activity levels were noted when recording started at approximately 3 h after exposure to isoflurane and persisted throughout the recording time of 2 h, such that the overall findings represented effects at as long as 5 h after brief anesthetic exposure (less than 1 min in an induction chamber). We did not anticipate this prolonged effect of brief isoflurane exposure.
Our testing methodology exposed animals to a tail biopsy, followed by measurement of anxiety and activity related directly to the tissue sampling procedure and anesthetic exposure. Within the experimental paradigm, responses in any given test could be influenced by both genetics and environment, in addition to sources of variation that might overwhelm other a priori robust effects.27 We were able to rule out any differences in responses between sex across both strains, but did note that the younger animals of weaning age were the most responsive to biopsy in acute behavioral measurements, while exhibiting trends of diminished activity on both the EPM and in locomotor boxes. All animals were changed from their home cages in the housing room into at least 2 additional clean cage set-ups between the EPM and locomotor activity boxes. Therefore, the influence of movement within the facility, and placement into novel environments independent of the EPM, likely contributed to behavioral outcomes. The degree of baseline stressors potentially experienced due to relocation and cage changes was assumed to be inherent in the response levels of all experimental animals described. Further, we acknowledge that the actual experience of being anesthetized with a vaporized inhalant (including placement in the induction box, overt physical reactions as mice succumb to anesthesia, and resultant immobility with loss of consciousness) might have contributed to outcomes detected within the anxiety and locomotor assessments of the experimental testing paradigm. The overall outcome on the EPM in our testing paradigm was that the biopsy itself did not have a significant effect on increased anxiety-like behaviors.
Tail biopsy should be performed in young mice to avoid transection of distal mature vertebrae, but our study indicated that isoflurane anesthesia does not appreciably enhance wellbeing over that of mice biopsied without anesthesia at weaning age up to 31 d of age. The use of anesthesia was of benefit to older animals, particularly B6 mice. However, mice that were exposed to isoflurane showed increased anxiety-like behavior on the EPM and decreased activity overall in the locomotor test. Further research on the application of topical anesthetics and analgesics in young mice is necessary to determine potential efficacy for amelioration of undesirable behavioral responses to biopsy. The effect of inhaled isoflurane indicated that acute and prolonged alterations in anxiety and activity must be considered when interpreting the impact of anesthesia on tail biopsy across various ages and strains of laboratory mice.
Acknowledgments
We are grateful to David Weiss and Britter Gunderson, Cedric Mombereau, Steve Mague, and Lisa Briand from the Blendy laboratory for their collective computer expertise and assistance with experimental set-up and data analysis. Thanks to Charles River Laboratories and Dr Laura Conour for the donation of founder breeding pairs. We thank Dr Kurt Hankenson for review of the manuscript. We also thank the American College of Laboratory Animal Medicine Foundation and the Office of the Vice Provost at the University of Pennsylvania for funding these endeavors and providing support for GBW. In addition, we are grateful to the ULAR animal care staff for the excellent oversight and care of our animals.
References
- 1.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45 [DOI] [PubMed] [Google Scholar]
- 2.Calatayud F, Belzung C, Aubert A. 2004. Ethological validation and the assessment of anxiety-like behaviours: methodological comparison of classical analyses and structural approaches. Behav Processes 67:195–206 [DOI] [PubMed] [Google Scholar]
- 3.Campbell DB, Hess EJ. 1997. Rapid genotyping of mutant mice using dried blood spots for polymerase chain reaction (PCR) analysis. Brain Res Brain Res Protoc 1:117–123 [DOI] [PubMed] [Google Scholar]
- 4.Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. 2002. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134:49–57 [DOI] [PubMed] [Google Scholar]
- 5.Castelhano-Carlos MJ, Sousa N, Ohl F, Baumans V. 2010. Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab Anim 44:88–103 [DOI] [PubMed] [Google Scholar]
- 6.Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M. 2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44:329–336 [DOI] [PubMed] [Google Scholar]
