Abstract
OBJECTIVES
The natural history of fecal incontinence (FI) in community subjects is uncertain and the onset rate is unknown. The aim of the study is to estimate the prevalence, new-onset rate, and risk factors for FI in community subjects.
METHODS
A random sample of 2,400 community subjects aged ≥ 50 years was surveyed in 1993, using a validated questionnaire. Responders were recontacted in 2003. FI was defined as self-reported problems with leakage of stool. Onset rate was calculated as the proportion of subjects without FI who became new cases. Logistic regression models were constructed to identify predictive factors for developing FI and changes in bowel habit associated with the onset of FI.
RESULTS
Overall, 1,540 (64%) subjects responded to the initial survey, and 674 (44%) of them responded to the second survey a median of 9 (8.8 – 9.5) years later. The prevalence of FI in the first survey was 15.3% (13.4 – 17.3%). In the second survey, 37 reported incident FI; thus, the onset rate of FI was 7.0% (5.0 – 9.6) per 10 years. Predictive factors at baseline for the onset of FI were self-reported diarrhea (odds ratio (OR) = 3.8 (1.5, 9.4)), incomplete evacuation (OR = 3.4 (1.2, 9.8)), and pelvic radiation (OR = 5.1 (1.01, 25.9)). Development of urgency was the primary predictor among the set of predictors reflecting changes in bowel symptoms that were associated with the onset of FI (OR = 24.9 (10.6, 58.4)).
CONCLUSIONS
The onset rate of FI is approximately 7% per 10 years in community subjects aged ≥ 50 years. Prevention may be possible if bowel habit is appropriately managed in high-risk individuals.
INTRODUCTION
Fecal incontinence (FI) is a devastating problem for those subjects who suffer it and their families (1 – 5). It worsens quality of life (1), has a profound psychological impact (2), reduces work productivity (3), impairs social life (4), and is one of the most common reasons for admittance to nursing homes (5). Costs associated with FI are remarkably high. A Dutch study calculated a global cost of $ 2,900 per affected outpatient each year (3), including both direct and indirect costs; the costs are likely to be much higher for institutionalized patients (6). However, the social relevance of FI is usually underestimated, because affected subjects do not report it to their physician or even to close relatives (7), so diagnosis and treatment are often delayed.
Several studies have evaluated the prevalence of FI but most of them have focused on specific populations, such as nursing home residents (5,8,9), patients attending medical facilities (10,11), or obstetric/gynecologic clinics (12 – 14), where a high prevalence is to be expected. There is a relative lack of true community-based data on the magnitude of the problem of FI, and reports on its prevalence have been very inconsistent, ranging from 0.5 to 24% in adult populations (1,15 – 20) albeit increasing with age (21). Most of the heterogeneity seen in prevalence studies is probably explained by methodological problems, specifically the use of nonreliable instruments to collect information and bias in sample selection; these problems may be overcome using validated questionnaires (22) and a true randomized population-based approach (1). Most recent studies overcome these problems and reported that the prevalence of FI in the general population is somewhere around 8 – 15% (1,23,24).
In addition to the need for well-designed studies assessing the prevalence and severity of FI in the community, information regarding risk factors associated with FI in the general population is scarce and somewhat contradictory (20,25 – 28). In referral centers, obstetrical injury, diabetes mellitus, neurological diseases, and chronic diarrhea of any cause are suggested to be important (29).
There are no population-based studies addressing the onset of FI symptoms. New onset has been evaluated only in specific populations or conditions, including women after delivery (30 – 33), the institutionalized elderly (9), those having anal or pelvic surgery (34 – 36), and after acute brain injury (37). Although these studies have provided valuable information, data on the onset rate of FI symptoms in the community would be more useful for clinicians and health policy makers. An estimation of how many people will become incontinent over time and what risk factors may account for it in the community may help to plan resource allocation and help clinicians individualize management.
For these reasons, we sought to more clearly delineate the epidemiology of FI in the general population that was 50 years of age and older, and to define the risk factors associated with the condition. Moreover, we aimed to estimate the cumulative 9 year new onset rate of FI symptoms in community subjects, and evaluate risk factors associated with onset.
METHODS
The population of Olmsted County, MN, is approximately 120,000 predominantly white (90%) people, and is similar sociodemographically to the United States. white population (38). Mayo Clinic is the primary provider of health care for this population. Comparison with 1990 census figures indicates that virtually all county residents of all ages have a Mayo Clinic identification number. Each year over 80% of the county population is seen at Mayo Clinic or another local care facility. During any given 4-year period, virtually all local residents have at least one contact with a local medical care provider. Pertinent clinical data for each of these patients are accessible because Mayo Clinic has maintained, since 1910, extensive indices based on clinical and histological diagnoses and surgical procedures. In addition, each of the local medical providers employs a dossier (or unit record) system, whereby all medical information for each individual is accumulated in a single record. The Rochester Epidemiology Project (REP) created a linkage system, whereby all patients treated by any medical care provider in Olmsted County are accessible through a single data bank (38). The REP records linkage system, therefore, provides what is essentially an enumeration of the population from which samples can be drawn.
