Abstract
The structures of the uracil and thiouracils were examined using NMR spectroscopy and crystal structure data when available. The relationships between the extent of polarization and the C5–C6 bond length as well as the H5–H6 coupling constants were probed. It was found that the bond length and coupling constants correlate well with the proton affinities at the carbonyl or thiocarbonyl groups at C4 but not C2. The possible implication in the tighter binding of thiouracil based nucleotides to orotidine-5’-monophosphate decarboxylase was discussed.
Keywords: uracil, thiouracil, dipole moment, polarization, ODCase, coupling constant
The structure and properties of uracil and derivatives have been examined quite extensively due to its status as one of the nucleic base in RNA.1–7 Some studies have focused on the protonation of the two carbonyl oxygens in the mechanistic studies of the reaction catalyzed by orotidine-5’-monophosphate decarboxylase (ODCase).8–12
The inhibition of ODCase by uridine-5’-monophosphate (UMP) and its thialated analogues 2-thioUMP and 4-thioUMP has been compared.13 The replacement of either carbonyl oxygen by sulfur was found to enhance the ability of these nucleotides to inhibit ODCase.13 In this Letter, we examine the structures of uracil (1) and thiouracils 2–4, especially the extent of polarization, as an attempt to provide insight into the enhanced inhibition seen in thioUMPs.
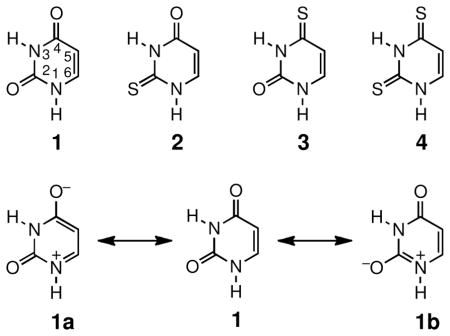
One way to estimate the extent of polarization in the structures of uracil and thiouracils is to probe the contribution of zwitterionic resonance forms to the overall structure. Structures 1a and 1b of uracil 1 are depicted as examples. It is evident that in these resonance forms, specifically 1a, the C5–C6 double bond has become single bond. The more the zwitterions such as 1a contributes to the overall structure, the less double-bond character for the C5–C6 bond. The decreased bond order will result in the lengthening of the C5–C6 bond. The bond lengths of the C5–C6 bonds in uracil 1, 2-thiouracil 2, and 2,4-dithiouracil 4 are available from published crystal structures as listed in Table 1.14–16 The impact of thialation on the uracil structure is evident from the comparison of the C5–C6 bond lengths. Thialation clearly increases the bond lengths and suggests increased contribution of zwitterionic resonance forms as well as enhanced polarization of the π-electrons.
Table 1.
Some structural and electronic properties of uracil and thiouracils and binding affinities of corresponding nucleotides
In addition to the examination of their crystal structures, the structures of uracil and thiouracils in solution were probed with NMR spectroscopy, which has been used to examine resonance effects in conjugated cyclic structures and the zwitterionic contribution to the structures of pyridone and thiopyridone.17,18 In NMR spectroscopy, the chemical shifts of the ring hydrogens are indicative of the extent of aromaticity and the coupling constants of the ring hydrogens are dependent on the bond length.17,18 For two hydrogens on the two ends of a C–C bond, a shorter bond length results in closer distance between the two hydrogens and thus a larger coupling constant.17,18 In the case of uracils 1–4, increased importance of zwitterions such as 1a to the overall structure will lead to the lengthening of the C5–C6 bond and thus smaller coupling constants. Interestingly, the H5–H6 coupling constants measured for 1–4 come in two distinct sets of values, one value for uracil 1 and 2-thiouracil 2 and a different one for 4-thiouracil 3 and 2,4-dithiouracil 4. Thialation of the carbonyl group at C4 results in smaller H5–H6 coupling constants, indicating decreasing bond order and thus more contribution from zwitterionic resonance forms. The greater importance of zwitterionic resonance forms with negative charge on sulfur, relative to oxygen, has been observed in thiopyridones and phosphorothioates.17,19
The polarity of uracil and its thio-analogues can also be assessed by their dipole moments. The polarization of uracil derivatives upon thialation is evident from their reported dipole moments listed in Table 1.20,21 Thialation, especially at C4, results in significant increases of the dipole moments of thiouracils.
