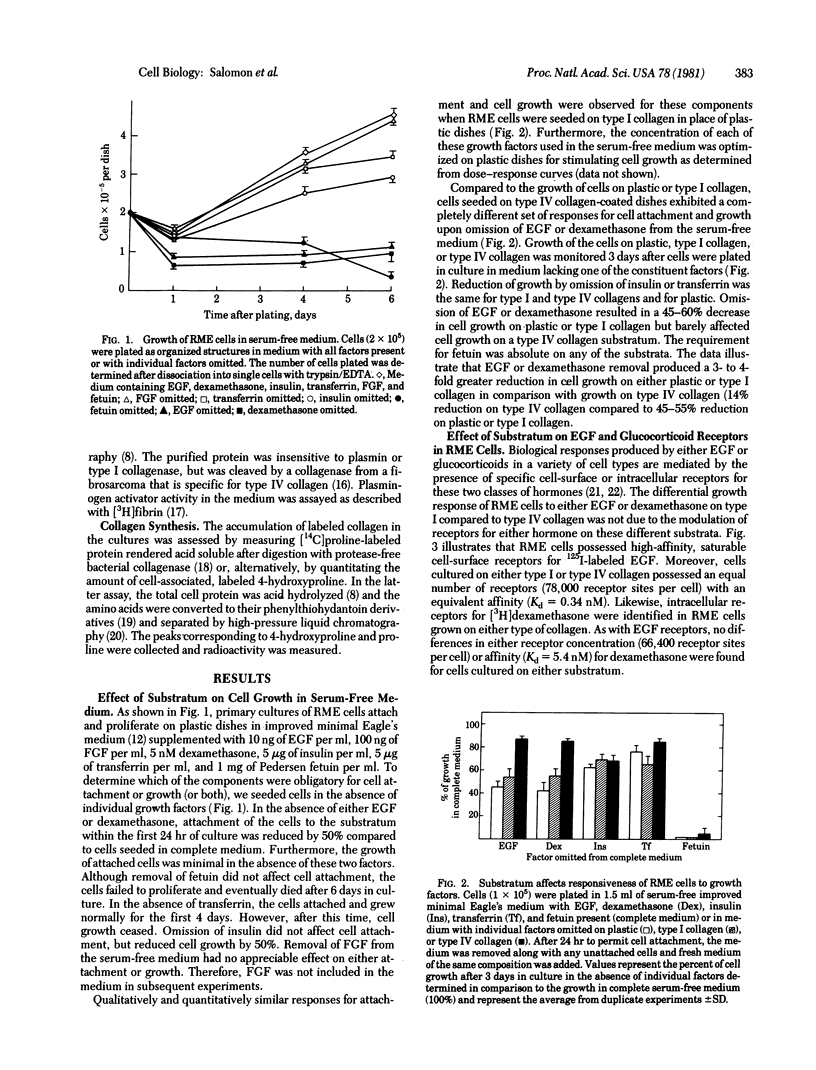
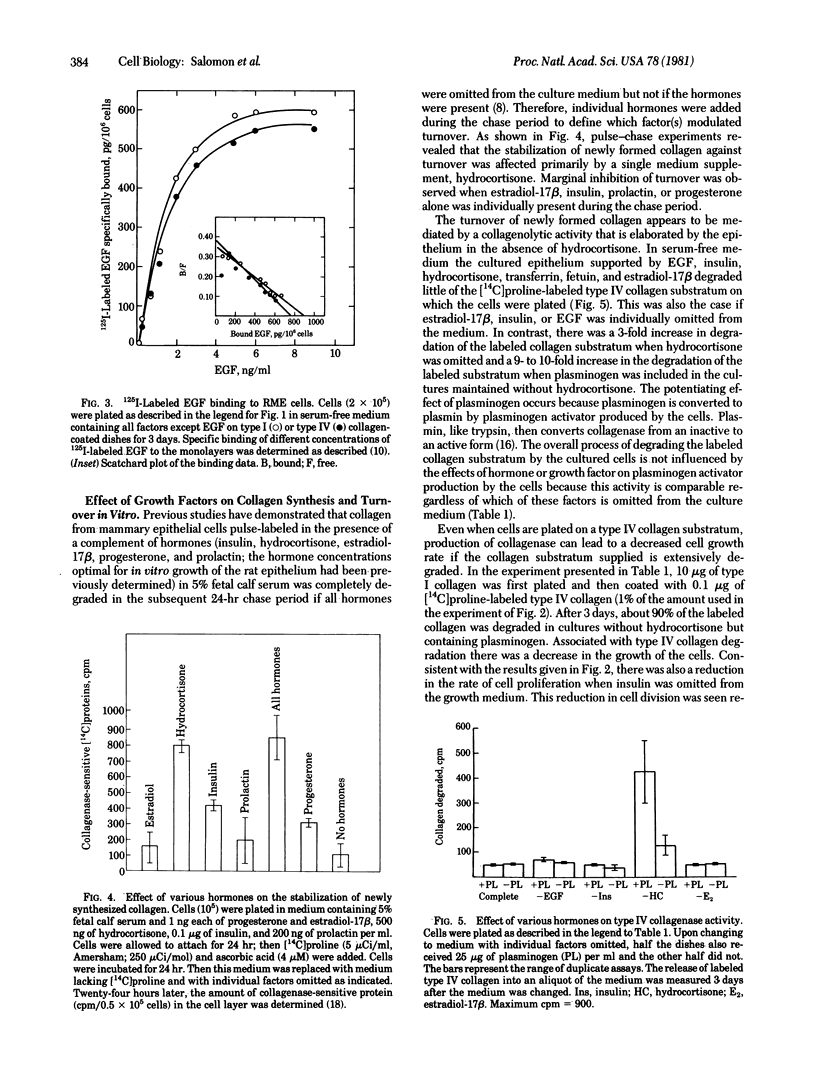
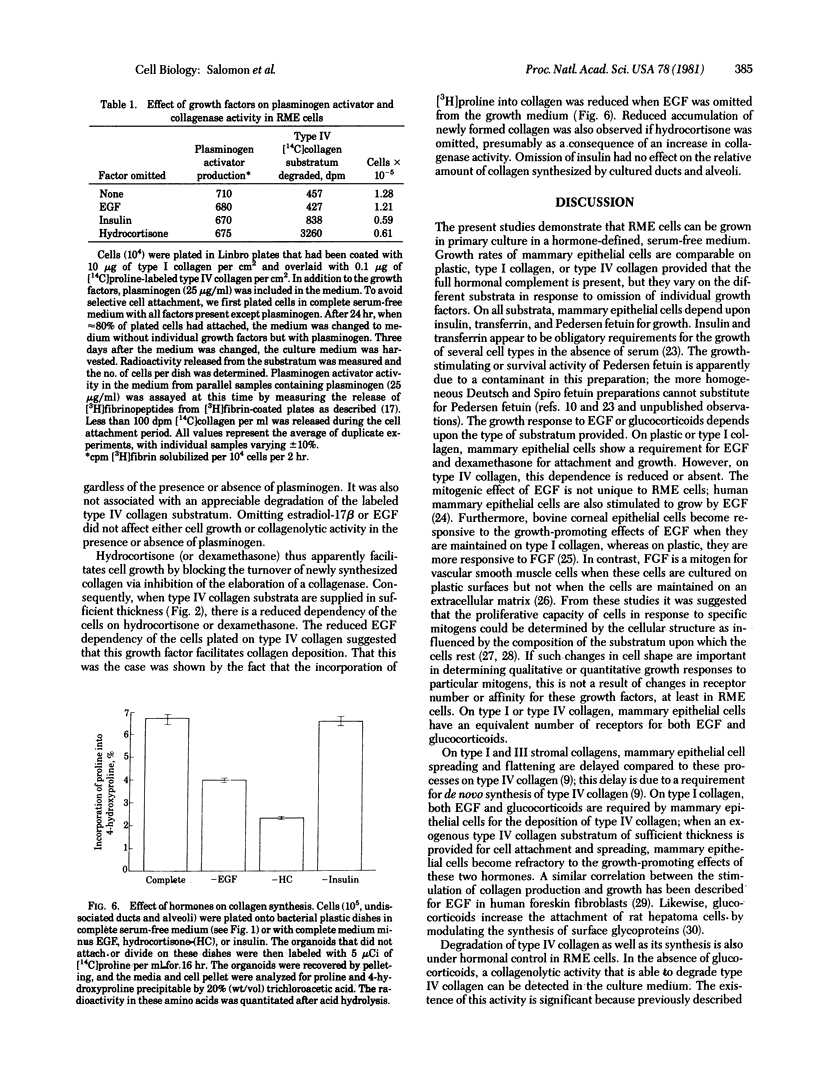
Abstract
Primary cultures of rat mammary epithelial cells proliferate and synthesize basement membrane collagen (type IV collagen) in a serum-free medium supplemented with epidermal growth factor (EGF), hydrocortisone or dexamethasone, insulin, transferrin, and Pedersen fetuin. The growth response of the cells to EGF and glucocorticoids but not to insulin or transferrin varies depending on the substratum on which the cells are plated. Cell growth is 4 times more sensitive to omission of EGF or glucocorticoid on type I collagen or plastic substratum than on type IV collagen substratum. The mechanism by which these two growth factors differentially affect cell growth appears to be linked to an increase in type IV collagen synthesis and a stabilization of secreted type IV collagen in the extracellular matrix. Glucocorticoids suppress the elaboration of type IV collagenolytic activity by the cells whereas EGF stimulates amino acid incorporation into type IV collagen. The results suggest that EGF and glucocorticoids affect mammary epithelial cell growth by facilitating the