First survey
A random sample of 2,400 subjects aged 50 years or older was selected for our study. This sample was stratified by 5-year age groups and gender. To evaluate the entire population, no exclusions were made on the basis of mental state or residence in nursing homes or other institutions. Where necessary, however, questionnaires were completed by proxy (family member or institutional caretaker after family consent) in a small number of cases. The postal survey was mailed in 1993. Subjects were invited to decline participation, if they wished, by returning a signed, unanswered form through mail. Nonresponders were delivered an additional questionnaire each 3 – 4 weeks for a total of three mailings. In this population, data on the baseline prevalence of urinary incontinence (39) and combined urinary and FI (using a more restrictive definition of FI) have been already reported (40).
In this survey, a validated self-report questionnaire was applied based on a previously developed instrument (41). Previous testing has shown that the instrument used was reliable on test-retest, and in comparison with a physician interview was a valid measure of symptoms and diagnoses (22). Intelligibility of the questionnaire was maximized by adhering to an assumed 6th grade reading level and was supported by the high literacy rate in the population surveyed.
Fecal incontinence was defined as a positive response to the question, “Have you had problems with leakage of stool (accidents or soiling because of the inability to control the passage of stool until you reached a toilet)”?
In addition, multiple questions assessing potential risk factors for FI were included in the questionnaire or identified by chart review. Questions were designed to evaluate (a) bowel habit dysfunction (self-reported bowel habit, frequency, urgency, incomplete evacuation, consistency, straining, self-digitation, feeling of anal blockage, position to defecate, time to defecate), (b) any history of anatomic perianal disease or injury (perianal infection, fissure, fistula, anorectal surgery, or trauma), and (c) factors associated with neurologic injury and subsequent neurogenic FI (diabetes mellitus, spinal cord injury, stroke, rectal prolapse, rectocele). Each of the questions assessed the presence or absence of risk factors, as well as quantification of the severity and frequency of symptoms where relevant. Where applicable, subjects were asked to answer all questions specifically regarding symptoms within “the last year, excluding temporary illnesses.” Quantification of symptoms was most often estimated by frequency: (1) never, (2) sometimes (less than 25% of the time), (3) oft en (25 – 75% of the time), or (4) usually (more than 75% of the time). The complete in-patient and outpatient medical records of all responders were reviewed, using a standard abstraction sheet, to identify the medical diagnoses at the time of baseline, including anorectal diseases, relevant surgery or trauma, rectal prolapse, rectocele, diabetes mellitus, spinal cord injury, and stroke.
Follow-up survey
All participants in the initial survey were mailed in 2003 a questionnaire about abdominal symptoms, which included the same question to evaluate FI. As in the first interview, subjects were invited to decline participation, if they wished; non-responders were delivered an additional questionnaire each 3 – 4 weeks for a total of three mailings. Subjects reporting FI, based on the same definition, at follow-up but not having the problem at the time of the first survey were considered as new cases.
Changes in bowel habits were identified by comparing the first and second survey interview. The following were considered as potential factors associated to the onset of FI: change in self-reported bowel habit, onset of looser stools, onset of urgency, onset of feelings of incomplete evacuation, onset of feeling of anal blockage, and laxative use.
Statistical analysis
Age- and gender-adjusted point prevalence rates (as of 1 January 1994) and 95% confidence intervals (CI) for FI were obtained and directly age- and gender-adjusted to the US white population in 2000. Logistic regression analysis was used to assess the association of potential risk factors with reporting FI on the initial survey. Odds ratios (OR) and 95% CI were calculated on the basis of the estimated coefficients from the logistic regression models and adjusted for age, gender, and diarrhea (the latter variable included due to its strong association with reported FI). In addition, a backward elimination approach was used to identify independent risk factors for reporting FI on the initial survey.
Onset of FI was defined for subjects not reporting FI at baseline but reporting it on the follow-up survey. The onset rate was calculated as the proportion of subjects without FI at baseline who become new cases, and reported as a percentage along with the 95% CI. On the basis of the data from the initial and follow-up survey, separate logistic regression models were used to examine factors predicting the onset of FI using changes in bowel habits anticipated to be associated with the onset of FI, adjusting for age and gender. Potential predictive factors for developing FI included symptoms reported at the first survey or identified by chart review to be present before the first survey interview. Changes in bowel symptom were considered when the symptom was reported in the follow-up survey, but not at the time of the first survey. Owing to the limited number of subjects reporting new onset FI, a multiple variable model examined only a few specific combinations of predictors, rather than a backward elimination approach.
All P values calculated were two-tailed; the α-level of significance was set at 0.05.
RESULTS
In the initial survey, there were 1,513 respondents (64% response rate). The median age of the respondents was 65 years (range 50 – 91) and 50.5% were men. Response to the survey was not associated with gender (P = 0.77), although older subjects had slightly decreased odds for response (OR = 0.99 per year of age (95% CI: 0.91 – 1.00), P = 0.02). A total of 14 subjects indicated that they required help in filling out the questionnaire. There were two subjects who were nursing home or institutional residents. A total of 971 of the 1,513 responders to the initial survey were mailed a second survey in 2003, and 683 returned a completed questionnaire (70% response rate) with a similar proportion of men (72%) and woman (69%) responding. A decreasing odds for responding was observed with increasing age (OR = 0.95 per year of age (95% CI: 0.93 – 0.97)), an increasing odds for responding in subjects reporting manual disimpaction (OR = 1.5 (1.0, 2.3)) and incomplete evacuation (OR = 2.2 (1.1, 4.5)) on the first survey, but a decreased odds in subjects experiencing an intervening stroke (OR = 0.4 (0.2, 0.8)). The median time between the first and the second surveys was 9 years (range 8.8 – 9.5 years).