To further understand the effect of thialation, the proton affinities of the carbonyl or thiocarbonyl groups at C2 and C4 were calculated. The calculated values of proton affinity are in good agreement with those previously reported.7,9,22 The values have further demonstrated the higher basicity of the carbonyl or thiocarbonyl groups at C4. This site benefits most from resonance form 1a, which is enhanced by the stretch in the C5–C6 distance. Interestingly, gas-phase calculations showed little difference in the structures (such as C5–C6 bond lengths) of uracil or thiouracils. This discrepancy between the experimentally determined and calculated structural parameters may be a result of self-interactions in the crystal form or in solution through hydrogen bonds. Indeed, crystal structures of uracil and thiouracils have revealed strong hydrogen bonding-mediated self-interactions between these molecules in the crystal lattice.14–16
In summary, theoretical studies indicate that thialation leads to higher basicity at the sulfur site, but not the oxygen site of uracil derivatives. Structural information on uracil and thiouracil from studies of these molecules in the crystal form and in solution has demonstrated that thialation results in a greater extent of polarization. The relationship between the polarity of the uracil derivative and the binding of their corresponding nucleotides to ODCase is worth exploring. Although outright proton transfer to the carbonyl groups11,23 appears unlikely due to the absence of adjacent basic residues at the active site of ODCase24–27, the enhanced polarization and larger dipole moments may allow thioUMPs to interact with ODCase more favorably. It has been proposed that the unique charge distribution at the active site of ODCase forms a polar environment that interacts with substrate analogues through dipole interactions.28–31 The more readily polarizable nature and the larger dipole moments of the thiouracil moieties may lead to stronger interactions at the active site of ODCase and thus the enhanced inhibitory ability of the thioUMPs as seen in Table 1.
These results have provided some preliminary support for the dipole interaction mechanism, which hypothesizes that ODCase selectively binds nucleotides with large and directionally matched dipole moment.28–31 However, other factors such as the lower pKa of the thiouracils may also play a role in the tighter binding of thioUMPs.13 We are currently examining other substrate analogues to further test this hypothesis.
Acknowledgments
This investigation was supported by the National Institutes of Health (MBRS SCORE Program – Grant S06 GM52588), by a grant from the Center for Computing for Life Sciences at SFSU, and the National Science Foundation (CHE–1011771). The NMR facility was funded by the National Science Foundation (DUE-9451624 and DBI 0521342). Computational studies were performed at the VCU Center for High-Performance Computing. S.H. was supported by a Summer Research Fellowship from the Department of Chemistry and Biochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Footnotes
- 1.Chandra AK, Nguyen MT, Zeegers-Huyskens T. J Phys Chem A. 1998;102:6010–6016. [Google Scholar]
- 2.Katritzky AR, Szafran M, Stevens J. J Chem Soc Perkin Trans. 1989;2:1507–1511. [Google Scholar]
