Abstract
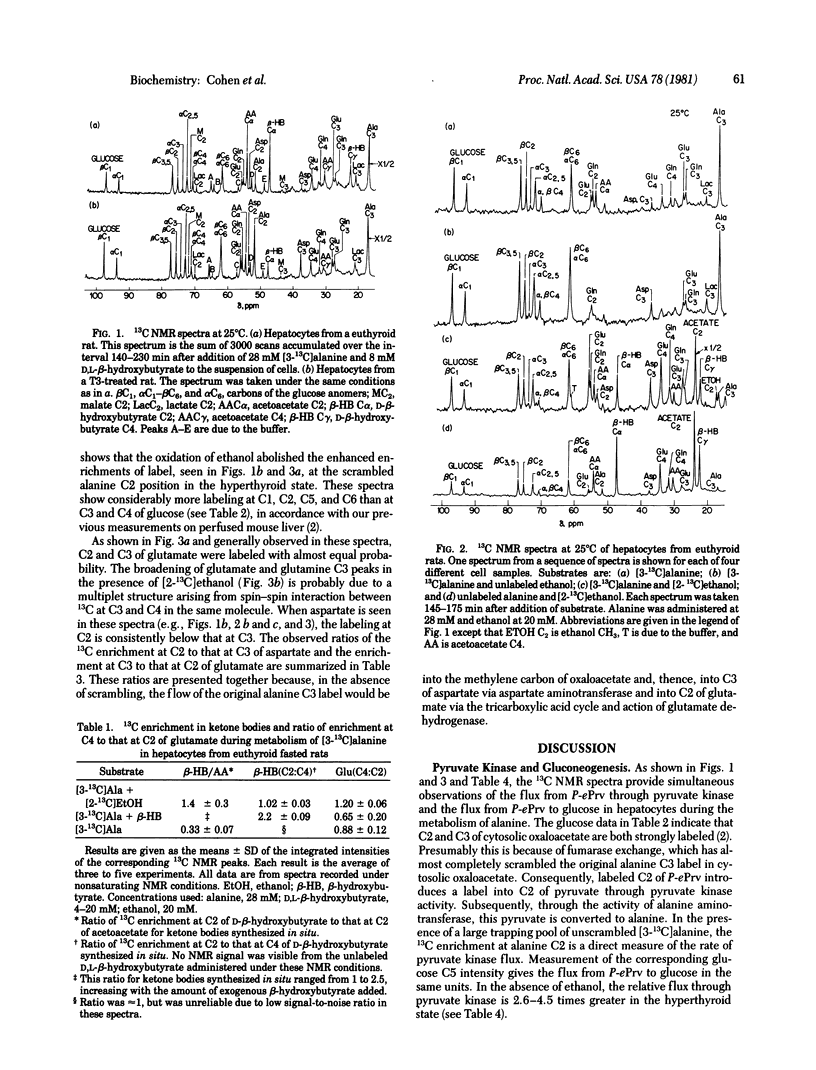
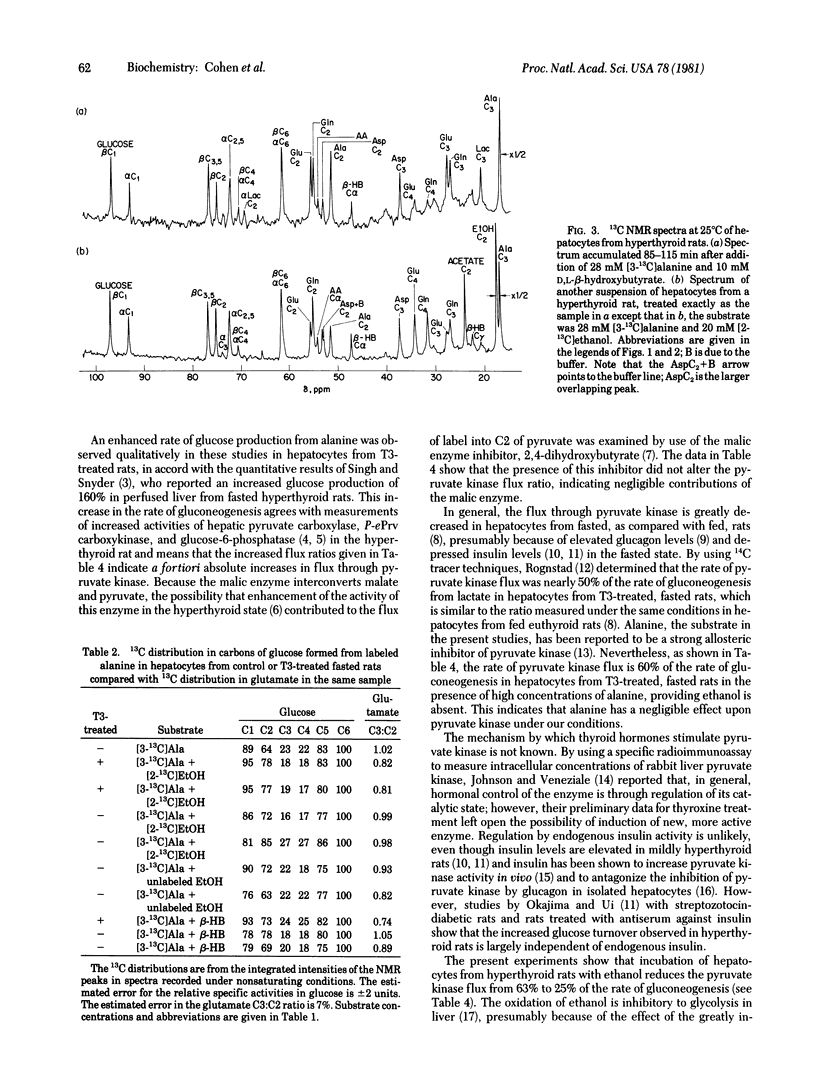
Metabolism of [3-13C]alanine in the presence and absence of beta-hydroxybutyrate or ethanol has been followed at 25 degrees C by 13C NMR at 90.5 MHz in primary hepatocytes from untreated rats and rats treated with triiodothyronine and not allowed to eat for 24 hr. The phosphoenolpyruvate/pyruvate futile cycle was followed in situ by comparing the concentration of 13C at the scrambled alanine C2 position with that at glucose C5. In the absence of ethanol, the flux through pyruvate kinase was 60% of the gluconeogenic flux in hepatocytes from hyperthyroid rats, compared with 25% in the controls. Incubation with ethanol reduced the pyruvate kinase flux in the hyperthyroid state to that measured in the controls. Under all conditions, the relative concentration of label at the aspartate C2 and C3 sites was 1:2, whereas at the corresponding carbons in glutamate, randomization was almost complete. These observations, which require flux of unscrambled label into aspartate, are consistent with intramitochondrial synthesis of aspartate only if there is incomplete mixing of the intramitochondrial oxaloacetate pool. The 13C enrichment measured in the ketone bodies is increased by the presence of exogenous beta-hydroxybutyrate. The greater labeling that we observe at C2 of beta-hydroxybutyrate compared with C4 under this condition is explained by the flow through 3-hydroxy-3-methylglutaryl-coenzyme A synthase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranda A., Montoya E., Herrera E. Effects of hypo- and hyper-thyroidism on liver composition, blood glucose, ketone bodies and insulin in the male rat. Biochem J. 1972 Jul;128(3):597–604. doi: 10.1042/bj1280597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. B., Cimbala M. A., Foster J. L., Morgan R. A. Hepatic pyruvate kinase. Regulation by glucagon, cyclic adenosine 3'-5'-monophosphate, and insulin in the perfused rat liver. J Biol Chem. 1976 Jun 25;251(12):3756–3762. [PubMed] [Google Scholar]