Prevalence and associated factors at baseline survey
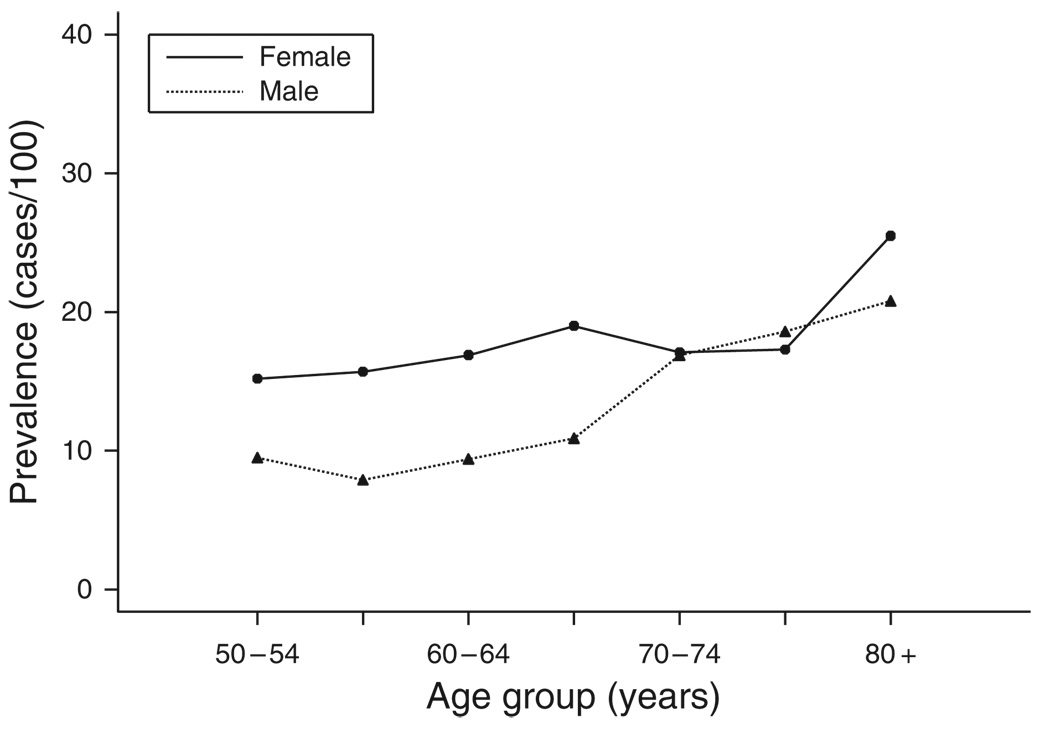
Two hundred and twenty-five subjects reported problems with leakage of stool; adjusted to the 2000 US Caucasian population, the prevalence of FI was 15.3 per 100 inhabitants (95% CI: 13.4 – 17.3). A greater proportion of women reported FI and the proportions reporting FI increased with age (Figure 1). The age-adjusted prevalence was 12.6 (95% CI: 10.1 – 15.1) in males and 17.7 per 100 (95% CI: 14.8 – 20.6) in females.
Figure 1.
Prevalence rates of fecal incontinence in 1993 survey by age and gender.
Table 1 shows the frequency of FI according to the risk factors evaluated, and a summary of the corresponding univariate and adjusted ORs. The multiple variable model indicated that age, gender, self-reported usual bowel pattern, number of bowel movements per week, urgency, loose/watery stools, straining, time needed for defecation, feeling of anal blockage, anal trauma, and pelvic radiation were independently associated with FI.
Table 1.
Factors associated with FI at baseline
| Variable | Total n |
FI at baseline n (%) |
Univariate OR (95% CI) |
Adjusted OR (95% CI)a |
Multiple variable OR (95% CI)b |
|---|---|---|---|---|---|
| Age (mean ± s.d.) | 67 ± 9 | 1.27 (1.09, 1.48)c | 1.35 (1.15, 1.58)c | 1.03 (1.01, 1.05)c | |
| Gender | |||||
| Male | 767 | 95 (12.4) | 1.0 | 1.0 | 1.0 |
| Female | 743 | 130 (17.5) | 1.50 (1.13, 2.00) | 1.47 (1.10, 2.01) | 1.47 (1.03, 2.10) |
| Usual bowel pattern (self-reported)# | |||||
| Normal | 1,069 | 97 (9.1) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Constipation | 224 | 34 (15.2) | 1.79 (1.18, 2.73) | 1.61 (1.05, 2.46) | 1.63 (0.98, 2.72) |
| Diarrhea | 162 | 71 (43.8) | 7.82 (5.38, 11.37) | 7.98 (5.46, 11.66) | 2.62 (1.55, 4.42) |
| Alternating | 49 | 23 (46.9) | 8.87 (4.87, 16.14) | 8.15 (4.45, 14.94) | 4.10 (1.89, 8.93) |
| Number of bowel movements | |||||
| < 3 Weekly | 52 | 6 (11.5) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 3 Weekly to 2 daily | 1,372 | 176 (12.8) | 1.13 (0.48, 2.68) | 1.19 (0.49, 2.92) | 2.70 (0.81, 9.01) |
| >2 Daily | 84 | 43 (51.2) | 8.04 (3.10, 20.84) | 6.08 (2.22, 16.69) | 8.60 (2.22, 33.31) |
| Urgency | |||||
| Nod | 1,426 | 171 (12.0) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yese | 75 | 54 (72.0) | 18.87 (11.12, 32.02) | 12.66 (7.05, 22.71) | 6.58(3.27, 13.24) |
| Loose or watery stools | |||||
| Nod | 1,384 | 159 (11.5) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yese | 122 | 63 (51.6) | 8.23 (5.56, 12.17) | 4.91 (3.08, 7.83) | 2.16 (1.19, 3.89) |
| Straining | |||||
| Nod | 1,441 | 211 (14.6) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yese | 68 | 14 (20.6) | 1.51 (0.83, 2.77) | 1.67 (0.89, 3.13) | 0.26 (0.09, 0.76) |
| Time to defecate | |||||
| < 5 min | 1,158 | 147 (12.7) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 5 – 30 min | 343 | 75 (21.9) | 1.93 (1.41, 2.62) | 2.08 (1.50, 2.89) | 1.69 (1.13, 2.53) |
| >30 min | 7 | 3 (42.9) | 5.16 (1.14, 23.28) | 5.45 (1.08, 27.52) | 2.00 (0.23, 17.84) |
| Feeling of anal blockage | |||||
| Nod | 1,478 | 215 (14.6) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yese | 21 | 9 (42.9) | 4.41 (1.83, 10.58) | 4.33 (1.74, 10.75) | 5.71 (1.37, 23.78) |