- 7.Chu DK, Jordan MC, Kim JK, Couto MA, Roos KP. 2006. Comparing isoflurane with tribromoethanol anesthesia for echocardiographic phenotyping of transgenic mice. J Am Assoc Lab Anim Sci 45:8–13 [PubMed] [Google Scholar]
- 8.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 9.Crabbe JC, Wahlsten D. 2003. Of mice and their environments. Science 299:1313–1314 [DOI] [PubMed] [Google Scholar]
- 10.Crabbe JC, Wahlsten D, Dudek BC. 1999. Genetics of mouse behavior: interactions with laboratory environment. Science 284:1670–1672 [DOI] [PubMed] [Google Scholar]
- 11.Crawley JN. 2003. Behavioral phenotyping of rodents. Comp Med 53:140–146 [PubMed] [Google Scholar]
- 12.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. 1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124 [DOI] [PubMed] [Google Scholar]
- 13.Depino AM, Gross C. 2007. Simultaneous assessment of autonomic function and anxiety-related behavior in BALB/c and C57BL/6 mice. Behav Brain Res 177:254–260 [DOI] [PubMed] [Google Scholar]
- 14.Garzel LM, Hankenson FC, Combs J, Hankenson KD. 2010. Use of quantitative polymerase chain reaction analysis to compare quantity and stability of isolated murine DNA. Lab Anim (NY) 39:283–289 [DOI] [PubMed] [Google Scholar]
- 15.Griebel G, Belzung C, Perrault G, Sanger DJ. 2000. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 148:164–170 [DOI] [PubMed] [Google Scholar]
- 16.Hankenson FC, Garzel LM, Fischer DD, Nolan B, Hankenson KD. 2008. Evaluation of tail biopsy collection in laboratory mice (Mus musculus): vertebral ossification, DNA quantity, and acute behavioral responses. J Am Assoc Lab Anim Sci 47:10–18 [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 18.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. 2004. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287:H1618–H1624 [DOI] [PubMed] [Google Scholar]
- 19.Laber K, Veatch LM, Lopez MF, Mulligan JK, Lathers DM. 2008. Effects of housing density on weight gain, immune function, behavior, and plasma corticosterone concentrations in BALB/c and C57BL/6 mice. J Am Assoc Lab Anim Sci 47:16–23 [PMC free article] [PubMed] [Google Scholar]
- 20.Lalonde R, Strazielle C. 2008. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Methods 171:48–52 [DOI] [PubMed] [Google Scholar]
- 21.Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. 2009. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci USA 106:10847–10852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthias N, Robinson MA, Goodwin BS. 2010. Local cryoanalgesia: an effective method for tail-tip biopsy in mice. J Am Assoc Lab Anim Sci 49:657–658 [PMC free article] [PubMed] [Google Scholar]
- 23.Milner LC, Crabbe JC. 2008. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav 7:496–505 [DOI] [PubMed] [Google Scholar]
- 24.Mombereau C, Gur TL, Onksen J, Blendy JA. 2010. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int J Neuropsychopharmacol 13:321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales ME, Gereau RW. 2009. The effects of tail biopsy for genotyping on behavioral responses to nociceptive stimuli. PLoS ONE 4:e6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulder GB, Pritchett K. 2004. The elevated plus-maze. Contemp Top Lab Anim Sci 43:39–40 [PubMed] [Google Scholar]
- 27.Ramos A. 2008. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 29:493–498 [DOI] [PubMed] [Google Scholar]
- 28.Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. 1999. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of 6 inbred mouse strains. Behav Brain Res 105:207–217 [DOI] [PubMed] [Google Scholar]
- 29.Schaefer DC, Asner IN, Seifert B, Burki K, Cinelli P. 2010. Analysis of physiological and behavioural parameters in mice after toe clipping as newborns. Lab Anim 44:7–13 [DOI] [PubMed] [Google Scholar]
- 30.Sorensen DB, Stub C, Jensen HE, Ritskes-Hoitinga M, Hjorth P, Ottesen JL, Hansen AK. 2007. The impact of tail-tip amputation and ink tattoo on C57BL/6JBomTac mice. Lab Anim 41:19–29 [DOI] [PubMed] [Google Scholar]
- 31.Valentim AM, Alves HC, Olsson IA, Antunes LM. 2008. The effects of depth of isoflurane anesthesia on the performance of mice in a simple spatial learning task. J Am Assoc Lab Anim Sci 47:16–19 [PMC free article] [PubMed] [Google Scholar]
- 32.Voikar V, Vasar E, Rauvala H. 2004. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav 3:27–38 [DOI] [PubMed] [Google Scholar]
- 33.Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. 2006. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA 103:16364–16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walf AA, Frye CA. 2007. The use of the elevated plus-maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams WO, Riskin DK, Mott AK. 2008. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci 47:8–10 [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC. 2004. The rat exposure test: a model of mouse defensive behaviors. Physiol Behav 81:465–473 [DOI] [PubMed] [Google Scholar]