- Böttger I., Kriegel H., Wieland O. Fluctuation of hepatic enzymes important in glucose metabolism in relation to thyroid function. Eur J Biochem. 1970 Apr;13(2):253–257. doi: 10.1111/j.1432-1033.1970.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Shulman R. G. 13C NMR studies of gluconeogenesis in rat liver cells: utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1603–1609. doi: 10.1073/pnas.76.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Shulman R. G., McLaughlin A. C. Effects of ethanol on alanine metabolism in perfused mouse liver studied by 13C NMR. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4808–4812. doi: 10.1073/pnas.76.10.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton D. G., Mehlman M. A., Ruegamer W. R. Effect of thyroxine and 5,5-diphenyl-2-thiohydantoin on enzyme activities of rat liver and kidney. Endocrinology. 1972 Jun;90(6):1521–1528. doi: 10.1210/endo-90-6-1521. [DOI] [PubMed] [Google Scholar]
- Duszynski J., Mueller G., LaNoue K. Microcompartmentation of aspartate in rat liver mitochondria. J Biol Chem. 1978 Sep 10;253(17):6149–6157. [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. L., Blair J. B. Acute hormonal control of pyruvate kinase and lactate formation in the isolated rat hepatocyte. Arch Biochem Biophys. 1978 Aug;189(2):263–276. doi: 10.1016/0003-9861(78)90212-6. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Veneziale C. M. Hormonal regulation of liver pyruvate kinase concentration and activity. Biochemistry. 1980 May 13;19(10):2191–2195. doi: 10.1021/bi00551a030. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G., Tischler M. E., Tager J. M., Williamson J. R. Interrelationships between gluconeogenesis and ureogenesis in isolated hepatocytes. J Biol Chem. 1978 Apr 10;253(7):2308–2320. [PubMed] [Google Scholar]
- Miziorko H. M., Lane M. D. 3-Hydroxy-3-methylgutaryl-CoA synthase. Participation of acetyl-S-enzyme and enzyme-S-hydroxymethylgutaryl-SCoA intermediates in the reaction. J Biol Chem. 1977 Feb 25;252(4):1414–1420. [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Minor role of endogenous insulin in thyroid-dependent changes in glucose turnover. Biochem J. 1979 Aug 15;182(2):577–584. doi: 10.1042/bj1820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R. Futile hydrogen cycling in liver cells from triiodothyronine treated rats. Biochem Biophys Res Commun. 