| Anorectal trauma | |||||
| No | 1,458 | 207 (14.2) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yes | 27 | 12 (44.4) | 4.84 (2.23, 10.48) | 4.37 (1.90, 10.05) | 4.25 (1.66, 10.86) |
| Pelvic radiation | |||||
| No | 1,450 | 206 (14.2) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yes | 42 | 15 (35.7) | 3.36 (1.76, 6.42) | 3.40 (1.68, 6.91) | 3.58 (1.63, 7.90) |
CI, confidence interval; FI, fecal incontinence; OR, odds ratio.
Adjusted for age, gender, and diarrhea, except as noted (#) for usual bowel pattern, which only adjusted for age and gender.
From backward elimination selection process, only those remaining in the model are shown.
Per 10 years of age.
Defined as never or sometimes.
Defined as often or usually.
Variables not retained in the model following the backward elimination approach included the following: days without stools, hard stools, incomplete evacuation, manual disimpaction, position other than sitting for defecation, medications to assist defecation, perianal infection, fistula, anorectal surgery, diabetes, spinal cord injury, stroke, vaginal births, rectal prolapse, and rectocele.
Among 225 subjects reporting FI, 195 (86.7%) had visited a physician in the prior year for any reason, but only 18 (8%) had consulted for leakage of stools.
Onset of FI
After excluding 89 subjects who reported FI symptoms at the first interview, 37 of the remaining 585 subjects reported FI on the second survey; thus the onset of FI in persons ≥ 50 years of age was 6.3% (95% CI: 4.5– 8.6%) over the approximately 9 years between surveys, corresponding to a 7.0% (5.0 – 9.6) onset rate per 10 years.
Risk factors for the onset of FI
Table 2 shows the univariate and adjusted ORs for factors predicting the onset of FI. Baseline survey factors associated with the onset of FI, in addition to a self-reported usual bowel of diarrhea, were (adjusting for age, gender, and self-reported diarrhea): feelings of incomplete evacuation, history of pelvic radiation before baseline survey and, in females, a diagnosis of rectocele before the first survey.
Table 2.
Predictors of the onset of fecal incontinence (FI) at follow-up (FU)
| Variable | Overall n |
FI at FU n (%) |
Univariate OR | Adjusted ORa |
|---|---|---|---|---|
| Ageb (mean ± s.d.) | 67 ± 9 | 61 ± 6 | 1.2 (0.7, 2.1) | 1.3 (0.8, 2.2) |
| Gender | ||||
| Male | 312 | 20 (6.4) | 1.0 | 1.0 |
| Female | 273 | 17 (6.2) | 1.0 (0.5, 1.9) | 0.9 (0.5, 1.9) |
| Usual bowel pattern (self-reported)# | ||||
| Normal | 456 | 25 (5.5) | 1.0 | 1.0 |
| Constipation | 79 | 4 (5.1) | 0.9 (0.3, 2.7) | 0.9 (0.3, 2.8) |
| Diarrhea | 40 | 7 (17.5) | 3.7 (1.5, 9.1) | 3.8 (1.5, 9.4) |
| Alternating | 8 | 1 (12.5) | 2.5 (0.3, 20.8) | 2.6 (0.3, 22.5) |
| Incomplete evacuation | ||||
| Noc | 553 | 32 (5.8) | 1.0 | 1.0 |
| Yesd | 32 | 5 (15.6) | 3.0 (1.1, 8.3) | 3.4 (1.2, 9.8) |
| Position other than sitting for defecation | ||||
| Noc | 583 | 36 (6.2) | 1.0 | 1.0 |
| Yesd | 2 | 1 (50.0) | 15.2 (0.9, 247.9) | 20.0 (1.2, 339) |
| Pelvic radiation | ||||
| No | 572 | 35 (6.1) | 1.0 | 1.0 |
| Yes | 9 | 2 (22.2) | 4.4 (0.9, 21.9) | 5.1 (1.1, 25.9) |
OR, odds ratio.
Adjusted for age, gender, and diarrhea at baseline, except as noted (#) for usual bowel pattern, which only adjusted for age and gender.