accumulation of the appropriate cell substratum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Ts'o P. O. Quantitation of fibrinolytic activity of Syrian hamster fibroblasts using 3H-labeled fibrinogen prepared by reductive alkylation. Cancer Res. 1977 Apr;37(4):1182–1185. [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Dürnberger H., Kratochwil K. Specificity of tissue interaction and origin of mesenchymal cells in the androgen response of the embryonic mammary gland. Cell. 1980 Feb;19(2):465–471. doi: 10.1016/0092-8674(80)90521-8. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fredin B. L., Seifert S. C., Gelehrter T. D. Dexamethasone-induced adhesion in hepatoma cells: the role of plasminogen activator. Nature. 1979 Jan 25;277(5694):312–313. doi: 10.1038/277312a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R. Do plasma and serum have different abilities to promote cell growth? Proc Natl Acad Sci U S A. 1980 May;77(5):2726–2730. doi: 10.1073/pnas.77.5.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Lembach K. J. Induction of human fibroblast proliferation by epidermal growth factor (EGF): enhancement by an EGF-binding arginine esterase and by ascorbate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):183–187. doi: 10.1073/pnas.73.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Abe S., Robey P. G., Martin G. R. Preferential digestion of basement membrane collagen by an enzyme derived from a metastatic murine tumor. Proc Natl Acad Sci U S A. 1979 May;76(5):2268–2272. doi: 10.1073/pnas.76.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Wicha M. S., Foidart J. M., Rennard S. I., Garbisa S., Kidwell W. R. Hormonal requirements for basement membrane collagen deposition by cultured rat mammary epithelium. Lab Invest. 1979 Dec;41(6):511–518. [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Salomon D. S. Correlation of receptors for growth factors on mouse embryonal carcinoma cells with growth in serum-free, hormone-supplemented medium. Exp Cell Res. 1980 Aug;128(2):311–321. doi: 10.1016/0014-4827(80)90067-1. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Stoker M. G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977 Dec 15;20(6):903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Vonderhaar B. K., Kidwell W. R. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980 Dec;80(2):253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]


