Abstract
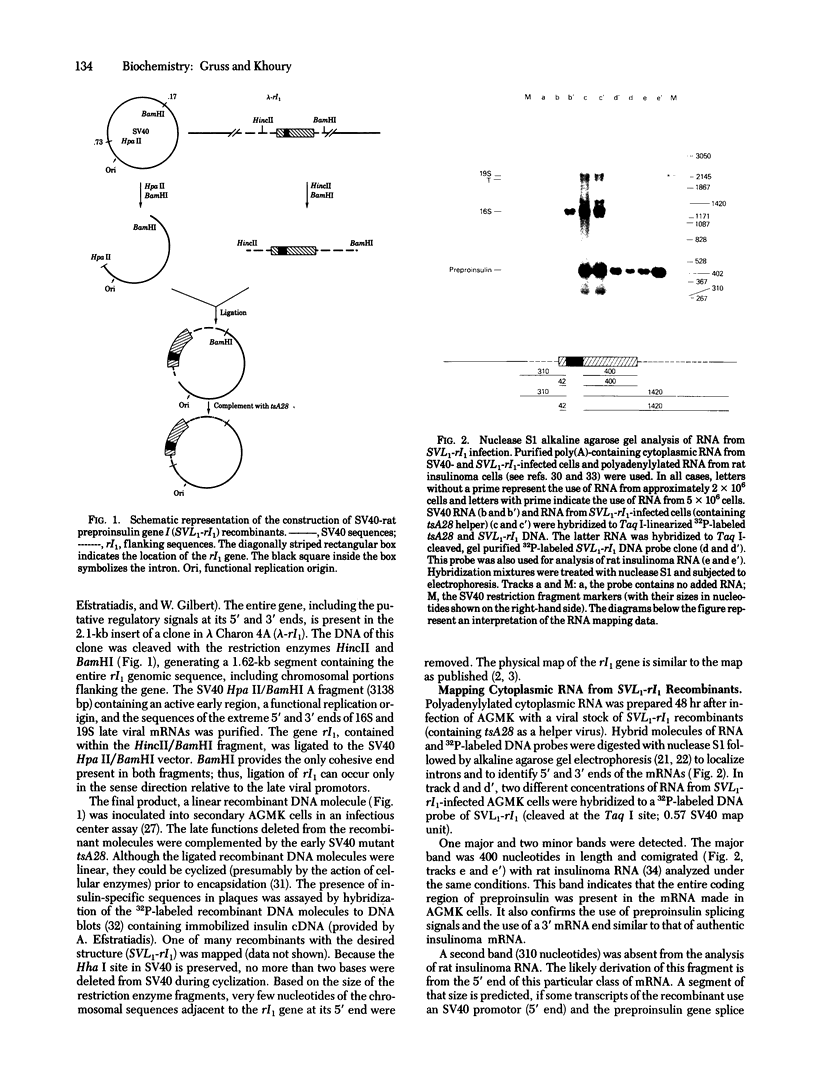
The complete rat preproinsulin gene I was cloned into a simian virus 30 (SV 40) vector. Most of the late region of the viral vector, including the SV40 intervening sequences (introns) and all of the major splice junctions, was deleted and replaced by the entire rat insulin gene. The recombinant molecules and a temperature-sensitive helper virus (tsA28) were inoculated into monkey kidney cultures. The formation of stable transcripts of the insulin insert was as efficient as the production of late SV40 mRNA. Analysis of these transcripts indicated that the rat preproinsulin gene nucleotide signals involved in RNA splicing and poly(A) addition were used. Examination of the 5' ends of the mRNAs showed several classes, one of which was the same size as the authentic rat insulinoma mRNA. This suggests that a portion of the transcripts may be initiated or processed faithfully, or both, at their 5' ends within rat insulin sequences. Significant quantities of a protein identified as rat proinsulin were synthesized. Detection of most of the proinsulin in the tissue culture medium suggests that this protein was secreted.
Full text
PDF



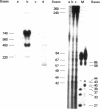
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Wahli W. Application of recombinant DNA technology to questions of developmental biology: a review. Dev Biol. 1979 Mar;69(1):305–328. doi: 10.1016/0012-1606(79)90294-x. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Steiner D. F., Chick W. L. Partial purification and characterization of the mRNA for rat preproinsulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3539–3543. doi: 10.1073/pnas.73.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Nussbaum A. L., Davoli D., Fareed G. C. Propagation of a segment of bacteriophage lamda-DNA in monkey cells after covalent linkage to a defective simian virus 40 genome. Cell. 1976 Mar;7(3):349–359. doi: 10.1016/0092-8674(76)90164-1. [DOI] [PubMed] [Google Scholar]
- Gerner E. W., Humphrey R. M. The cell-cycle phase synthesis of non-histone proteins in mammalian cells. Biochim Biophys Acta. 1973 Nov 26;331(1):117–127. doi: 10.1016/0005-2787(73)90424-3. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells. Cell. 1976 Dec;9(4 Pt 2):695–705. doi: 10.1016/0092-8674(76)90133-1. [DOI] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Davoli D., Thomas C. A., Jr, Fareed G. C. Simian virus 40 carrying an Escherichia coli suppressor gene. J Mol Biol. 1977 May 15;112(2):155–182. doi: 10.1016/s0022-2836(77)80137-x. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Expression of the chromosomal mouse Beta maj-globin gene cloned in SV40. Nature. 1979 Sep 6;281(5726):35–40. doi: 10.1038/281035a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Smith K. D., Boyer S. H., Leder P. SV40 recombinants carrying rabbit beta-globin gene coding sequences. Cell. 1979 Jul;17(3):725–735. doi: 10.1016/0092-8674(79)90279-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Khoury G. Deletion mutants of simian virus 40 defective in biosynthesis of late viral mRNA. Proc Natl Acad Sci U S A. 1979 Jan;76(1):71–75. doi: 10.1073/pnas.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Chan S. J., Steiner D. F., Saunders G. F. Immunological and chemical characterization of bovine preproinsulin. J Biol Chem. 1977 Nov 25;252(22):7971–7978. [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Relationship between the methionine tryptic peptides of simian virus 40 and BK virus tumor antigens. J Virol. 1977 Oct;24(1):319–325. doi: 10.1128/jvi.24.1.319-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Peptide hormones. Annu Rev Biochem. 1974;43(0):509–538. doi: 10.1146/annurev.bi.43.070174.002453. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P., Skolnik H., Upcroft J. A., Solomon D., Khoury G., Hamer D. H., Fareed G. C. Transduction of a bacterial gene into mammalian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2117–2121. doi: 10.1073/pnas.75.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain B. S., Roberts R. J. Sequences from the beginning of the fiber messenger RNA of adenovirus-2. J Mol Biol. 1979 Jun 25;131(2):341–352. doi: 10.1016/0022-2836(79)90080-9. [DOI] [PubMed] [Google Scholar]