Per 10 years of age.
Defined as never or sometimes.
Defined as often or usually.
Additional variables considered but not significant were number of bowel movements, urgency, loose or watery stools, days without stools, straining, hard stools, manual disimpaction, time to defecate, feeling of anal blockage, medications to assist defecation, perianal infection, fistula, anorectal surgery, anorectal trauma, diabetes mellitus, spinal cord injury, stroke, vaginal births, rectal prolapse, rectocele.
Changes in bowel symptoms associated with the onset of FI
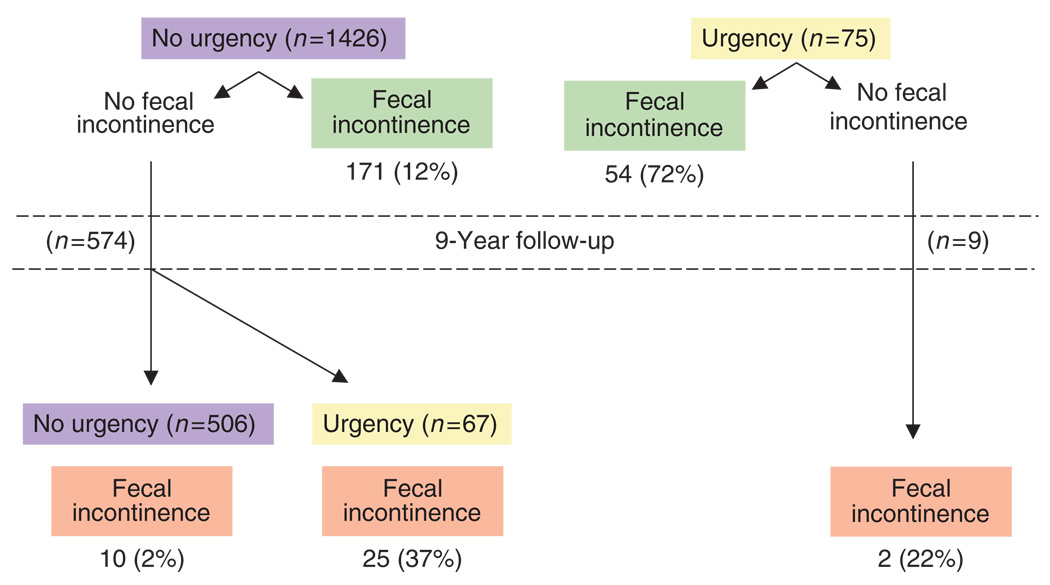
Changes in bowel habits identified to be associated with the onset of FI symptoms on the follow-up survey were the new onset of urgency, self-reported diarrhea, loose/watery stools, and onset of increased defecatory frequency to more than two bowel movements daily (adjusted for age and gender). However, a multiple variable model (including these variables, along with age and gender) implied that only the development of urgency was independently associated with the onset of FI (OR 25.9 (10.6, 58.4)) among these specific changes in bowel symptoms. Figure 2 shows the association between urgency and FI over the approximately 9-year follow-up.
Figure 2.
Association of urgency with fecal incontinence in community subjects over a 9-year follow-up.
DISCUSSION
Our study provides a comprehensive picture of the problem of FI in the general population, addressing simultaneously, the prevalence of FI, the 9-year onset rate, and risk factors associated with both suffering and developing FI in middle aged and elderly community subjects.
The adjusted prevalence of FI in white US community subjects aged 50 years or older was estimated to be 15.2%. This estimate is in agreement with several cross-sectional population-based surveys (1,23,28,42), and specifically with the 12.1% in females found in Olmsted County by Bharucha et al. (1) in an independent study. Recently, using the NHAMES survey, a prevalence of 8.3% for FI for noninstitutionalized adults in the US general population has been estimated (24); however, the reported prevalence in women aged 60 – 79 years old was 14.4% (23). In addition, our estimate is consistent with population-based surveys in Australia (28) and Sweden (42). FI is likely to be suffered in silence by many who have the condition. In fact, in our study just 8% of the community subjects reporting FI had consulted a physician for it. Thus, our estimate of the prevalence of silent FI compliments the literature (15).
Until now, data on the onset rate of FI have come from studies in specific populations followed over relatively short periods of time. These previous studies have shown an onset rate of 7.5% in institutionalized elderly people at 1 year (9), 29% 3– 6 months after delivery (30), 5.4% at 1 year after rehabilitation from acute brain injury (37), and 10.2% at 66 months after lateral sphincterectomy for anal fissure (35). However, these figures cannot be extrapolated to the community. We found that the 9-year onset rate of FI in people 50 years of age and older is 6.3%. This onset rate in community people is striking, and emphasizes the magnitude of the problem in the community. We conclude that prevention of FI deserves greater attention by health authorities and heath-care providers.
Most studies report that the prevalence of FI is higher in females and increases with age (21). Our study supports that women are more likely to suffer from FI, but notably we observed that the onset rate is not different in males and females. These results suggest that females are at a higher risk before 50 years of age, but not later. Although obstetric trauma is frequently argued to be the underling reason (29), it does not seem to be the main explanation from a population-based perspective. In our study, previous vaginal deliveries were not associated with FI, which is in agreement with several other population-based studies (20,25,43,44), but not all (27,45). Other factors, such as a higher prevalence of diarrhea and a higher probability of pelvic dysfunction (46), may account for the higher prevalence of FI at a younger age in women.
