Abstract
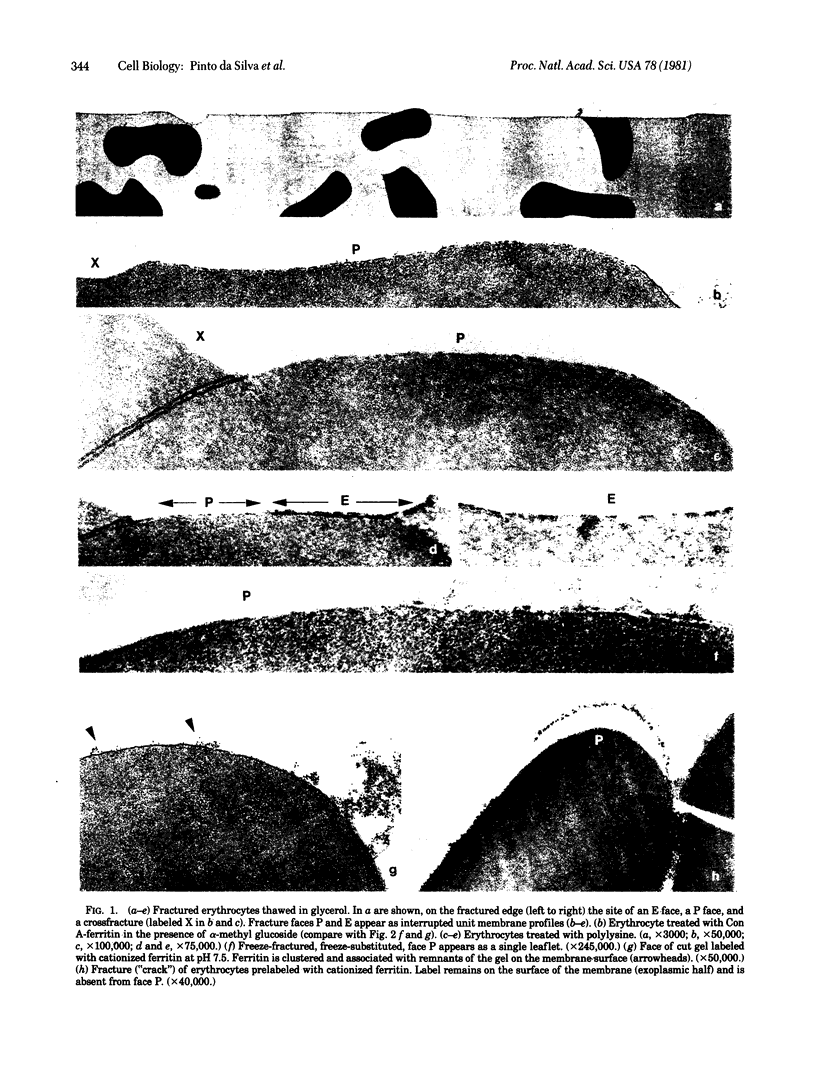
A method--"fracture label"--is described for the cytochemical labeling of the membrane faces produced by freeze-fracture. Human erythrocytes embedded in a crosslinked matrix are frozen, fractured in liquid nitrogen, thawed, labeled, and cut into thin sections. Electron microscope observation of the fracture faces shows preferential partition of concanavalin A binding sites with the inner half of the membrane. This signifies that, during freeze-fracture, binding sites are dragged from the outer surface across the outer ("exoplasmic") half of the membrane and retained on the protoplasmic fracture face (face P). The fracture process results in exposure of new anionic sites on face P. Fracture-label can be applied to the cytochemical characterization of the cellular components exposed by freeze-fracture of isolated cells and tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Edwards H. H., Mueller T. J., Morrison M. Distribution of transmembrane polypeptides in freeze fracture. Science. 1979 Mar 30;203(4387):1343–1346. doi: 10.1126/science.424755. [DOI] [PubMed] [Google Scholar]
- Findlay J. B. The receptor proteins for concanavalin A and Lens culinaris phytohemagglutinin in the membrane of the human erythrocyte. J Biol Chem. 1974 Jul 25;249(14):4398–4403. [PubMed] [Google Scholar]
- Fisher K. A. Analysis of membrane halves: cholesterol. Proc Natl Acad Sci U S A. 1976 Jan;73(1):173–177. doi: 10.1073/pnas.73.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Glycophorin in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4653–4657. doi: 10.1073/pnas.71.12.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979 Jan;16(1):3–20. [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Fudenberg H. H. Anionic sites on the membrane intercalated particles of human erythrocyte ghost membranes. Freeze-etch localization. Exp Cell Res. 1973 Sep;81(1):127–138. doi: 10.1016/0014-4827(73)90119-5. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Nogueira M. L. Membrane fusion during secretion. A hypothesis based on electron microscope observation of Phytophthora Palmivora zoospores during encystment. J Cell Biol. 1977 Apr;73(1):161–181. doi: 10.1083/jcb.73.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. P., Martínez-Palomo A., Gonzalez-Robles A. Membrane structure and surface coat of Entamoeba histolytica. Topochemistry and dynamics of the cell surface: cap formation and microexudate. J Cell Biol. 1975 Mar;64(3):538–550. doi: 10.1083/jcb.64.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. P., Nicolson G. L. Freeze-etch localization of concanavalin A receptors to the membrane intercalated particles of human erythrocyte ghost membranes. Biochim Biophys Acta. 1974 Sep 23;363(3):311–319. doi: 10.1016/0005-2736(74)90071-6. [DOI] [PubMed] [Google Scholar]
- Sjöstrand F. S. The interpretation of pictures of freeze-fractured biological material. J Ultrastruct Res. 1979 Dec;69(3):378–420. doi: 10.1016/s0022-5320(79)80055-6. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The band 3 protein of the human red cell membrane: a review. J Supramol Struct. 1978;8(3):311–324. doi: 10.1002/jss.400080309. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Marchesi V. T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J Cell Biol. 1970 Jun;45(3):649–653. doi: 10.1083/jcb.45.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Branton D. Reconstitution of intramembrane particles in recombinants of erythrocyte protein band 3 and lipid: effects of spectrin-actin association. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3891–3895. doi: 10.1073/pnas.73.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Verkley A. J., van Echteld C. J., Gerritsen W. J., Mombers C., Noordam P. C., de Gier J. The occurrence of lipidic particles in lipid bilayers as seen by 31P NMR and freeze-fracture electron-microscopy. Biochim Biophys Acta. 1979 Aug 7;555(2):200–209. doi: 10.1016/0005-2736(79)90160-3. [DOI] [PubMed] [Google Scholar]