Abstract
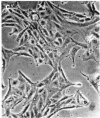
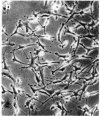
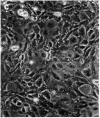
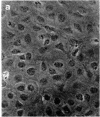
A nontransformed and a spontaneously transformed clone of BALB/c 3T3 cells were compared for their capacity to multiply in decreased concentrations of Mg2+. Cells of the nontransformed clone were flat, formed regularly patterned, nonoverlapping arrays, required high serum concentration for multiplication, had a low saturation density, and did not make colonies in agar. Cells of the transformed clone were slender and spiky, formed random, overlapping arrays, multiplied in low serum concentrations, and had no fixed saturation density, and 20-30% of them formed colonies in agar. The saturation density of the nontransformed clone was decreased in a growth-limiting supply of Mg2+ in proportion to the reduction in initial rate of multiplication. At very low Mg2+ concentrations, saturation occurred when less than half of the surface of the dish was covered with cells. The transformed cells did not reach a stable saturation density in low Mg2+ concentrations, but their growth rate did slow down when they became crowded, and a transient saturation density was reached at the lowest Mg2+ concentrations that allowed multiplication. Limiting the supply of Mg2+ caused the transformed cells to flatten and to assume a regularly patterned, non-overlapping relationship to one another, resembling that of the nontransformed cells. This also occurred in BALB/c 3T3 cells transformed by infection with Moloney mouse sarcoma virus. After 1 week in low concentrations of Mg2+, the nontransformed cells began to multiply and to incorporate [3H]thymidine at a rapid rate. The transformed cells did so also and, in addition, reverted to their transformed appearance. The intracellular content of Mg2+ was not significantly decreased when the extracellular concentration was decreased to 1/50th. The results suggest that: (a) limited contact among cells already multiplying at a reduced rate is sufficient to halt further multiplication; (b) a very small decrease in intracellular Mg2+ content or in membrane-associated Mg2+ causes transformed cells to assume aspects of the appearance and behavior of nontransformed cells (i.e., Mg2+-regulated reactions may be involved in determining the transformed phenotype); and (c) cells multiplying at a slow rate in low concentrations of Mg2+ begin to multiply faster after about 1 week, due either to an adaptation of the cells or to a change in the cellular microenvironment.
Keywords: saturation density, normalization, phenotypic variation
Full text
PDF

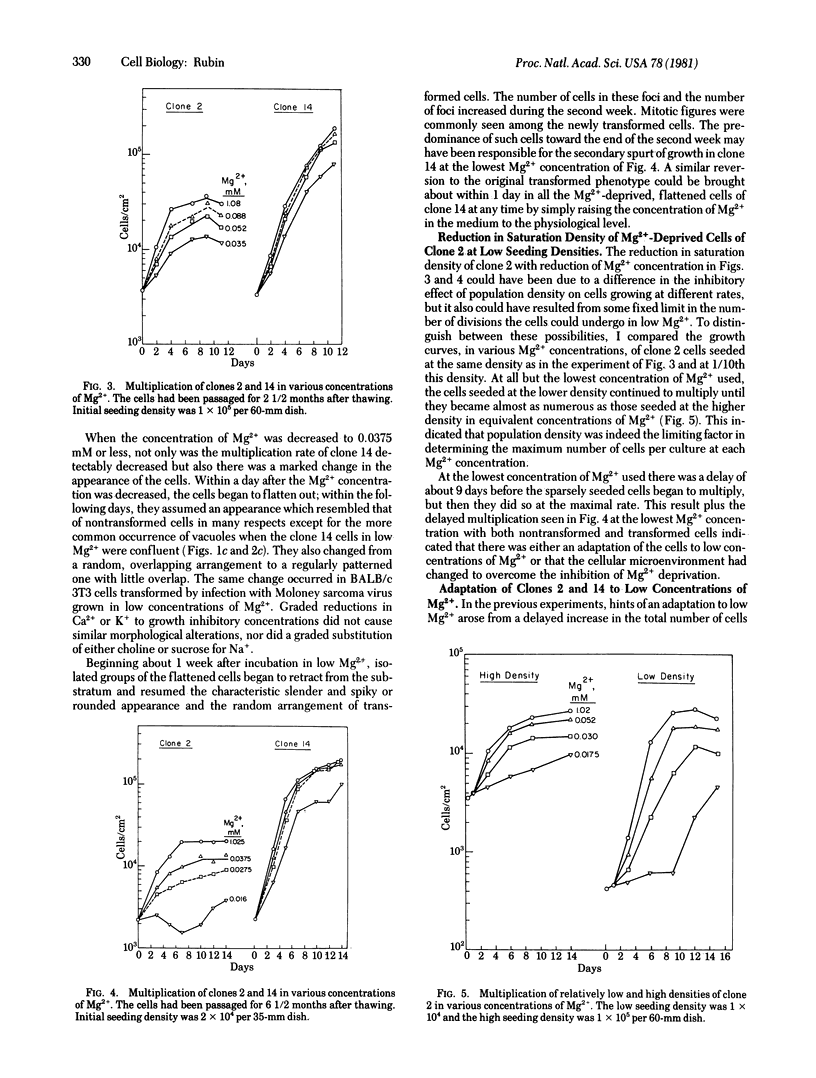

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