Our study not only provides a snapshot of FI in the community, but also provides a new information regarding risk factors for the onset of FI. This information is relevant as it provides clues for answering the following questions: (1) Who should be especially questioned about the possibility of FI?, (2) Who is more likely to suffer with FI in subsequent years and therefore deserves closer follow-up?, and (3) What changes in bowel habit should make us aware of a higher risk of FI?
A diarrhea bowel pattern and symptoms of diarrhea emerged as the most important factor associated with suffering and developing FI. Previous studies have shown that diarrhea symptoms and especially urgency is a risk factor for suffering FI (43). Our study goes further: urgency is the strongest factor associated with having FI. Although it may be surprising that baseline urgency was not a risk factor for the onset of FI, the most likely explanation is that urgency is so highly related to FI that if the subject did not experience leakage when suffering urgency, they are unlikely to experience it in the future. This interpretation is supported by the fact that the onset of urgency in the follow-up is a strong risk factor for becoming incontinent. In fact, among those reporting urgency, 72% actually suffered FI and 22% developed FI over 9 years; indeed, 37% of those who developed urgency became incontinent. Urgency seems to be a good marker of FI, and it may be viewed as a “natural test” of continence mechanisms. Loose stools and number of bowel movements somewhat mirrored urgency, but the association was weaker.
Various symptoms suggestive of constipation are also risk factors for FI. Specifically, feelings of incomplete evacuation, feelings of anal blockage, and time to defecate are likely associated with evacuatory defects and pelvic floor dysfunction (47), and in this study were also associated with FI. Moreover, they may also mark a future risk for FI, as suggested by the fact that reporting feelings of incomplete evacuation is a risk factor for the onset of FI. Forty percent of those with feelings of incomplete evacuation actually suffered FI and 15% developed it in the following 9 years. Similar to urgency, feelings of incomplete evacuation seems to be a good marker for FI.
Another risk factor for developing FI in the following 9 years is self-reported bowel habit. This is remarkable as it is well recognized, there is a discrepancy between self-reported bowel habit and symptom-based definitions (48). If fact, self-reported diarrhea pattern was a predictor of the onset of FI, but specific symptoms of diarrhea were not, suggesting that the subjective perception of bowel habit captures additional information. The most likely explanation is that milder forms of altered bowel pattern, with occasional (less than 25% of times) loose stools, urgency or increase in number of bowel movements are self-perceived as diarrhea; these symptoms, although less relevant from a clinical perspective, may be enough to lead to FI sometime in the future in predisposed individuals.
A history of previous anorectal trauma or surgeries and pelvic radiation were associated with FI, as have been found by others (27,28), but they were not related to the onset of FI. The most likely explanation is that FI occurs shortly after these events and, if it does not happen, subjects are not more likely to develop FI in the long term. The same interpretation can be applied to diabetes mellitus; although 25% of subjects with diabetes suffered FI, none developed FI in the subsequent 9 years, although this interval may be too short to detect diabetic complications such as neuropathy. An association between FI and diabetes is well recognized (49), and it may be related to poor glycemic control and diabetic complications (50). We do not have information about the control of diabetes or its complications in this study, but the most likely explanation for our findings is that there is a low incidence of complications and relatively good glycemic control in most community diabetics followed up by the Mayo Clinic (51).
Our study has several strengths. First, it is a true population-based study, not restricted to specific subgroups aside from age, using the unique resources of the Rochester Epidemiology Project (38). Second, the response rate at the baseline interview was quite acceptable and the response rate at follow-up was high enough to be confident of the results; although we cannot exclude response bias, there is no reason to believe that subjects suffering FI are more or less likely to respond; also, ascertainment bias is unlikely because the follow-up survey was not specifically directed at FI. Moreover, a recent study in Olmsted County has shown no relevant differences between responders and nonresponders to our surveys, including in terms of bowel symptoms (52). Information was collected with a validated instrument, and key questions were the same at baseline and follow-up. For those with FI, this is especially critical, as it is well known that people tend to underreport FI; the questions we used to collect information have been previously shown to produce reliable and valid responses (22,41).
On the other hand, we have to recognize the potential limitations. Our study population and our conclusions are limited to those older than 50 years; in fact, the onset rate of FI below 50 years old is likely to be different and specifically may vary by gender, although this is speculative. Unfortunately, the size of the follow-up cohort with FI was not enough to permit a multivariate regression analysis to identify independent risk factors for the onset of FI. Finally, the severity of FI could not be evaluated, so we are unable to separate risk factors for mild/occasional FI and moderate-to-severe/frequent incontinence; although some scales suitable for population-based studies have been published, we could not apply these to the available baseline information (53,54).
In summary, 15% of the population over 50 years of age suffers with FI and an additional 7% will develop FI in the following 10 years. Symptoms of diarrhea and constipation are associated with FI and, specifically, urgency seems to be an especially useful marker of risk.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
-
✓
Fecal incontinence (FI) is common in the community, with a prevalence around 9 – 15%, and is notably higher in older people.
-
✓
Studies of risk factors for FI are scarce and somewhat contradictory.
WHAT IS NEW HERE
-
✓
The 10-year onset rate of FI is 7% in community subjects aged 50 years and older.
-
✓
Factors that predict the onset of FI after 10 years are self-reported diarrhea, feelings of incomplete evacuation, and pelvic irradiation.