- 3.Wolken JK, Tureček F. J Am Soc Mass Spectrom. 2000;11:1065–1071. doi: 10.1016/s1044-0305(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 4.Kurinovich MA, Lee JK. J Am Chem Soc. 2000;122:6258–6262. [Google Scholar]
- 5.Huang Y, Kenttämaa H. J Phys Chem A. 2003;107:4893–4897. [Google Scholar]
- 6.Whittleton SR, Hunter KC, Wetmore SD. J Phys Chem A. 2004;108:7709–7718. [Google Scholar]
- 7.González Moa MJ, Mosquera RA. J Phys Chem A. 2005;109:3682–3686. doi: 10.1021/jp044529k. [DOI] [PubMed] [Google Scholar]
- 8.Lee JK, Houk KN. Science. 1997;276:942–945. doi: 10.1126/science.276.5314.942. [DOI] [PubMed] [Google Scholar]
- 9.Kurinovich MA, Phillips LM, Sharma S, Lee JK. Chem Commun. 2002:2354–2355. doi: 10.1039/b207112f. [DOI] [PubMed] [Google Scholar]
- 10.Gronert S, Feng WY, Chew F, Wu W. Int J Mass Spectrom. 2000;195/196:251–258. [Google Scholar]
- 11.Feng WY, Austin TJ, Chew F, Gronert S, Wu W. Biochemistry. 2000;39:1778–1783. doi: 10.1021/bi992553w. [DOI] [PubMed] [Google Scholar]
- 12.Shem DL, Gronert S, Wu W. Bioorg Chem. 2004;32:76–81. doi: 10.1016/j.bioorg.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Smiley JA, Saleh L. Bioorg Chem. 1999;27:297–306. [Google Scholar]
- 14.Stewart RF. Acta Cryst. 1967;23:1102–1105. [Google Scholar]
- 15.Shefter E, Mautner HG. J Am Chem Soc. 1967;89:1249–1253. doi: 10.1021/ja00981a035. [DOI] [PubMed] [Google Scholar]
- 16.Munshi P, Guru Row TN. Acta Cryst. 2006;B62:612–626. doi: 10.1107/S0108768106017393. [DOI] [PubMed] [Google Scholar]
- 17.Stewart WE, Siddall TH., III J Phys Chem. 1970;74:2027–2029. [Google Scholar]
- 18.Smith WB, Watson WH, Chiranjeevi S. J Am Chem Soc. 1967;89:1438–1441. [Google Scholar]
- 19.Frey PA, Sammons RD. Science. 1985;228:541–545. doi: 10.1126/science.2984773. [DOI] [PubMed] [Google Scholar]
- 20.Zadorozhnaya AA, Krylov AI. J Chem Theory Comput. 2010;6:705–717. doi: 10.1021/ct900515a. [DOI] [PubMed] [Google Scholar]
- 21.Schneider WC, Halverstadt IF. J Am Chem Soc. 1948;70:2626–2631. doi: 10.1021/ja01188a005. [DOI] [PubMed] [Google Scholar]
- 22.Lamsabhi M, Alcamí M, Mó O, Bouab W, Esseffar M, Abboud JL-M, Yáñez M. J Phys Chem A. 2000;104:5122–5130. [Google Scholar]
- 23.Beak P, Siegel B. J Am Chem Soc. 1976;98:3601–3606. doi: 10.1021/ja00428a035. [DOI] [PubMed] [Google Scholar]
- 24.Appleby TC, Kinsland C, Begley TP, Ealick SE. Proc Natl Acad Sci. 2000;97:2005–2010. doi: 10.1073/pnas.259441296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller BG, Hassell AM, Wolfenden R, Milburn MV, Short SA. Proc Natl Acad Sci USA. 2000;97:2011–2016. doi: 10.1073/pnas.030409797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu N, Mo Y, Gao J, Pai EF. Proc Natl Acad Sci. 2000;97:2017–2022. doi: 10.1073/pnas.050417797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris P, Poulsen J-CN, Jensen KF, Larsen S. Biochemistry. 2000;39:4217–4224. doi: 10.1021/bi992952r. [DOI] [PubMed] [Google Scholar]
- 28.Wu N, Pai EF. J Biol Chem. 2002;277:28080–28087. doi: 10.1074/jbc.M202362200. [DOI] [PubMed] [Google Scholar]
- 29.Warshel A, Strajbl M, Villa J, Florian J. Biochemistry. 2000;39:14728–14738. doi: 10.1021/bi000987h. [DOI] [PubMed] [Google Scholar]
- 30.Wong FW, Capule CC, Wu W. Org Lett. 2006;8:6019–6022. doi: 10.1021/ol0624981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Wong FM, Gassner GT, Wu W. Tetrahedron Lett. 2011;52:3960–3962. doi: 10.1016/j.tetlet.2011.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