1977 Oct 10;78(3):881–888. doi: 10.1016/0006-291x(77)90505-8. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Gluconeogenesis in the kidney cortex. Flow of malate between compartments. Biochem J. 1970 Feb;116(3):493–502. doi: 10.1042/bj1160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Role of pyruvate kinase in the regulation of gluconeogenesis from L-lactate. J Biol Chem. 1977 Mar 25;252(6):1831–1833. [PubMed] [Google Scholar]
- Schimerlik M. I., Cleland W. W. Inhibition and alternate-substrate studies on the mechanism of malic enzyme. Biochemistry. 1977 Feb 22;16(4):565–570. doi: 10.1021/bi00623a001. [DOI] [PubMed] [Google Scholar]
- Schoolwerth A. C., LaNoue K. F. The role of microcompartmentation in the regulation of glutamate metabolism by rat kidney mitochondria. J Biol Chem. 1980 Apr 25;255(8):3403–3411. [PubMed] [Google Scholar]
- Seitz H. J., Müller M. J., Krone W., Tarnowski W. Coordinate control of intermediary metabolism in rat liver by the insulin/glucagon ratio during starvation and after glucose refeeding. Regulatory significance of long-chain acyl-CoA and cyclic AMP. Arch Biochem Biophys. 1977 Oct;183(2):647–663. doi: 10.1016/0003-9861(77)90399-x. [DOI] [PubMed] [Google Scholar]
- Singh S. P., Snyder A. K. Effect of thyrotoxicosis on gluconeogenesis from alanine in the perfused rat liver. Endocrinology. 1978 Jan;102(1):182–187. doi: 10.1210/endo-102-1-182. [DOI] [PubMed] [Google Scholar]
- Taunton O. D., Stifel F. B., Greene H. L., Herman R. H. Rapid reciprocal changes in rat hepatic glycolytic enzyme and fructose diphosphatase activities following insulin and glucagon injection. J Biol Chem. 1974 Nov 25;249(22):7228–7239. [PubMed] [Google Scholar]
- Thurman R. G., Scholz R. Interaction of glycolysis and respiration in perfused rat liver. Changes in oxygen uptake following the addition of ethanol. Eur J Biochem. 1977 May 2;75(1):13–21. doi: 10.1111/j.1432-1033.1977.tb11499.x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]