-
✓
Development of urgency was the main bowel-symptom predictor for new onset of FI.
-
✓
This study identifies who is at higher risk of developing FI and who may deserve closer follow-up.
Acknowledgments
Financial support: Enrique Rey was supported by grant BA08/90038 from the Carlos III Institute, Ministry of Health, Spain. This study was in part funded by a Rochester Epidemiology project grant (RO1-AR30582) from NIAMS.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Nicholas J. Talley, MD, PhD.
Specific author contributions: Talley has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Talley, Locke, Zinsmeister, Rey; analysis and interpretation: Rey, Choung, Zinsmeister, Locke, and Talley; drafting the manuscript: Rey, Schleck, Zinsmeister, and Talley; critical review of the manuscript for important intellectual content: Rey, Choung, Zinsmeister, Locke, and Talley; statistical analysis: Schleck, Zinsmeister.
Potential competing interests: None.
REFERENCES
- 1.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards NI, Jones D. The prevalence of faecal incontinence in older people living at home. Age Ageing. 2001;30:503–507. doi: 10.1093/ageing/30.6.503. [DOI] [PubMed] [Google Scholar]
- 3.Deutekom M, Dobben AC, Dijkgraaf MG, et al. Costs of outpatients with fecal incontinence. Scand J Gastroenterol. 2005;40:552–558. doi: 10.1080/00365520510012172. [DOI] [PubMed] [Google Scholar]
- 4.Miner PB., Jr Economic and personal impact of fecal and urinary incontinence. Gastroenterology. 2004;126:S8–S13. doi: 10.1053/j.gastro.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 5.Nelson R, Furner S, Jesudason V, et al. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998;41:1226–1229. doi: 10.1007/BF02258218. [DOI] [PubMed] [Google Scholar]
- 6.Borrie MJ, Davidson HA. Incontinence in institutions: costs and contributing factors. CMAJ. 1992;147:322–328. [PMC free article] [PubMed] [Google Scholar]
- 7.Macmillan AK, Merrie AE, Marshall RJ, et al. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum. 2004;47:1341–1349. doi: 10.1007/s10350-004-0593-0. [DOI] [PubMed] [Google Scholar]
- 8.Johanson JF, Irizarry F, Doughty A. Risk factors for fecal incontinence in a nursing home population. J Clin Gastroenterol. 1997;24:156–160. doi: 10.1097/00004836-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chassagne P, Landrin I, Neveu C, et al. Fecal incontinence in the institutionalized elderly: incidence, risk factors, and prognosis. Am J Med. 1999;106:185–190. doi: 10.1016/s0002-9343(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 10.Enck P, Bielefeldt K, Rathmann W, et al. Epidemiology of faecal incontinence in selected patient groups. Int J Colorectal Dis. 1991;6:143–146. doi: 10.1007/BF00341234. [DOI] [PubMed] [Google Scholar]
- 11.Ho YH, Muller R, Veitch C, et al. Faecal incontinence: an unrecognised epidemic in rural North Queensland? Results of a hospital-based outpatient study. Aust J Rural Health. 2005;13:28–34. doi: 10.1111/j.1440-1854.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths AN, Makam A, Edwards GJ. Should we actively screen for urinary and anal incontinence in the general gynaecology outpatients setting?--A prospective observational study. J Obstet Gynaecol. 2006;26:442–444. doi: 10.1080/01443610600747272. [DOI] [PubMed] [Google Scholar]
- 13.Bano F, Barrington JW. Prevalence of anorectal dysfunction in women attending health care services. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:57–60. doi: 10.1007/s00192-006-0095-9. [DOI] [PubMed] [Google Scholar]
- 14.Boreham MK, Richter HE, Kenton KS, et al. Anal incontinence in women presenting for gynecologic care: prevalence, risk factors, and impact upon quality of life. Am J Obstet Gynecol. 2005;192:1637–1642. doi: 10.1016/j.ajog.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Nelson R, Norton N, Cautley E, et al. Community-based prevalence of anal incontinence. JAMA. 1995;274:559–561. [PubMed] [Google Scholar]
- 16.Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895–901. doi: 10.1016/0016-5085(92)90175-x. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 18.Kok AL, Voorhorst FJ, Burger CW, et al. Urinary and faecal incontinence in community-residing elderly women. Age Ageing. 1992;21:211–215. doi: 10.1093/ageing/21.3.211. [DOI] [PubMed] [Google Scholar]
- 19.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–484. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritel X, Ringa V, Varnoux N, et al. Mode of delivery and fecal incontinence at midlife: a study of 2,640 women in the Gazel cohort. Obstet Gynecol. 2007;110:31–38. doi: 10.1097/01.AOG.0000266981.69332.db. [DOI] [PubMed] [Google Scholar]
- 21.Pretlove SJ, Radley S, Toozs-Hobson PM, et al. Prevalence of anal incontinence according to age and gender: a systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:407–417. doi: 10.1007/s00192-005-0014-5. [DOI] [PubMed] [Google Scholar]