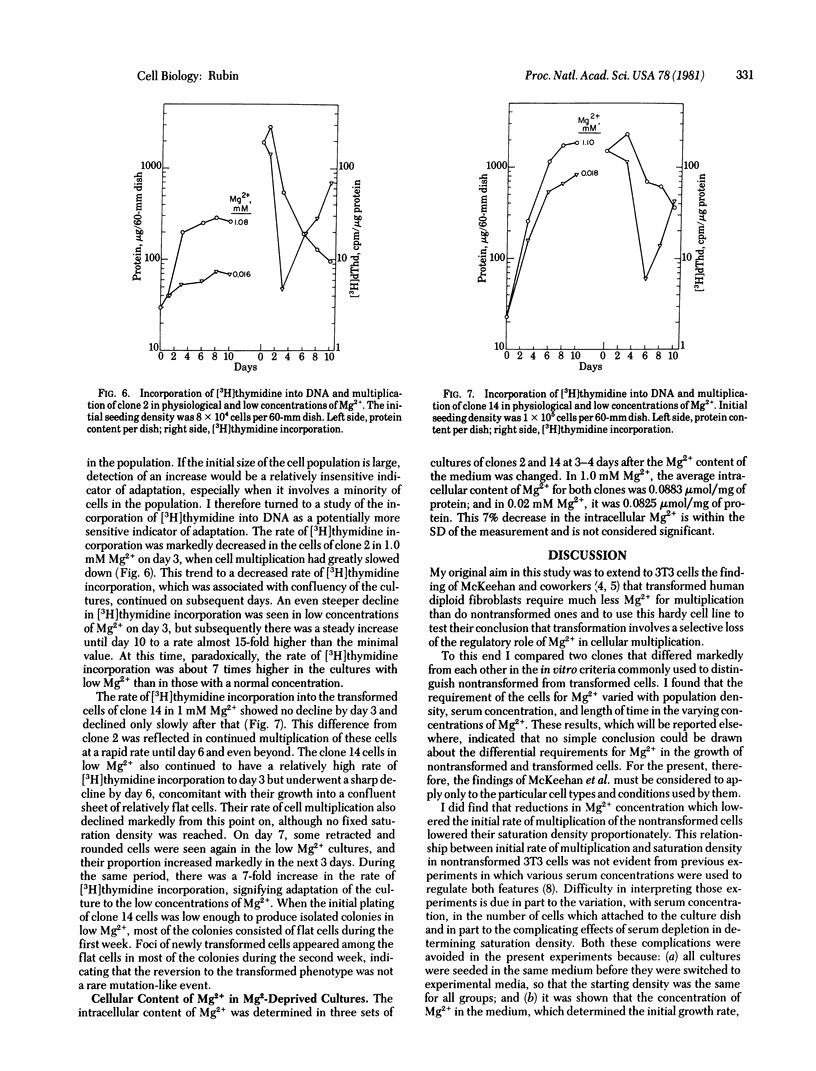
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953 Sep;5(1):111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- Bartholomew J. C., Yokota H., Ross P. Effect of serum on the growth of Balb oT3 A31 mouse fibroblasts and an SV40-transformed derivative. J Cell Physiol. 1976 Jul;88(3):277–286. doi: 10.1002/jcp.1040880303. [DOI] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Grimes W. J., Schroeder J. L. Dibutyryl cyclic adenosine 3'5' monophosphate, sugar transport, and regulatory control of cell division in normal and transformed cells. J Cell Biol. 1973 Feb;56(2):487–491. doi: 10.1083/jcb.56.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Biochem Soc Symp. 1968;27:61–86. [PubMed] [Google Scholar]
- Mastro A. M., Rozengurt E. Endgoenous protein kinase in outer plasma membrane of cultured 3T3 cells. Nature of the membrane-bound substrate and effect of cell density, serum addition, and oncogenic transformation. J Biol Chem. 1976 Dec 25;251(24):7899–7906. [PubMed] [Google Scholar]
- McKeehan W. L., Ham R. G. Calcium and magnesium ions and the regulation of multiplication in normal and transformed cells. Nature. 1978 Oct 26;275(5682):756–758. doi: 10.1038/275756a0. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A. Calcium, magnesium, and serum factors in multiplication of normal and transformed human lung fibroblasts. In Vitro. 1980 Jun;16(6):475–485. doi: 10.1007/BF02626460. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Rubin A. H., Terasaki M., Sanui H. Major intracellular cations and growth control: correspondence among magnesium content, protein synthesis, and the onset of DNA synthesis in BALB/c3T3 cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3917–3921. doi: 10.1073/pnas.76.8.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Central role for magnesium in coordinate control of metabolism and growth in animal cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3551–3555. doi: 10.1073/pnas.72.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Koide T. Early cellular responses to diverse growth stimuli independent of protein and RNA synthesis. J Cell Physiol. 1975 Aug;86(1):47–58. doi: 10.1002/jcp.1040860107. [DOI] [PubMed] [Google Scholar]
- Rubin H. Magnesium deprivation reproduces the coordinate effects of serum removal or cortisol addition on transport and metabolism in chick embryo fibroblasts. J Cell Physiol. 1976 Dec;89(4):613–625. doi: 10.1002/jcp.1040890418. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin A. H. Measurement of total, intracellular and surface bound cations in animal cells grown in culture. J Cell Physiol. 1979 Aug;100(2):215–226. doi: 10.1002/jcp.1041000203. [DOI] [PubMed] [Google Scholar]