- 22.Reilly WT, Talley NJ, Pemberton JH, et al. Validation of a questionnaire to assess fecal incontinence and associated risk factors: Fecal Incontinence Questionnaire. Dis Colon Rectum. 2000;43:146–153. doi: 10.1007/BF02236971. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–517. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varma MG, Brown JS, Creasman JM, et al. Fecal incontinence in females older than aged 40 years: who is at risk? Dis Colon Rectum. 2006;49:841–851. doi: 10.1007/s10350-006-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eva UF, Gun W, Preben K. Prevalence of urinary and fecal incontinence and symptoms of genital prolapse in women. Acta Obstet Gynecol Scand. 2003;82:280–286. doi: 10.1034/j.1600-0412.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen GD, Hu SW, Chen YC, et al. Prevalence and correlations of anal incontinence and constipation in Taiwanese women. Neurourol Urodyn. 2003;22:664–669. doi: 10.1002/nau.10067. [DOI] [PubMed] [Google Scholar]
- 28.Kalantar JS, Howell S, Talley NJ. Prevalence of faecal incontinence and associated risk factors; an underdiagnosed problem in the Australian community? Med J Aust. 2002;176:54–57. [PubMed] [Google Scholar]
- 29.Bharucha AE. Fecal incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 30.Guise JM, Morris C, Osterweil P, et al. Incidence of fecal incontinence after childbirth. Obstet Gynecol. 2007;109:281–288. doi: 10.1097/01.AOG.0000254164.67182.78. [DOI] [PubMed] [Google Scholar]
- 31.Norderval S, Nsubuga D, Bjelke C, et al. Anal incontinence after obstetric sphincter tears: incidence in a Norwegian county. Acta Obstet Gynecol Scand. 2004;83:989–994. doi: 10.1111/j.0001-6349.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinta TM, Kylanpaa ML, Teramo KA, et al. Sphincter rupture and anal incontinence after first vaginal delivery. Acta Obstet Gynecol Scand. 2004;83:917–922. doi: 10.1111/j.0001-6349.2004.00346.x. [DOI] [PubMed] [Google Scholar]
- 33.Groutz A, Fait G, Lessing JB, et al. Incidence and obstetric risk factors of postpartum anal incontinence. Scand J Gastroenterol. 1999;34:315–318. doi: 10.1080/00365529950173753. [DOI] [PubMed] [Google Scholar]
- 34.Bishoff JT, Motley G, Optenberg SA, et al. Incidence of fecal and urinary incontinence following radical perineal and retropubic prostatectomy in a national population. J Urol. 1998;160:454–458. [PubMed] [Google Scholar]
- 35.Rotholtz NA, Bun M, Mauri MV, et al. Long-term assessment of fecal incontinence after lateral internal sphincterotomy. Tech Coloproctol. 2005;9:115–118. doi: 10.1007/s10151-005-0208-3. [DOI] [PubMed] [Google Scholar]
- 36.Kirschner-Hermanns R, Borchers H, Reineke T, et al. Fecal incontinence after radical perineal prostatectomy: a prospective study. Urology. 2005;65:337–342. doi: 10.1016/j.urology.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Foxx-Orenstein A, Kolakowsky-Hayner S, Marwitz JH, et al. Incidence, risk factors, and outcomes of fecal incontinence after acute brain injury: findings from the Traumatic Brain Injury Model Systems national database. Arch Phys Med Rehabil. 2003;84:231–237. doi: 10.1053/apmr.2003.50095. [DOI] [PubMed] [Google Scholar]
- 38.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 39.Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc. 1998;46:467–472. doi: 10.1111/j.1532-5415.1998.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 40.Roberts RO, Jacobsen SJ, Reilly WT, et al. Prevalence of combined fecal and urinary incontinence: a community-based study. J Am Geriatr Soc. 1999;47:837–841. doi: 10.1111/j.1532-5415.1999.tb03841.x. [DOI] [PubMed] [Google Scholar]
- 41.Talley NJ, Phillips SF, Melton J, III, et al. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 42.Walter S, Hallbook O, Gotthard R, et al. A population-based study on bowel habits in a Swedish community: prevalence of faecal incontinence and constipation. Scand J Gastroenterol. 2002;37:911–916. doi: 10.1080/003655202760230865. [DOI] [PubMed] [Google Scholar]
- 43.Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101:1305–1312. doi: 10.1111/j.1572-0241.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 44.Rizk DE, Hassan MY, Shaheen H, et al. The prevalence and determinants of health care-seeking behavior for fecal incontinence in multiparous United Arab Emirates females. Dis Colon Rectum. 2001;44:1850–1856. doi: 10.1007/BF02234467. [DOI] [PubMed] [Google Scholar]
- 45.Uustal FE, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004;83:383–389. doi: 10.1111/j.0001-6349.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 46.Chang L, Toner BB, Fukudo S, et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 47.Locke GR, III, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000;119:1766–1778. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- 48.Talley NJ, Weaver AL, Zinsmeister AR, et al. Functional constipation and outlet delay: a population-based study. Gastroenterology. 1993;105:781–790. doi: 10.1016/0016-5085(93)90896-k. [DOI] [PubMed] [Google Scholar]
- 49.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 50.Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–611. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 51.Montori VM, Dinneen SF, Gorman CA, et al. The impact of planned care and a diabetes electronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes Care. 2002;25:1952–1957. doi: 10.2337/diacare.25.11.1952. [DOI] [PubMed] [Google Scholar]
- 52.Von Blumenthal J, Choung R, Locke GR, III, et al. Differences between nonresponders and responders in bowel disease surveys in Olmsted County residents. Neurogastro Motil. 2008;20 Supp 2:138–139. [Google Scholar]
- 53.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bharucha AE, Locke GR, III, Seide BM, et al. A new questionnaire for constipation and faecal incontinence. Aliment Pharmacol Ther. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]